Application of LC-MS based glutathione-trapped reactive metabolites in the discovery of toxicity of traditional Chinese medicine
Xiao-Mei Liu,Hong Lv,Xiao-Ming Wang,Ya-Qing Guo,Ting-Ting Li,Gui-Xiang Pan
1Tianjin State Key Laboratory of Modern Chinese Medicine,Tianjin University of Traditional Chinese Medicine,Tianjin,China.2SecondAffiliated hospital of Tianjin University of Traditional Chinese Medicine,Tianjin,China.
Background
Some drugs are converted into chemically reactive metabolites by enzyme-mediated bioactivation.These reactive metabolitescan covalently modify cellular macromolecules such as proteins and DNA causing cell damage and ultimately lead to drug induced toxicity and subsequently negative outcomes[1].Some of traditional Chinese medicine(TCM),especially those containing pyrrolizidine alkaloids,quinoid species and furans,can produce toxic reactive metabolites.In order to reduce the elimination rate of candidate drugs,it is necessary to conduct drug candidate compounds for reactive metabolite screening in drug design and early development of new drugs to predict possible interactions between drugs and organisms.As a ubiquitous substance,glutathione(GSH)can capture reactive metabolites and prevent damage to important proteins and nucleic acid[2].Adding GSH to liver microsome or hepatocyte incubation system,and detecting or identifying GSH conjugates of reactive metabolites by liquid chromatography-tandem mass spectrometry(LC-MS)is the basic method for studying reactive metabolites[3].
GSH captures reactive metabolites
Many exogenous compounds are absorbed in the body,and then an electrophilic active intermediate is formed by metabolic reaction.These metabolites can interact with cellular components in a variety of way,such as covalent bonding with biomacromolecules and stimulate lipid peroxidation[4].This process of converting relatively inert compounds into active intermediate metabolites is commonly referred to as metabolic activation or biological activation[5,6].The biological activation process is the root cause of many TCM poisonings.However,due to the short half-life and unstable nature of reactive metabolites,they are not easily detected.Most of the reactive metabolites are electrophilic and can react with nucleophilic substances[7].In vitro capture methods are generally used to examine the bioactivation potential of these candidate drugs.The capture agents are mainly thiols(GSH,cysteine orN-acetylcysteine),amines(semicarbazide and methoxyamines)and cyano anions,among them GSH is generally used to trap reactive metabolites.GSH,a tripeptide consisting of glutamate,cysteine and glycine,is the most ubiquitous non-protein intracellularthiolin mammalian systems and its nucleophilic cysteinyl thiol group allows GSH to react with a variety of electrophilic species to form GSH conjugates[8].An importantphysiologicaldefense mechanism against chemically reactive intermediates in vivo is GSH conjugation.Based on the site of conjugation,GSH conjugates are mainly divided into five structural classes,namely aliphatic,aromatic,benzylic,disulfide,and thioester,shown in Figure 1.The GSH S-transferase orients the substrate and the GSH in a position that increases nucleophilicity of-SH towards the substrate.GSH conjugates are excreted in bile and its breakdown product(the N-acetylcysteine conjugate or mercapturic acid)is excreted in urine[9].In the liver,dipeptidases can hydrolyze glycine and glutamate from the GSH conjugate to form a cysteine conjugate,and then this conjugate is transported to the bile orblood.In the kidney,N-acetyltransferase acetylates theprimary amine of cysteine to form the N-acetylcysteine conjugate[10].Furthermore,β-lyase can cleave the carbon-sulfur bond to form a free thiol in the kidney.Excretion of mercapturic acid is often taken as a sign of the formation of the reactive species.
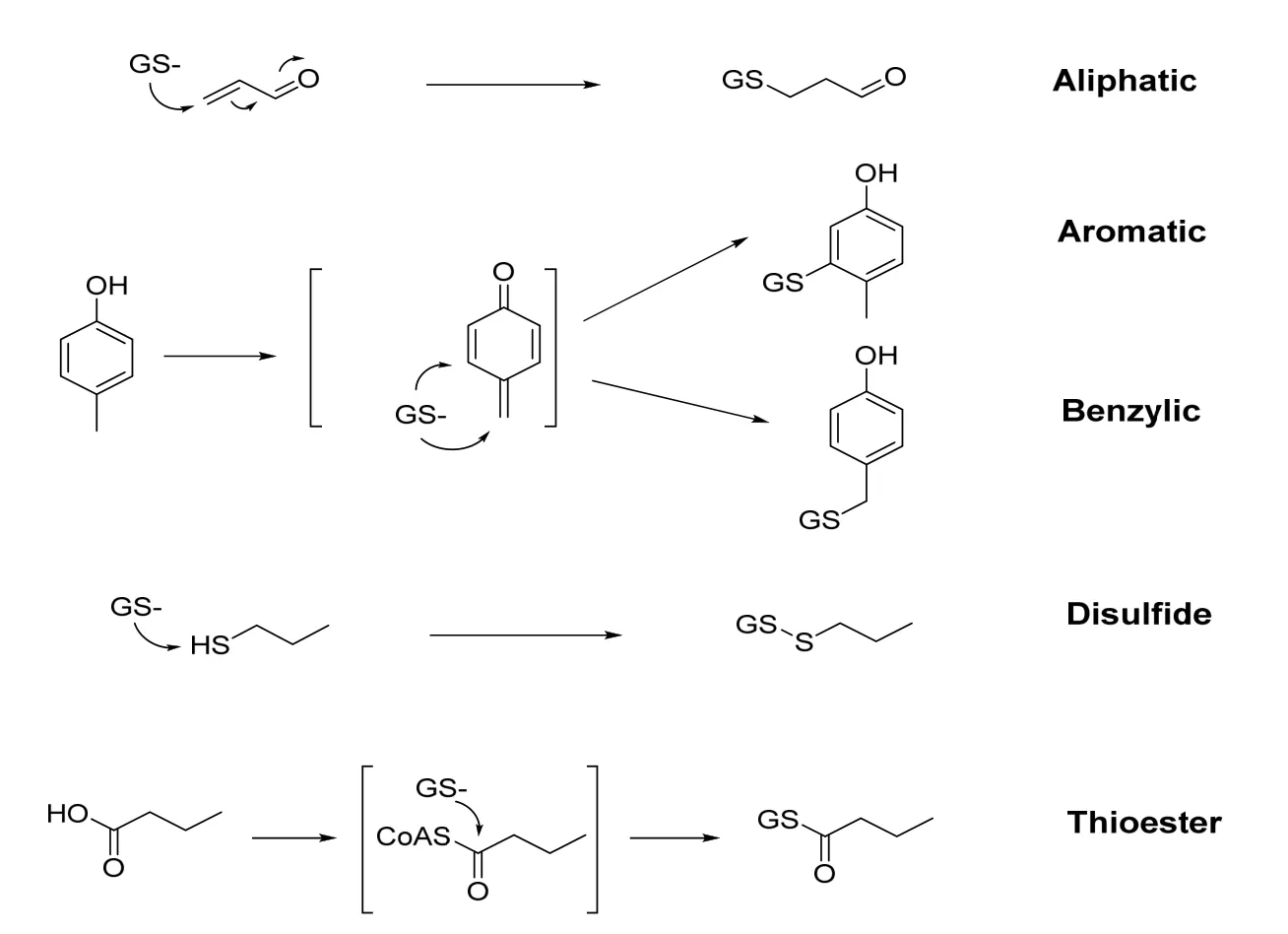
Figure 1.Different conjugation sites of GSH with reactive species
Technology for capturing toxic substances based on GSH
Although drug metabolism is a complex progress in terms of metabolic pathways and biochemical reactions,the detection of GSH conjugates can identify most of the reactive metabolites formed by drugs.GSH is a tripeptide in mammalian systems and its nucleophilic cysteinyl thiol group can trap electrophilic species to form GSH conjugates. Reactive metabolite detection using microsomal incubations in vitro with GSH is one of the most crucial steps in assessing potential toxicity of pharmaceutical compounds[11].And the identification of GSH conjugates by LC-MS(Figure 2)techniques is also an integral part of pharmaceutical research.The indirect but valuable information regarding the nature of reactive species and supporting pharmaceutical lead optimization was provided by the structural characterization of the resulting stable conjugates.
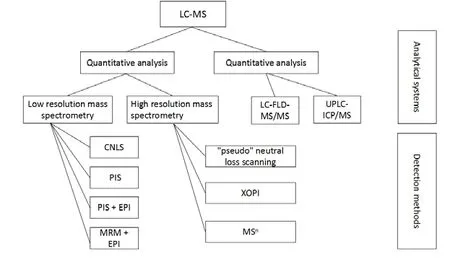
Figure 2.Qualitative and quantitative analysis techniques by LC-MS for capturing toxic substances based on GSH.
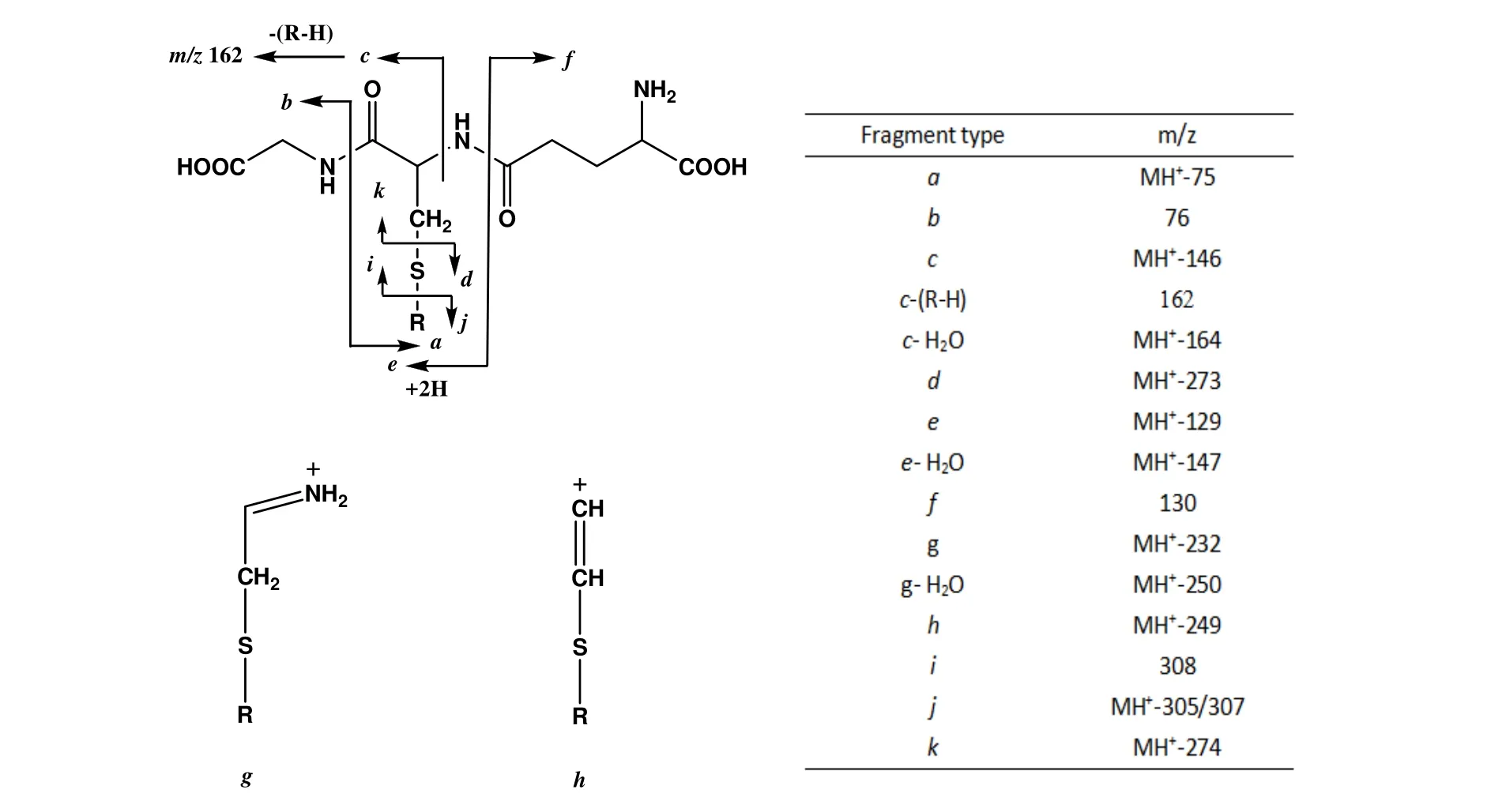
Figure 3.Characteristic fragment ions of glutathione conjugates under collision-induced dissociation[15]
Qualitative analysis by LC-MS
The constant neutral loss method is a classic one which is based on the observation of the pyroglutamic acid moiety(m/z 129 Da)cleaving from GSH conjugates upon collision-induced dissociation(CID).Therefore,neutral loss scanning of 129 Da in positive ion mode has been widely embraced as the gold standard for GSH conjugate screening.The fragments in the GSH conjugate are mainly derived from the cleavage of the peptide backbone in the structure.Although the relative abundance of different types of fragment ions sometimes depends on the nature of the bound species,GSH binders generally lose 129 Da(pyroglutamic acid)neutrally,producing e-type fragment ions[12],showed in Figure 3.Therefore,GSH conjugates can be detected by 129 Da neutral loss scan(CNLS)[13].However,this method may lead to a false negative result due to its poor selectivity.In order to avoid this situation,Dieckhaus et al.demonstrated that negative ion MS/MS is promising to overcome this limitation because the MS/MS spectrum of the deprotonated molecule[M-H]-of GSH and the major class GSH conjugate has a common anion fragment at m/z 272(deprotonated γ-glutamyl-dehydroalanyl-glycine)[14].Therefore,the precursor ion m/z 272 can be scanned in negative ion mode to detect the GSH conjugate and then switch the polarity to the positive ion mode to obtain the full scan product ion mass spectrum of the conjugate MH+.In structural analysis,the combination of these two modes may provide more information.According to the above description of neutral loss scan and precursor ion scan,such a strategy can be adopted.Wen et al.developed a method for high-throughput detection of GSH conjugates by polarity switching on a quadrupole linear ion trap mass spectrometry(Q-Trap)[15].In the same LC-MS/MS test,this method not only exerts the advantages of high selectivity and sensitivity of negative ion precursor ion scanning,but also exerts the structural analysis ability of full scan of product ions in positive ion mode,which enhances the throughput of screening GSH conjugate and greatly shortens the analysis time.Zheng et al.used a new method of Q-Trap mass spectrometer combined with multiple reaction detection(MRM)as a probing screening[16].An enhanced product ion(EPI)scan was also performed to screen for reactive metabolites[17].Accordingto thecommon P450 metabolic activation reaction,a series of MRM transformations were constructed from protonated molecules of potential GSH conjugates by losing 129 Da,305 Da and 307 Da(Figure 3)to their product ions.Due to the high scanning speed oftheQ-Trap mass spectrometer,the probe scan can be set to track up to more than 100 MRM conversions without significant loss of sensitivity.However,it can only be used to detect conventional predictable GSH conjugates,while for those unconventional conjugates,neutral loss and precursor ion scanning are more effective.
In recent years,Castro-Perez et al.reported a method for LC/MS.Accurate neutral loss of 129.0426 Da(corresponding to the exact mass of pyroglutamic acid)was used in a triple quadrupole-time-of-flight mass spectrometer(Q-TOF)to detect GSH conjugates to eliminate false positives[18].Q-TOF mass spectrometry does not have a true neutral loss scan,and the detector mass spectrum is obtained by successively switching the collision energy at 5-20 eV.Precursor ions are obtained at low energy(5 eV)and product ions are obtained at high energy(20 eV)for"pseudo"neutral loss scanning.This method allows for the selective detection and identification of GSH conjugates,providing greater selectivity and greatly reducing analysis time.GSH conjugates produce anionic fragments in the negative ion mode(deprotonated γ-glutamyl-dehydroalanyl-glycine).Using this property,Zhu et al.detected product ions with a charge-to-mass ratio in the range of m/z 269.5-274.5 underCID conditionsand collected corresponding high-resolution mass spectrometry data.Extracting m/z 272.0888±5 ppm of product ions from these product ions, such that the deprotonated γ-glutamyl-dehydroalanyl-glycine(m/z 272.0888)will have a major peak and almost no interference peaks[19].Therefore,the peaks in this range are mainly derived from the binding products of GSH.GSH conjugate precursor ions were determined by precursor ion full-scan mass spectrometry and their structures were identified by MS/MS mass spectrometry.MS data from MS and MS/MS scan are not sufficient to determine the structure of the metabolites and how they are broken.Therefore,multi-stage full-scan mass spectrometry is required to provide sufficient data to determine the fragmentation pattern and structure ofmetabolitesormetabolite fragments,and also infer the biotransformation process of the drug in the body.
For the screen of active metabolites, the development of Q-TOF,LTQ-FTMS,and LTQ Orbitrap high resolution mass spectrometry promotes the improvement in analytical methods [20]. These instruments have high sensitivity with fast data acquisition capability,and also have the function of multi-stage mass spectrometry scanning(MSn).However,these mass spectra differ from traditional triple quadrupole mass spectrometers in that they do not have true neutral loss,precursor ion,and MRM scan modes,which limit their usage in detecting reactive metabolites in complex biological matrices.So as to overcome this defect,Zhu etal.combined high-resolution mass spectrometry with post-data processing technology mass defect filtering (MDF) for unconventional and unanticipated metabolite screening [21].With the development of data mining technology,scholars have developed some new MDF technologies based on the conventional method.MMDF technology,multi-quality loss filtering,is to set up multiple structural filtering templates based on MDF technology to reveal different types of GSH conjugates[22].Quality loss filtering is a kind of data processing technology after acquisition,followed by background deduction and noise reduction software[23].The software has proven to be very effective in detecting metabolites in complex matrices.Metabolite detection and identification can be achieved by comparing the detected sample data with control data using accurate mass data and subtracting background-related signals.Zhu et al.improved the background deduction procedure by adding noise reduction algorithms to further clean up residual matrix noise.The program effectively reduces matrix ions and cleans up the ion chromatogram,and the processed mass spectral data helps identify the molecular ions of the metabolite.
Quantitative analysis by LC-MS
Gan et al.used LC-FLD-MS/MS method to quantify reactive metabolitesin livermicrosome incubation solution with dansyl glutathione(d-GSH).d-GSH is formed by a series of reactions between GSH and the fluorophore dansyl [24].This method has been successfully applied to the quantitative detection of active metabolitesin somemodeldrug livermicrosome incubation solutions.d-GSH and GSH have similar reactivity,but d-GSH is not a coenzyme of glutathione transferase.Thus,screening for compounds that require glutathione transferase catalysis is not applicable.
The concentration of drug metabolites in organisms and their pharmacokinetic parameters are useful for characterizing the toxicity of candidate drugs.GSH conjugates are key metabolites in two-phase metabolism.If GSH does not bind to one-phase metabolites,it will cause toxicity in response.Therefore,it is particularly important to monitor the level of GSH conjugates early in drug development. Stable isotope-labeled internal standard compounds have been widely used in the detection of two-phase metabolites.Li et al.used an isotope-labeled GSH conjugates as an internal standard,and quantitatively determined the two-phase metabolite GSH conjugate in the liver microparticle incubation solution by LC-MS[25].The precision and accuracy were good.
Quantification of drug-related substances in biological samples is a problem in the absence of radiolabeled drugs or standard controls. Liquid chromatography coupled with inductively coupled plasma mass spectrometry(ICP MS)can provide a novel method for determining GSH conjugates.MacDonald et al.quantified the GSH conjugate of clozapine in human liver microsome incubation solution by UPLC/ICP MS[26].However,ICP MS cannot provide relevant structural information and it’s not applicable to screen GSH conjugates.
In summary,most findings about the TCM reactive metabolic toxicity were qualitatively studied by LC-MS.The quantitative study of reactive metabolites is not perfect enough,and still need further improvement.
Application in the discovery of toxic components of TCM
Metabolism of TCM can produce some metabolites with physicochemical and pharmacological properties that significantly different from the parent drug.Therefore,in order to reduce the risk of expensive clinical stage loss due to the production of toxic reactive metabolites by drug candidates,reliable and efficient methods are needed to capture and detect reactive metabolites.GSH is a nucleophile that binds well to most reactive metabolism.GSH is incubated in vitro with hepatic microsomes or hepatocytes to capture the TCM metabolites,and then the GSH conjugates are detected by qualitative analysis and quantitative analysis by LC-MS(Table 1).This method has been widely applied to discover and detect the toxic components of TCM,and the elimination rate of drug candidates is reduced.Some TCM toxic components captured by LC-MS-based GSH are summarized as follows.
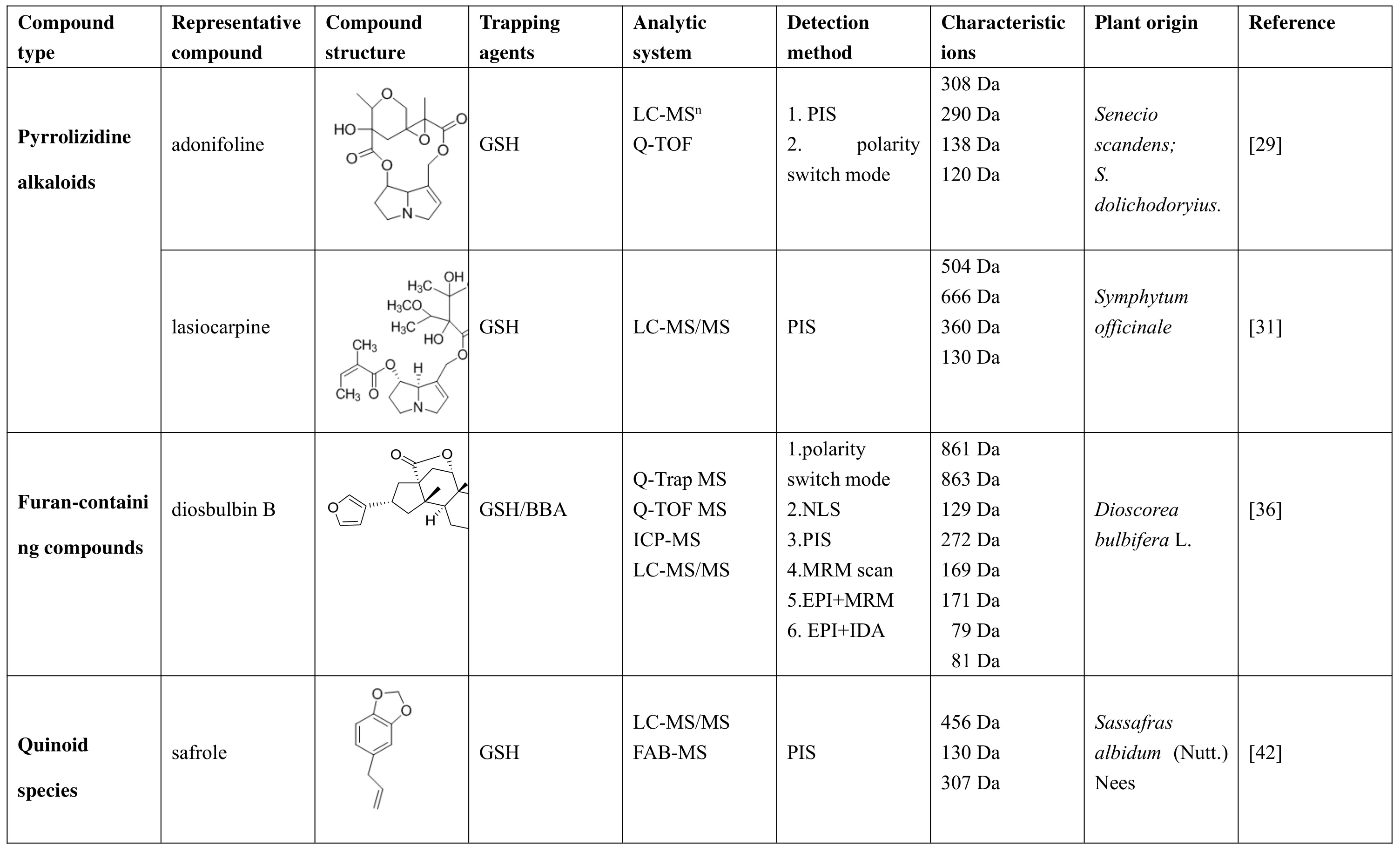
Table1.Summarized characteristic of the adonifoline,lasiocarpine,diosbulbin B and safrole were detected by LC-MS.
Pyrrolizidine alkaloids
Pyrrolizidine alkaloids(PAs)is widely found in plants with flowers,mainly distributed in Boraginaceae,Compositae,Legum inosae and Orchidaceae.At least 40 TCM or folk medicines in China contain PAs.Some of them,such as senecio,coltsfoot flower,perrin and comfrey,are recorded in the Chinese Pharmacopoeia,and they arewidely used in Chinesepatentmedicine preparations.PAs is called hepatotoxic pyrrolizidine alkaloids(HPAs)because of its significant liver toxicity[27].The toxicity of PAs depends to a large extent on their metabolic activation through liver enzymes,including cytochrome P450,which formscovalent adducts with cellular nucleophiles to become chemically reactive pyrrole derivatives to exhibit toxicity.Capturing reactive metabolites with nucleophilic GSH to produce structurally stable GSH conjugates has been widely exploited to evaluate preclinical bioactivation potential drug candidates[28].Research has shown that GSH captured theirpyrrole metabolites to form GSH conjugates including 7-glutathione-6,7-dihydro-1-hydroxymethyl-5H-pyrrole-azine(7-GSH-DHP); and 7,9-diglutathione-6,7-dihydro-1-hydroxymethyl-5H-pyraz ine(7,9-di-GSH-DHP);to compare the metabolic activity of PAs,and those GSH conjugates were quantified by LC-MS/MS[29].This method was highly sensitive and selective forthe estimation and quantification of GSH-DHP conjugates.Using the mostpowerful quantitative MRM method,the metabolites can be separated into two majorpyrrole metabolites by LC-MS/MS,namely 7-GSH-DHP and 7,9-di-GSH-DHP.A large number of studies have been conducted on the metabolism of PAs,but they are mainly concentrated in vitro,and there are relatively few studies in vivo.Aster contains a variety of hepatotoxic pyrrolizidine alkaloids,ofwhich clivorine isthe main component.The combination with pyrrole to form a pyrrole conjugate during metabolism in the body is an important indicator of its toxicity.There are experimental studies using Ligularia hodgsonii Hook to extract a certain amount of clivorine[30].Hepatic toxicity was studied by detecting serum alanine aminotransferase(ALT)and aspartate aminotransferase(AST)activities,binding to pyrrole and reduced GSH in liver tissue.Senecionine is a 12-membered macrolide 1,2-unsaturated PA,which has attracted extensive attention all over the world because of its strong hepatotoxicity and genotoxicity.It is also one of theearliestand moststudied PAs.Among these metabolites in bile,urine and feces of rats after oral senecio, GSH-DHP conjugate, 7-GSH-DHP,7,9-diGSH-DHP and dehydroretronecin were inferred by retention time and neutral loss of 129 Da using LC-MSn.The DHP formed by dehydrogenation of sub-alkali is a poisonous intermediate of PAs,and its binding to DNA and protein will directly cause irreversible damage.Therefore,the identification of DHP indirectly proves the mechanism of the toxicity of Senecionine.Comfrey contains as many as 14 PAs, including 7-acetylintermedine,7-acetyllycopsamine,echimidine,intermedine,lasiocarpine,lycopsamine,myoscorpine,symlandine,symphytine,and symviri-dine.However,the mechanism of geranium-induced genotoxicity and carcinogenicity is still not fully understood.Available evidence suggests that active metabolites of comfrey PA interactwith DNA in liverendothelialcellsand hepatocytes,leading to DNA damage mutations that induce cancerprogression [31].Since the limited conditions,the GSH can be used to capture the PAs metabolite of comfrey.This method requires further verification.HPAs exists in many medicinal plants,but there are few studies on its toxicity and safety.The research on its mechanism of toxicity is not deep enough,and there is no detoxification drug at present.Therefore,LC-MS based GSH captured reactive metabolites are useful for detecting and identifying the metabolic toxicity of PAs.However,methods based on LC-MS detection need further optimization.
Furan-containing compounds
Furan compounds are abundant in foods and herbs.Many furan-containing compounds have been reported to be cytotoxic and carcinogenic agents[32].The toxicity of the furan compound is mainly from the electrophilic intermediate produced by furan epoxidation reaction[33].The intermediate is an epoxide or cis-enedione which can react with nuclear proteins such as DNA or DNA to initiate toxicity.Previous studies have shown that furan is oxidized to reactive R,β-unsaturated dialdehyde,cis-2-butene-1,4-diol(BDA),which is catalyzed by cytochrome P450 in the reaction.Lu et al.proposed seven additional characteristics of urinary furan metabolites,and the presence of abundantfuran metabolites in urine was determined by LC-MS/MS,indicating that oxidized furans were followed by BDA and BDA with cellular cysteine and lysine residues.The base reaction may represent an important in vivo pathway for furan biotransformation.The metabolites are markers of furan exposure and biological activation because they are derived from cellular BDA reaction products.And these matabolites can be explored as potential biomarkers in human studies.Metabolism of hepatotoxic furans results in the formation of protein adducts in target organs.An important step in furan toxicity may be the protein adduct formation.In vitro,semicarbazide or GSH can capture furan active metabolites and they are identified as BDA.Studies in rat hepatocytes have shown that BDA cross-link GSH with various amines,including proteins,in a metabolically dependent process.Phillips et al.analyzed the tryptic digests of alkylated proteins by LC-MS/MS,indicating that most adducts occur on lysine residues,and the selectivity of BDA reactions is lower than that of GSH-BDA[34].This type of adducts may contribute to the toxic effects of furan experimented by the use of LC-MS/MS and NMR as an adduct of O-methyl hydrazine of the open-loop furan moiety of L-739,010 with liver microsomes[35].The furan moiety undergoes oxidative cleavage to highly reactive 2-butene-1,4-dialdehyde,representing the major site biotransformation of L-739,010.Many furan-containing compounds are biologically activated to produce toxicity,so the furan portion of L-739,010 can be considered undesirable.Wang et al.reported that the crude extract obtained from the TCM Dioscorea bulbifera L.contains furan dioxime,and the GSH/BBA-derived pyrrole can be produced by incubation of 2,5-dimethylfuran(DMF)in rat liver microsomes with GSH and 4-bromobenzylamine(BBA)as capture agents[36].A neutral loss scan of 129 Da and a precursor ion scan of m/z 272,169 were obtained by LC-MS,and then the incubation mixture was monitored for 171 polarity switching mode.And the formation of bromine-labeled pyrrole derivatives by ICP MS.Previous LC-MS may be sufficient to detect those furans of known structure,but it has a limiting effect on screening complex mixtures of samples,especially those containing no structural information.However,new applications of LC-ICP MS,as well as the use of bromine-labeled compounds as capturing and labeling agents,are particularly unique,allowing us to quickly detect the formation of active metabolites.
Quinoid species
An important toxicity of herbal medicines stems from their metabolic biotransformation into reactive metabolites(RMs).CYP catalyzes the synthesis of xenobioticsinto chemically reactive metabolitesor intermediates and stable metabolites.An in vitro assay using pulsed ultrafiltration and LC-MS/MS has been developed to screen plant extracts for the formation of electrophilic and potentially toxic quinoid species following hepatic cytochrome P450 bioactivation[37].Some of the constituent compounds in herbal medicines can be activated by P450 as electrophilic intermediates capable of alkylating cellular biomolecules or participating in a redox cycle reaction and causing cell damage [38]. Covalent binding of hepatic macromolecules can lead to liver toxicity.Plant electrophiles contain phenols substituted with alkoxy-or o-hydroxy groups that can be metabolized to quinone methides or o-quinones,respectively[39].The human body's important defense against this reactive intermediate is the nucleophile GSH.GSH is covalently bound to an electrophile to form a stable hydrophilic binder.Safrole is the main component of the volatile oil of Xixin and has antifungal effects.However,it has been shown that safrole has a certain relationship in the carcinogenesis of hepatocytes.Methylenedioxyphenyl and allyl-benzene substructures of safrole can cause inhibition of enzyme activity and toxic effects,as a result of the mechanism-based inhibition(MBI)of CYP450 enzymes(CYPs)and producing RMs.Safrole combines with GSH to form two reactive metabolites of 1,2-dihydroxy-4-allylbenzene (M1) and 1'-hydroxyxanthine (M2). Yang et al. used the UPLC-MS/MS method to identify RMs by analysis of the safrole lysis process and GSH-M1 adduct.It can be concluded that the hepatotoxicity mechanism of jaundice is related to CYP1A2-mediated RMs and the cleavage process of GSH-M1/M2 adduct is analyzed in detail.Significant information has been obtained for predicting drug-induced liver damage in vivo[40].Bolton et al.selectively oxidized hydroxychavicol to the corresponding o-quinone (HC-quinone,4-allyl-3,5-cyclohexadiene-1,2-dione) or p-quinone methide (HC-QM,2-hydroxy-4-allylidene-2,5-cyclohexadien-l-one) and used GSH trapped these reactive electrophiles.And then used UV, NMR, and mass spectrometry fully characterized the GSH conjugates.By altering the GSH incubation conditions and other factors,experiments have shown that Safrole has another biological activation pathway that leads to toxicity.The toxic effects is initial O-dealkylation of themethylenedioxy ring forming hydroxychavicol,2-electron oxidation to the o-quinone,and isomerization forming the more electrophilic p-quinone methide[41].Sassafras albidum(Nutt.)Nees extract(sassafras),Symphytum officinale L.(Purple Grass)and Rosemary (Rosmarinus officinalis L.)contain compounds that are carcinogenic or toxic to mammals.All of them can produce GSH adducts during this screening assay.Johnson et al.used database search and other LC-MS/MS studies to identify several compounds that form GSH conjugates,including new metabolites of rosmarinic acid.These results demonstrate the feasibility ofusing pulsed ultrafiltration LC-MS/MS forthe screening of botanical extracts and dietary supplements for compounds.Not knowing the chemical composition of the sample does not affect LC-MS detection,as only GSH adducts can be detected during selective LC-MS/MS analysis using precursor ion scanning and the chemical diversity in the extract does not interference with the identification of active metabolites[42].After detection of the GSH adduct,LC-MS/MS with product ion scanning can be used for further characterization to facilitate structural resolution,and a search database(such as the NAPRALERT database)can help identify active metabolites and their plant precursors.
Conclusion
TCM reactive metabolites,especially Chinese medicines containing pyrrolizidine alkaloids,furans and quinoid species may cause liver toxicity,leading to liver failure or other adverse reactions[43].Therefore,this is an important topic worthy of discussion.Screening of the reactive metabolites of candidate drug is a key part of the discovery and development of TCM.The early study of reactive metabolitesisbeneficialto determinethe structural information of candidate Chinese medicines,thus effectively reducing the cost of TCM development.Massspectrometry based GSH captureof reactive metabolites has become an important research method for screening and identifying active metabolites.At present,mass spectrometry techniques for capturing GSH binding reactive metabolites include triple quadrupole,ion trap,quadrupole-linear ion trap, high resolution mass spectrometry,and data processing methods MDF and background subtraction,which greatly promote these methods.Studiesofreactivemetabolitesand high throughputscreening were achieved.Recently,the application of predictive software in the structure of reactive metabolites has helped to identify the structure of candidate drugs,thereby reducing the chances of drug development failure.Due to the complexity of the TCM ingredients and the multi-targeteffect,the mass spectrometric responseoftheconjugateisusually differentfrom the originalshape afterthe drug metabolism intermediate is combined with GSH.Therefore,the conventional mass spectrometry method cannot quantitatively determine the reactive metabolite.The scale of this metabolic pathway is also unknown.Consequently,quantification ofreactive metabolites remainsahuge challenge.Accurately and rapidly quantitative analysis of reactive metabolites still requires sustained efforts.
1.Park K,Williams DP,Naisbitt DJ,et al.Investigation oftoxic metabolitesduring drug development.Toxicol Appl Pharmacol 2005,207:425-434.
2.Jia Z,Dan W,You S,et al.Progress in research of glutathione.ShenyangYao Ke Da Xue Xue Bao 2009,26:238-242.
3.Ma S,Subramanian R.Detecting and characterizing reactive metabolites by liquid chromatography tandem mass spectrometry.J Mass Spectrom 2006,41:1121-1139.
4.Chen WG,Zhang C,Avery MJ,et al.Reactive metabolite screen for reducing candidate attrition in drug discovery.Adv Exp Med Biol 2001,500:521-524.
5.Evans DC,Watt AP,Nicoll-Griffith DA,et al.Drug-protein adducts:an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development.Chem Res Toxicol 2004,17:3-16.
6.Zhou S,Chan E,Duan W,et al.Drug bioactivation covalent binding to target proteins and toxicity relevance.Drug Metab Rev 2005,37:205-213.
7.Pohl LR,Branchflower RV.Covalent binding of electrophilic metabolites to macromolecules.Methods Enzymol 1981,77:43-50.
8.Blair IA.Endogenous glutathione adducts.Curr Drug Meta 2006,7:853-872.
9.Waldon DJ,Teffera Y,CollettiAE,etal.Identification of quinone imine containing glutathione conjugates of diclofenac in rat bile.Chem Res Toxicol 2010,23:1947-1953.
10.Zhang XY,Elfarra AA.Toxicity mechanism-based prodrugs:glutathione-dependent bioactivation as a strategy for anticancer prodrug design.Expert Opin Drug Discov 2018,13:1-10.
11.Cao L,Waldon D,Teffera Y,et al.Ratios of biliary glutathione disulfide(GSSG)to glutathione(GSH):a potentialindex to screen drug-induced hepatic oxidative stress in rats and mice.Anal Bioanal Chem 2013,405:2635-2642.
12.Xie W,Zhong DF,Chen XY,et al.Determination of Reactive Metabolites by Liquid Chromatography-Tandem Mass Spectrometry.J Chin Mass Spectrom Soc 2011,32:1-12.
13.Yan Z,Caldwell GW,Maher N,et al.Unbiased high-throughput screening of reactive metabolites on the linear ion trap mass spectrometer using polarity switch and mass tag triggered data-dependent acquisition.Anal Chem 2008,80:6410-6422.
14.Dieckhaus CM,Fernández-Metzler CL,King R,et al.Negative ion tandem mass spectrometry for the detection ofglutathioneconjugates.Chem Res Toxicol 2005,18:630-638.
15.Wen B,Ma L,Nelson SD,et al.High-throughput screening and characterization of reactive metabolites using polarity switching of hybrid triple quadrupole linear ion trap mass spectrometry.Anal Chem 2008,80:1788-1799.
16.Zheng J,Ma L,Xin B,et al.Screening and identification of GSH-trapped reactive metabolites using hybrid triple quadruple linear ion trap mass spectrometry.Chem Res Toxicol 2007,20:757-766.
17.Lim HK,Chen J,Cook K,et al.A generic method to detectelectrophilic intermediatesusing isotopic pattern triggered data-dependent high-resolution accurate mass spectrometry.Rapid Commun Mass Spectrom 2008,22:1295-1311.
18.Castro-PerezJ,Plumb R,Liang L,etal.A high-throughput liquid chromatography/tandem mass spectrometry method for screening glutathione conjugates using exact mass neutral loss acquisition.Rapid Commun Mass Spectrom 2005,19:798-804.
19.Zhu X,Kalyanaraman N,Subramanian R.Enhanced screening of glutathione-trapped reactive metabolites by in-source collision-induced dissociation and extraction ofproduction using UHPLC-high resolution mass spectrometry.Anal Chem 2011,83:9516-9523.
20.Tang C,Zhang W,Dai C,et al.Identification and quantification of adducts between oxidized rosmarinic acid and thiol compounds by UHPLC-LTQ-Orbitrap and MALDI-TOF/TOF tandem mass spectrometry.J Agric Food Chem 2015,63:902-911.
21.Zhu M,Ma L,Zhang H,et al.Detection and Structural Characterization of Glutathione-Trapped Reactive Metabolites Using Liquid Chromatography-High-Resolution Mass Spectrometry and Mass Defect Filtering.Anal Chem 2007,79:8333-8341.
22.Ruan Q,Peterman S,Szewc MA,et al.An integrated method for metabolite detection and identification using a linear ion trap/Orbitrap mass spectrometer and multiple data processing techniques:application to indinavir metabolite detection.J Mass spectrom 2008,43:251-261.
23.Prasad B,Garg A,Takwani H,et al.Metabolite identification by liquid chromatography-mass spectrometry.TrAC Trends Anal Chem 2011,30:360-387.
24.Gan J,Harper TW,Hsueh M M,et al.Dansyl glutathione as a trapping agent for the quantitative estimation and identification of reactive metabolites.Chem Res Toxicol 2005,18:896-903.
25.Li P,Li Z,Beck W D,et al.Bio-generation of stable isotope-labeled internal standards for absolute and relative quantitation of phase II drug metabolites in plasma samples using LC–MS/MS.Anal Bioanal Chem 2015,407:4053-4063.
26.MacDonald C,Smith C,Michopoulos F,et al.Identification and quantification of glutathione adducts of clozapine using ultra-high-performance liquid chromatography with orthogonal acceleration time-of-flight mass spectrometry and inductively coupled plasma mass spectrometry.Rapid Commun Mass Spectrom 2011,25:1787-1793.
27.Wang J,Wang CH,Wang YT,et al.Progress in the Cytotoxicity and Toxicity Mechanism of Pyrrolizidine Alkaloids.Int J Pharm Res 2007,34:246-249+258.
28.Gan J,Ruan Q,He B,et al.In Vitro Screening of 50 Highly Prescribed Drugs for Thiol Adduct Formation Comparison of Potential for Drug-Induced Toxicity and Extent of Adduct Formation.Chem Res Toxicol 2009,22:690-698.
29.Tamta H,Pawar RS,Wamer WG,et al.Comparison ofmetabolism-mediated effectsofpyrrolizidine alkaloids in a HepG2/C3A cell-S9 co-incubation system and quantification oftheir glutathione conjugates.Xenobiot 2012,42:1038-1048.
30.Cheng M,Tang J,Gao QF,et al.Analysis of the main alkaloids in the extract of Aster sinensis Clivorine and its preliminary study on hepatotoxicity in rats.ChineseTradiHerb Drugs2011,42:2507-2511.
31.MeiN,Guo L,Fu PP,etal.Metabolism,genotoxicity, and carcinogenicity of comfrey.Environ Health Toxicol 2010,13:509-526.
32.Lu D,Peterson LA.Identification of Furan Metabolites Derived from Cysteine-cis-2-Butene-1,4-dial-Lysine Cross-Links.Chem Res Toxicol 2009,23:142-151.
33.Li C, Lin D, Gao H, et al. N-Acetyl lysine/glutathione-derived pyrroles as potential Ex Vivo biomarkers of bioactivated furan-containing compounds.Chem Res Toxicol 2014,28:384-393.
34.Phillips MB,Sullivan MM,Villalta PW,et al.Covalent modification of cytochrome c by reactive metabolites of furan.Chem Res Toxicol 2013,27:129-135
35.Zhang KE,Naue JA,Arison B,et al.Microsomal metabolism of the 5-lipoxygenase inhibitor L-739,010:evidence for furan bioactivation.Chem Res Toxicol 1996,9(2):547-554.
36.Wang K,Zheng L,Peng Y,et al.Selective and sensitive platform for function-based screening of potentially harmful furans.Anal Chem 2014,86:10755-10762.
37.Van Breemen RB,Nikolic D,Bolton JL.Metabolic screening using on-line ultrafiltration mass spectrometry.Drug Metab Dispos 1998,26:85-90.
38.Thompson DC,BarhoumiR,BurghardtRC.Comparative toxicity of eugenol and its quinone methide metabolite in cultured liver cells using kinetic fluorescence bioassays. Toxicol Appl Pharmacol 1998,149:55-63.
39.Thompson D,Constantin-Teodosiu D,Egestad B,et al.Formation ofglutathione conjugatesduring oxidation of eugenol by microsomal fractions of rat liver and lung.Biochem Pharmacol 1990,39(10):1587-1595.
40.Yang AH,Zhang L,Zhi DX,et al.Identification and analysis of the reactive metabolites related to the hepatotoxicity of safrole.Xenobiot2018,48:11164-1172.
41.Bolton JL,Acay NM,Vukomanovic V.Evidence that 4-allyl-o-quinones spontaneously rearrange to their more electrophilic quinone methides:potential bioactivation mechanism for the hepatocarcinogen safrole.Chem Research Toxicol 1994,7:443-450.
42.Johnson BM,Bolton JL,van Breemen RB.Screening botanical extracts for quinoid metabolites.Chem Res Toxicol 2001,14:1546-1551.
43.Wu H,Zhong RL,Xia Z,et al.Progress in the study of components of potential hepatotoxic Chinese medicines.Chin J Tradi Chin Med 2016,41:3209-3217.
 TMR Modern Herbal Medicine2018年4期
TMR Modern Herbal Medicine2018年4期
- TMR Modern Herbal Medicine的其它文章
- Effect of TongFengNing Decoction on UricAcid Levels and Xanthine Oxidase Activity in Hyperuricemia Rats
- Clinical experience in treating 78 cases of upper limb edema after breast cancer operation by WenYang HuoXue Washing Prescription
- Clinical observation of Furongtongmai capsule on the lower extremity Atherosclerotic Occlusive Disease after Intervention Operation
- The progress in the chemical constituents of the genus Picrasma during 2007-2017
- Survey of dose-effect relationship in Chinese materia medica
- Analysis of pharmacological action and clinical application of DaXueTeng based on its anti-inflammatory action
