Factors influencing direct shoot regeneration from leaves,petioles,and plantlet roots of triploid hybrid Populus sect.Tacamahaca
Yan Zhang•Beibei Wang•Liqin Guo•Wenting Xu•Zewei Wang•Bailian Li,2•Jinfeng Zhang
Abstract Since the generation of full-sib artificial triploid families,rapid clone establishment and genetic improvements have been needed.Here,we report an in vitro method of direct shoot regeneration of a triploid hybrid poplar[(Populus simonii× P.nigra ‘Italica’)× (P.× ‘popularis’)].Using different randomized block designs,we selected one triploid to evaluate the explant type,optimal concentrations of plant growth regulators and agar,and culture time under light or dark conditions over 60 days.The highest rate of shoot induction,80.0%,was obtained using Murashige and Skoog(MS)medium supplemented with 0.2 mg/L benzyladenine,0.04 mg/L naphthaleneacetic acid(NAA),and 5.5 g/L agar for the first 30 days in the dark,then 3 g/L agar for the next 30 days in light.This last medium yielded the best rate of shoot induction(6.32 shoots/explant).These three media were also used to evaluate the influence of the genotypes of the parents and hybrid triploids on regeneration.Two parents and three of the four full-sib triploids were regenerated successfully;different genotypes and explant types significantly affected the rate of shoot induction and average number of shoots.Leaves but not petioles were a suitable explant.One genotype produced the highestrate of shoot induction of 96.67%.Half-strength MS medium supplemented with 0.2 mg/L indole butyric acid and 0.04 mg/L NAA was the most effective for rooting;rooting rate was 96.67%,survival rate of transplants was 73.33%,and rooting frequency surpassed 85%for each genotype.Overall,this in vitro regeneration system will be useful for the propagation and genetic modification of triploid poplars.
Keywords Direct organogenesis·Dark incubation ·Twostep culture
Introduction
Populus belongs to the Salicaceae family and is found throughout the Northern Hemisphere.Because it provides many useful products,such as wood, fibre,fuelwood,and other forestry products and can contribute to sustainable development,poplars are recognized internationally,especially in developing countries (Isebrands and Richardson 2014).As a result of its small genome,short rotation cycle,rapid growth rate,and easy vegetative propagation,poplar has become a model system in forest tree biotechnology(Confalonieri et al.2003;Yang et al.2009).In China,some superior poplar cultivars,progenies of Populus simonii×Populus nigra var.Italica(Moench)Koehne,such as P. ‘baicheng2#’and P.× ‘popularis’,have been widely cultivated in the Three Norths Forest Protection system.Genetic breeding of these poplars is ongoing,especially with respect to hybridization and allopolyploid breeding(Guo et al.2017).
Allopolyploids result from the merging of two or more genomes by intraspecific or interspecific hybridization(Song and Chen 2015;Tamayo-Ordóñez et al.2016).Their high biomass and fitness has benefited agriculture(Leitch and Leitch 2008;Chen 2013).Polyploidy breeding of poplars has also progressed greatly since the first triploid Populus was discovered(Müntzing 1936;Nilsson-Ehle 1936).Hybridization to yield different ploidy levels,by physical and chemical induction and endosperm culture,including triploids of Leuce,Aigeiros,and Tacamahaca,or selected for by natural selection(Zhu et al.1995;Xi et al.2012;Guo et al.2017).In addition,some cultivars exhibit superior traits,including rapid growth,long fibre length and high fibre content(Weisgerber et al.1980;Zhu et al.1995;Zhang et al.2004).However,several agricultural disadvantages have been revealed.Moreover,variations in gene structure and function following poplar polyploidization remain elusive;however,the use of genetic transformation to modify specific sites in genes of interest in the forestry industry could help solve this problem.For woody plants,a highly efficient regeneration system is essential for genetic modification.To date,in vitro triploid poplar regeneration systems have only been successful for Leuce(Hao et al.1999;Cai et al.2003;Wang et al.2014),so establishing a regeneration system for other polyploid poplar species is important.
Direct regeneration is favoured over indirect regeneration in poplars.Moreover,leaves,stem internodes,petioles,and root segments can be regenerated directly,as done for Populus alba×grandidentata(Son and Hall 1990),cottonwood hybrids(Han et al.2000),P.deltoides clone G48(Thakur et al.2012)and P.tomentosa(Wei et al.2017).Leaf explants have been commonly used for the genetic transformation of Populus.In Populus nigra,the percentage generation of ß-glucuronidase-positive plants from the leaves was significantly higher than that from stem segments(Confalonieri et al.1993).Petioles have greater resistance to genetic variation compared with other explants(Pierik 1991)and thus would be an ideal material for in vitro plant regeneration.
Increasing the proliferation rate and capacity for bud elongation are critical processes in plant tissue culture.It has been shown that in vitro incubation under dark conditions positively affects adventitious shoot formation and elongation in several species(Vieitez and San-José1996;Gao and Bao 2005;Huang et al.2005),and liquid medium or medium with a lower agar concentration promotes elongation of adventitious shoots but is often followed by vitrification(Arnold and Eriksson 1984).Liquid medium or semi-liquid medium also has a positive influence on shoot multiplication and elongation in Populus alba×P.grandidentata(Chun et al.1986)and Populus deltoides(Yadav et al.2009).However,a systematic study regarding the effects of different agar concentrations and a dark incubation period on shoot multiplication and elongation has not been tested for poplar species.
Indole butyric acid(IBA)and naphthaleneacetic acid(NAA),alone and in combination,are commonly used for bud rooting.However,in a previous study of Populus domestication,more attention was focused on the nutrition substrates,as for Populus hopeiensis(Feng et al.2003),Populus davidiana×bolleana(Wu et al.2007)and Populus×euramericana cv.Guariento(Liu and Li 2014),and the relationship between root growth and survival rate was not assessed.In that study,the primary objective was to establish an efficient system for triploid regeneration from the leaves and petioles to evaluate the effects of plant growth regulators(PGRs),duration in the dark,agar concentration,and genotype on adventitious shoot regeneration.Finally,we investigated the influence of PGRs on rooting and survival percentages.The resulting protocol should prove useful for forestry breeding and genetic modification.
Materials and methods
Plant material and explant manipulation
Coppice shoots from the female parent tree,P.simonii×P.nigra var.Italica,were collected from Chajintai forestry centre(Tongliao City,Inner Mongolia Autonomous Region,P.R.China),and coppice shoots from the male parent tree,P.× ‘popularis’,were collected from the Dadongliu Nursery(Changping District,Beijing,P.R.China).The five genotypes of the full-sib triploid family were induced in our lab(Guo et al.2017)and named T1,T2,T3,T4,and T5,respectively.In this study, five genotypes were selected randomly from the triploid group,and T1 was selected randomly from the five genotypes.All materials were cultivated in a greenhouse at Beijing Forestry University.
In the spring of 2014,young branchlets were cut to 2–3 cm and immersed in washing powder for 3 min,washed with tap water for 20 min,sterilized in 75%ethanol(v/v)for 45 s,rinsed once with sterile distilled water,followed by 0.2%w/v sodium hypochlorite solution for 12 min,and they were then washed three times with sterile distilled water.Sterilized shoots were then added to Murashige and Skoog(MS)basal medium(Murashige and Skoog 1962)supplemented with 5.5 g/L agar,30 g/L sucrose,0.2 mg/L IBA,and 0.02 mg/L NAA in magenta boxes with 50 mL medium.The pH was adjusted to 5.8 before autoclaving.Explants were incubated at 25± 2°C under cool white fluorescent light and a 16 h light/8 h dark photoperiod.
Plant propagation
Plants were subcultured and propagated in rooting medium(half-strength MS medium supplemented with 0.2 mg/L IBA and 0.02 mg/L NAA),and some of the plants were incubated in the dark for 20–25 days for lateral bud elongation.
Plant propagation and regeneration
The second to fourth in vitro leaves and petioles were collected from T1 plantlets that had been subcultured for 40 days.The leaf samples were proximal halves cut twice through the main vein,and the petioles were cut into 0.5–0.8 cm pieces;both were placed in shoot regeneration medium,with the adaxial side in contact with the medium.All tests were conducted using a randomized block design.The basal MS medium was supplemented with 30 g/L sucrose.Different concentrations of BA(0.2,0.4,and 0.6 mg/L)and NAA(0,0.02,0.04,and 0.08 mg/L)were added to the medium alone and in combination to determine the optimum combination of growth regulators,for a total of 12 treatments.The initial dark incubation period tested was 0,15,30,45,and 60 days,followed by incubation in the light.Other light–dark treatments were initially tested:light for 0,15,30,45,and 60 days,followed by 60 days in the dark,then 5 days in the light for greening,for a total of 65 days;eight different light treatments were tested.To determine the optimal concentration of agar,the culture period was divided into two periods,each 30 days long,and the first stage was supplemented with 3,5.5,and 8 g/L agar,and the second stage was supplemented with 3,5.5,and 8 g/L agar,for a total of nine treatments.The parents and four triploids were cultured on the three selected media,containing H3 and A4,and were combined with a randomly chosen medium from the group,such that the overall rate of shoot induction was greater than 50%,to measure the regeneration capacity of the different genotypes.
Each treatment was done in triplicate,using a total of 30 explants.The magenta boxes contained 40 mL medium,and five leaves and five petioles were placed on the surface and subcultured after 30 days.The cultures were incubated at 25± 2°C under cool white fluorescent light and a 16/8 h photoperiod.After 60–65 days,the number of adventitious shoots(≥1 cm)and the percentage shoot induction and vitrification were recorded.All of the adventitious shoots mentioned in this study were greater than 1 cm.
Shootinduction percentage:Numberofshoot/All explants×100%
Number of shoots/explant:Total number of shoots/Induced explants
Vitrification percentage:Number of vitrificated shoot/Induced explants×100%
Rooting and acclimatization
Regenerated shoots from A4 medium were excised,cut to approximately 1.5 cm,rooted in half-strength MS medium with different concentrations of IBA(0.1 and 0.2 mg/L)and NAA(0–0.12 mg/L),5.5 g/L agar,and 30 g/L sucrose,and placed in magenta boxes with 50 mL medium.The pH was adjusted to 5.8 before autoclaving.Each treatment consisted of 10 magenta boxes with three shoots per box.After 45 days,the rooting rate,number of main roots,and plantlet length were recorded.On the same day,20 mL sterile distilled water were added to the boxes,and the boxes were kept partially open for 5 days.All treated plantlets were picked up gently with forceps.After three washes,the plantlets were transplanted into plastic pots containing a 1:2:1:1 sterilized mixture (autoclaving,121°C,30 min)of garden soil,peat,perlite,and vermiculite and were maintained in a greenhouse at 25± 5°C with a natural photoperiod.The survival rate was examined after 1 month.
Rooting rate: Number of rooting plantets/All shoots×100%
Transplant survival rate:Number of survivaled plantet/All plantets×100%
Statistical analysis
The significance differences among treatments were evaluated at p=0.05 using Dennett’s T3 test.Welch’s t-test was applied to a one-way ANOVA with nonhomogeneous data.Analysis of variance and Dennett’s T3 test were performed using SPSS version 18.0(SPSS,Chicago,IL,USA).Arcsine transformation was applied to percentage data before the ANOVA using Excel(2010,Microsoft,Redmond,WA,USA).
Results
Propagation and effects of PGRs on the rate of shoot induction
The majority of buds rooted successfully following sterilization.Other buds were incubated in the dark for 20–25 days for elongation(Fig.1a),and the majority turned green after culture in light for 5 days.After 6–7 days,nodules could be seen on the main vein of the leaf,and the first buds longer than 1 cm were found after 25–30 days of culture.The regeneration capacity of the leaf was significantly higher than that of the petiole(Table 1).In the leaf,from the data of H1–H8 medium,the levels of cytokinin and auxin had a significant effect on the average number of shoots and the rate of shoot induction,as did their interaction(Table 2).H3 medium containing 0.2 mg/L BA and 0.04 mg/L NAA was the most effective for the leaf explants and petiole segments(Table 1),whereas the rate of shoot induction of the leaf,76.67%(Fig.1b),was much higher than that of the petiole,33.33%(Fig.1c).However,36.11%of the leaf explants that acquired adventitious shoots were vitrified(Fig.2k).
Effects of culture in the dark on shoot regeneration
During the first 30 days,the development of adventitious buds on the petioles was slower than that on the leaf(Fig.2a–c).The maximum rate of shoot induction,80%,and the greatest number of shoots,4.56/explant,were observed with leaves cultured in L3 medium (dark:30 days;light:30 days)and incubated in the dark for 30 days followed by light for 30 days(Fig.2j,Table 3).For the petiole,L2 medium(dark:45 days;light:15 days)was the most effective for shoot regeneration(36.67%)and 1.75 shoots/explant(Fig.2k).Meanwhile vitrification markedly increased as the incubation period in the dark increased.A significant difference was found in shoot regeneration between the leaf segments and petioles.The rate of shoot induction and the number of shoots from the petiole were much lower than those from the leaf.
Effects of agar concentration on shoot regeneration

Fig.1 Propagation,regeneration,and acclimatization from shoots,leaves,petioles and plantlets of triploid[(P.simonii×P.nigra‘Italica’) × (P.× ‘popularis’)]respectively.a Axillary bud elongation in the dark after cultured 25 days;b direct shoot regeneration from leaves after cultured 60 days;c direct shoot regeneration from petioles after cultured 60 days;d morphology of plantlets acclimatization after 1 month under 25± 5°C with a natural photoperiod in greenhouse
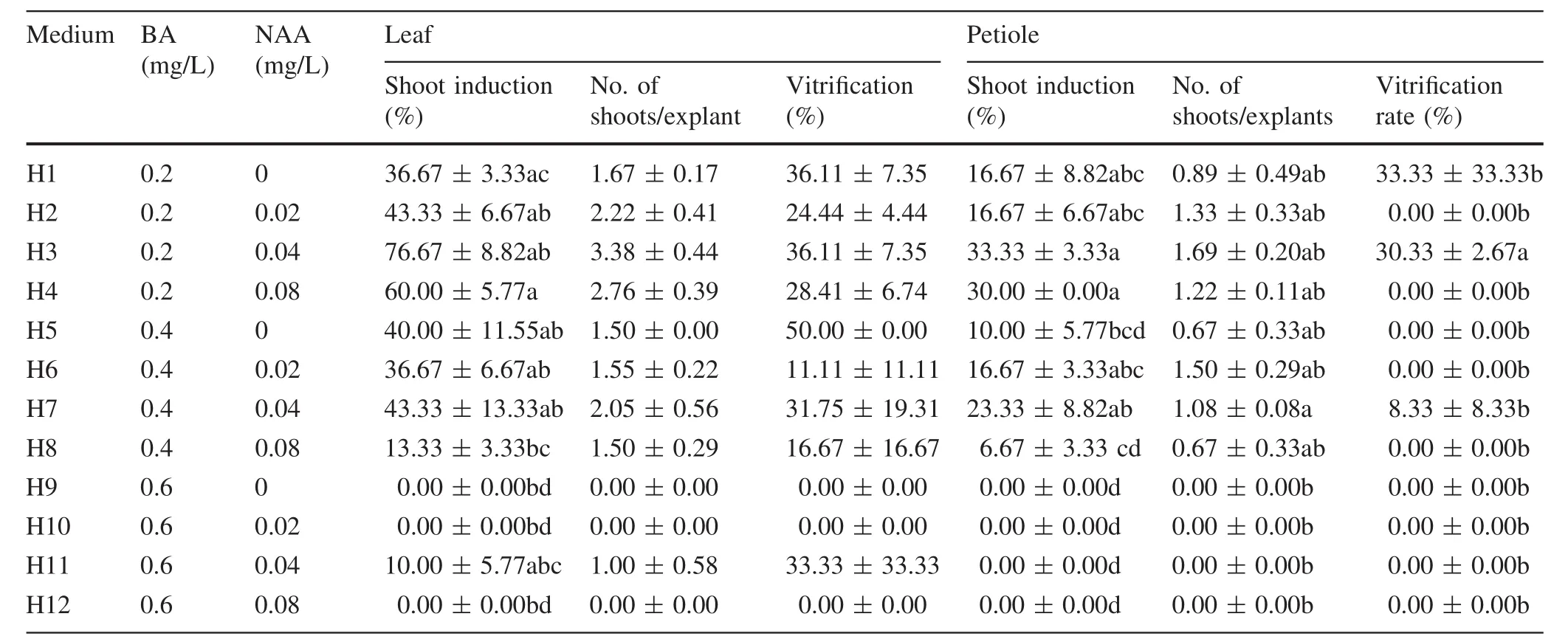
Table 1 Effects of BA and NAA in different media on shoot induction from T1 leaves and petioles of triploid[(P.simonii×P.nigra‘Italica’) × (P.× ‘popularis’)]and vitri fication
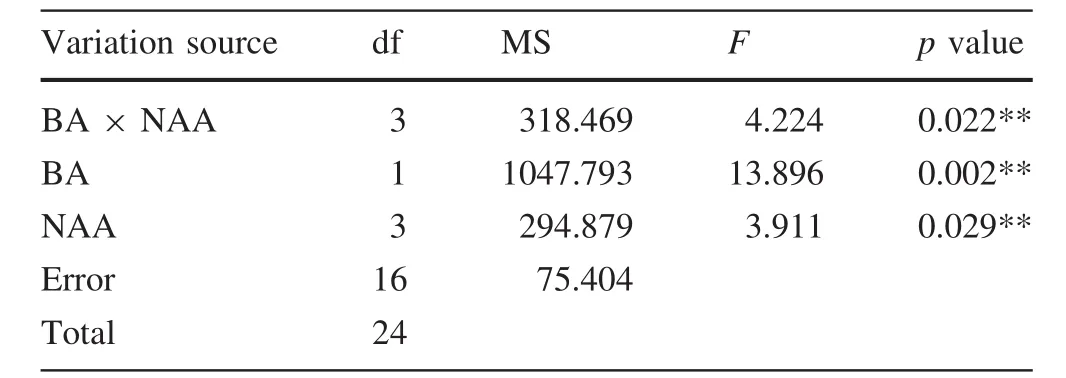
Table 2 Variation analyses of shoot induction rate for different cytokinin and auxin combinations
The regeneration capacity of the leaf was significantly higher than that of the petiole(Fig.3).The effects of the agar concentration on the rate of shoot induction and the number of shoots differed signi ficantly between the leaf and petiole.For the leaf,the A4 medium was supplemented with 5.5 g/L agar during the first stage and 3 g/L agar during the second stage,and the average number of shoots was 6.32/explant,with a rate of shoot induction of 80.00%(Fig.3e,Table 4).For the petiole,the maximum number of shoots was 1.56/explant,and only 23.33%of the petioles were regenerated in medium A5(Fig.3h).
Effect of genotype on shoot regeneration in the explants
The parents and two genotypes of the triploid progenies were regenerated on A4 medium,and T3 was regenerated successfully on H4 medium(Table 5).The optimal genotype for shoot regeneration was T2.For the T2 leaves and petioles,the highest rate of shoot induction,96.67%,was obtained on H3,and the highest average numbers of shoots,4.56/plant and 1.78/plant,were obtained on A4 and H3 media,respectively.
Rooting and acclimatization
Shoots regenerated in A4 medium containing 0.2 mg/L BA,0.04 mg/L NAA,and 5.5 g/L agar during the first stage and 3 g/L agar during the second stage were selected for evaluation.After 45 days,morphological data were recorded(Figs.4 and 5,Table 6).None of the unrooted plants survived acclimatization.The first root appeared after the shoots were cultured for 6 days on R6 medium supplemented with 0.2 mg/L IBA and 0 mg/L NAA.The rooting rates of R1 and R2 were significant lower than for the other treatments.The levels of IBA and NAA had a significant effect on survival rate of the transplants and the rate of shoot induction,but their interaction had no significant effect(Table 7).The survival rate of the transplants was slightly positively correlated with the number of roots(Fig.6).The highest survival rate of the transplants,73.33% (Fig.1d,Table 6),the highest plant height,3.52 cm(Fig.5i),and the greatest number of roots/explant,6.05/plant(Fig.4i),occurred in R9 medium.
Discussion
We have described a method of in vitro regeneration and rooting for triploid hybrid poplars that is optimized for explant type,agar concentration and PGRs,time in dark
conditions,and genotype.We found that the percentage of shoot induction differed significantly different between the leaf and petiole.We assessed the effect of different incubation times under dark conditions on elongation.We also highlighted the importance of the agar concentration on promoting bud formation and elongation,as well as the influence of exogenous hormones on shoot rooting and plant growth.The potential of the application of this system in the future is also discussed.

Fig.2 Morphology of shoot regeneration from the T1 leaf and petiole of triploid[(P.simonii× P.nigra ‘Italica’)× (P.× ‘popularis’)]following different intervals in the dark.a Dark:15 days.b Dark:30 days.c Dark:45 days.d Dark:60 days.e Dark:15 days,f Light:30 days.g Light:45 days.h Light:60 days.i Dark:15 days;light:45 days.j Dark:30 days;light:30 days.k Dark:45 days;light:15 days.l Light:15 days;dark:60 days.m Light:30 days;dark:30 days.n Light:45 days;dark:15 days.o Light:15 days;dark:45 days;light:5 days.p Light:30 days;dark:30 days;light:5 days.q Light:45 days;dark:15 days;light:5 days.r Light:60 days;dark:0 days;light:5 days
The leaf and petiole are generally used for in vitro regeneration of Populus.In a previous study,the average regeneration rate from leaves of Populus tremula ‘Erecta’(53%)was lower than that from petioles(91.3%)in a zeatin medium(Huang and Dai 2011).In Populus ciliata Wall.,the average percentage regeneration from leaves was 72 and 86%from petioles and(Thakur and Srivatava 2006;Aggarwal et al.2015).In this study,the leaves and petioles of the parents and five triploids were considered from the beginning to end.The results showed that the regeneration ability of the leaf is greater than that of the petiole.It is possible that regeneration of the leaves and petioles of the progeny is determined by their parents,suggesting that the differences between leaf and petiole regeneration may be apparent among different species or cultivars of Populus spp.Moreover,the petiole,unlike the leaf,did not adapt well to the low agar concentration,but the vitrification rate of the leaf was higher than that of the petiole.
In vitro bud elongation is important in adventitious shoot formation.The majority of the scrubby buds could not continue to grow optimally when placed in rooting medium.In our preliminary experiments,we were able to easily acquire multiple shoots(<0.5 cm)during the early stages of regeneration;however,most of them did not elongate.We found that culturing in the dark for the first 30 days was the most effective treatment for increasing the number of shoots.Light for 30 days followed by dark for 30 days or light for 45 days followed by dark for 15 days was also better than no incubation in the dark. ‘Qinmei’kiwifruit(Actinidia deliciosa)(Zhang et al.2016)and P.tremula(Huang and Dai 2011)also prefer to be incubated in the dark for the first 30 days.Apples incubated for 10 days in the light followed by 10–15 days in the dark were found to have the highest propagation efficiency(Shi et al.2004).Our results suggest that a suitable amount of time in the dark,whether at the beginning or a later,may also promote adventitious shoot formation and elongation.However,too much time in the dark also led to shoot vitrification when the explants were exposed to light(Fig.2p,r).Similar results have been observed for Cydonia oblonga(Baker and Bhatia 1993);incubation in the dark for longer than 5 weeks led to adventitious shoot vitrification.The shade avoidance syndrome is a well-known phenomenon in plants grown in the dark or low light;a decreased ratio of red(R)/far-red(FR)light promotes stem and petiole elongation to avoid shadeing.Thus,monitoringof the R:FR ratio is an important mechanism to avoid the challenge from shade(Smith and Whitelam 1997;Franklin 2008).Several genes that respond to phytohormones that contribute to shoot elongation in shade(Devlin et al.2003),such as auxin(Morelli and Ruberti 2000),brassinosteroids(BRs)(Luccioni et al.2002),ethylene and gibberellins(Pierik et al.2004),have also been con firmed.We suggest that these genes may be the principle contributor to adventitious shoot elongation in the dark.To the best of our knowledge,few reports have mentioned this mechanism for tissue culture.
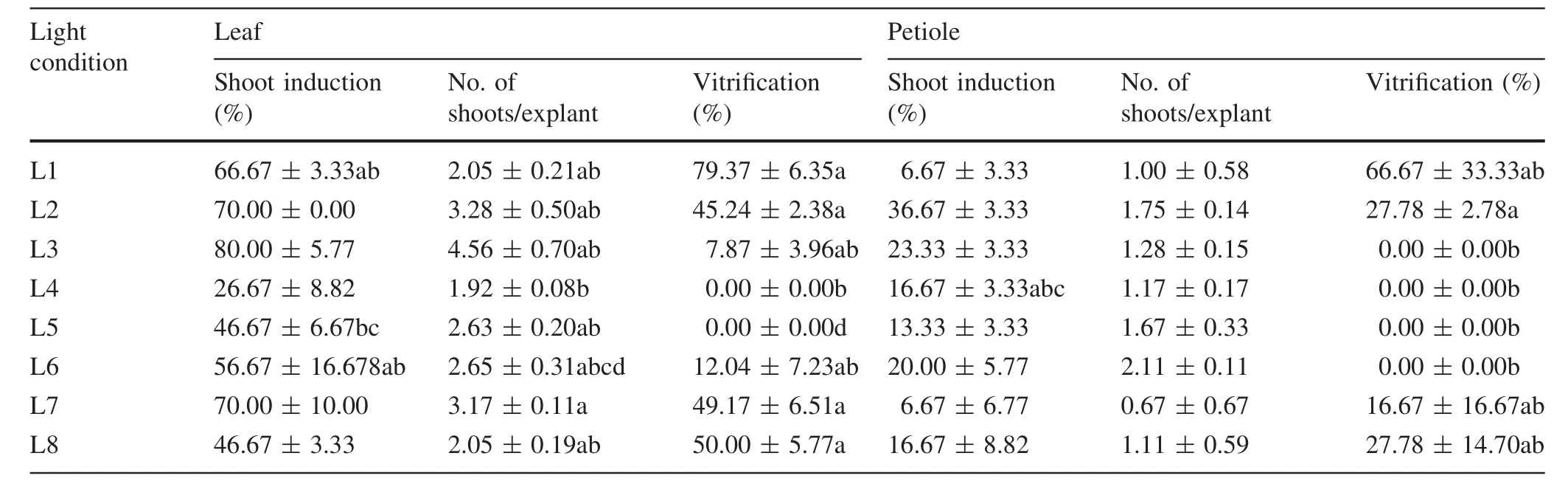
Table 3 Effects of dark conditions on the adventitious rate of T1 shoot induction

Fig.3 Morphology of shoot regeneration from the T1 leaf and petiole of triploid[(P.simonii× P.nigra ‘Italica’)× (P.× ‘popularis’)]at different stages of growth and with different agar concentrations.a–c 30 days old,d–l 60 days old
In general,the nutrients in liquid media are more available than is semi-solid media.However,the use of liquid medium is often accompanied by vitrification,which can damage shoots;the excessive synthesis of plant hormones,such as ethylene,when the explants are grown in liquid medium,could be responsible for vitrification(Dolev 1986;Ziv 1991).To reduce this problem,we used 3 g/L agar to support the explant.In this study,with the exception of the A8 medium,none of the evaluated treatments seemed to induce vitrification.The most shoots were produced on medium with 5.5 g/L agar in the first stage and 3 g/L agar in the stage second;the plants benefited from the low concentration of agar during the second stage.The rate of shoot induction,however,was lower than expected,perhaps because some of the leaves were submerged in medium at the beginning of the experiment.In future studies,we will reduce thewater level or use a holder to allow the explants to approach the water surface more closely.Compared with 5.5 g/L agar,3 g/L agar increased the average number of shoots by 3/explant.In a previous study,shoot propagation in liquid medium was more effective than in solid medium for Populus alba×P.grandidentata(Chun et al.1986),Liquidambar styraci flua(Kim et al.1997)and Populus deltoides(Yadav et al.2009).Moreover,incubation in the dark and in liquid medium can preserve space,energy,and reagents,a primary reason this method is being explored for use in an increasing number of species.

Fig.4 Morphology of triploid[(P.simonii× P.nigra ‘Italica’)× (P.× ‘popularis’)]roots treated with different concentrations of plant growth regulators.a–j 45 days old,bottom view

Fig.5 Growth conditions of T1 plants of triploid[(P.simonii× P.nigra ‘Italica’) × (P.× ‘popularis’)]treated with different concentrations of plant growth regulators.a–j 45 days old,front view
IBA,NAA,and a combination of IBA and NAA are commonly used to induce rooting.In this study,the root number and shoot length were associated with the concentrations of the two hormones.In lime(Citrus aurantifolia),the rooting ratio ranged from 25 to 90%depending on the different concentrations of IBA,NAA,or the IBA and NAA combination.The root number and root and shoot lengths were also affected(Al-Bahrany 2002).Different concentrations of IBA and NAA influenced in vitro rooting in Populus,including Populus×euramericana‘Bofeng 1’(Cui et al.2012),P.simonii Carr.× P.deltoides ‘Shanhaiguanensis’(Fu et al.2012)and Populus× euramericana ‘Neva’(Jiang et al.2015).In our study,the survival rate of the transplants was closely associated with the number of roots.We suggest that this phenomenon may be a result of the higher number of roots,and greater shoot length may promote the absorption of additional water.In addition,a high concentration of NAA promotes callus formation and negatively influences rooting,which may explain the reason that the number of roots and the transplant survival rate were lower when the concentration of NAA was increased to 0.12 mg/L(Table 6).Few studies have reported an association between the number of roots and the survival rate of transplants;however,one report suggested that the highest percentage of rooting by cuttings(63.3%)of Olea europaea‘Domat’was associated with the most roots(4.1/cutting)(İs fendiyaroğlu and Özeker 2008).
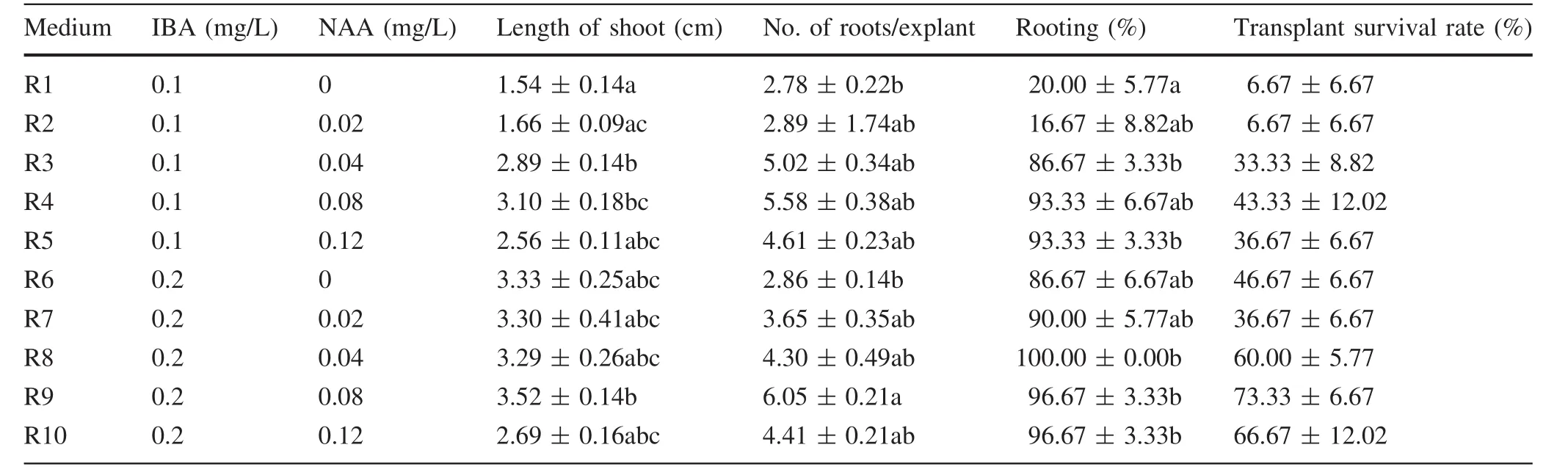
Table 6 Effects of plant growth regulators on rooting from T1 shoots and transplant survival after rooting or acclimating 1 month
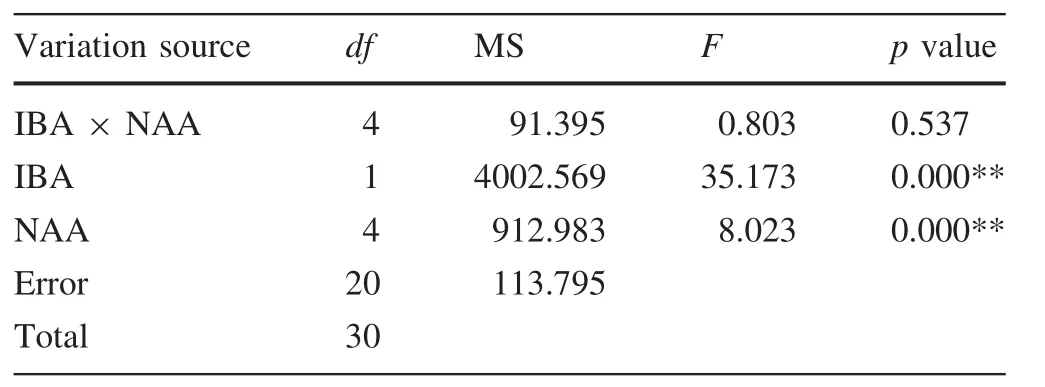
Table 7 Variation analyses of transplant survival(%)for different indole butyric acid and naphthaleneacetic acid combinations
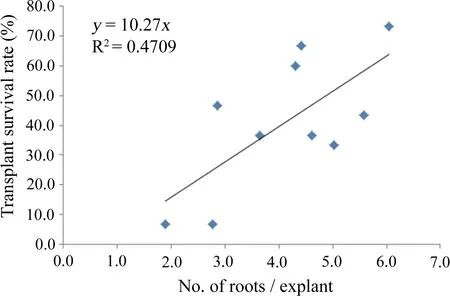
Fig.6 Correlation between the number of roots/explant and transplant survival (%) of triploid [(P. simonii×P. nigra‘Italica’)× (P.× ‘popularis’)]
To the best of our knowledge,this is the first report describing direct regeneration from leaves,petioles,and roots of triploid (P.simonii×P.nigra var.Italica)× (P.× ‘popularis’)in vitro.In the future,this method could be used for rapid propagation and genetic transformation to improve the traits of triploid poplar and to explore the mechanism of polyploidization.
AcknowledgementsThis work was supported by the Project of National Natural Science Foundation of China(31370658),Medium and Long Scientific Research Project for Young Teachers in Beijing Forestry University(2015ZCQ-SW-02),National Key R&D Program of China(2017YFD0600404-1),Program for Changjiang Scholars and Innovative Research Team in University(IRT13047),and the Project of Beijing Gardening and Greening Bureau(CEG-2016-01).
 Journal of Forestry Research2018年6期
Journal of Forestry Research2018年6期
- Journal of Forestry Research的其它文章
- Black locust(Robinia pseudoacacia L.)as a multi-purpose tree species in Hungary and Romania:a review
- The impact of the environmental factors on the photosynthetic activity of common pine(Pinus sylvestris)in spring and in autumn in the region of Eastern Siberia
- Osmoregulators in Hymenaea courbaril and Hymenaea stigonocarpa under water stress and rehydration
- Effect of nitrogen levels on photosynthetic parameters,morphological and chemical characters of saplings and trees in a temperate forest
- Free amino acid content in trunk,branches and branchlets of Araucaria angustifolia(Araucariaceae)
- Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress
