Effect of Biodiesel Soot on Tribological Behavior of Liquid Paraffin
Li Chuan; Wei Daozhu; Zhuang Yuan; Song Ruhong; Hu Xianguo
(1. School of Mechanical Engineering, Hefei University of Technology, Hefei 230009;2. School of Automobile and Transportation Engineering, Hefei University of Technology, Hefei 230009)
Abstract: Biodiesel soot (BDS) was collected from the combustion of biodiesel using a self-made soot trap. The effect of BDS on the tribological behavior of liquid paraffin (LP) was investigated using a four-ball tribometer. A rotating viscometer was used to investigate the effect of BDS on the viscosity of LP. The morphology, composition, and tribological mechanism of BDS were studied by means of FETEM, XRD, XPS, SEM/EDS, and the 3D laser scanning microscopy. Test results showed that the BDS aggregates were chain-like, and the average diameter of BDS was 35 nm. The BDS existed in the form of graphitic layers and amorphous carbon. The oxygen-containing functional groups in BDS consisted of the (C-O-C)and (C-O-H). With an increasing BDS content, the dynamic viscosity of LP increased and the maximum non-seizure load increased initially and became stable later. In addition, the average wear scar diameter (AWSD) of LP increased and the average friction coefficient of LP decreased at first and then increased later. The tribological mechanisms could be ascribed to the variation in content of BDS: BDS could act as a friction modifier for a lower friction coefficient in case of low BDS content. However, the BDS aggregates could lead to increase of abrasive wear to influence the lubricating oil film at higher content of BDS, which would reduce the friction reduction ability and wear resistance of LP.
Key words: biodiesel; soot; tribological behavior; liquid paraffin
1 Introduction
To mitigate the global climate change, reduce the dependence on fossil fuels, and increase the national energy security, there is an urgent need to develop renewable fuels to replace fossil fuels[1]. Biodiesel is one of the promising alternative fuels for automotive engines.Biodiesel, which is produced from vegetable oils, animal fats, and waste cooking oil by transesterification, is a mixture of fatty acid methyl esters[2]. Biodiesel with low sulfur content does not contain aromatic hydrocarbons, so it can greatly reduce the content of carcinogens in exhaust gas, and also reduce the environmental pollution. On the other hand, biodiesel is higher in oxygenates content and can burn more completely than petroleum diesel, which will result in higher combustion efficiency[3-4]. As a result,many countries are taking measures to promote the use of biodiesel.
The problem of soot generation during the fuel combustion process is apparent[5]. Most of the soot generated during the combustion process is exhausted,but some soot can contaminate the lubricating oil in the sump as a result of blow-by gasses[6]. This situation can be worsened when the exhaust gas recirculation(EGR) technology is applied[7]. Since the soot particles are charged, they can easily agglomerate in lubricating oil. Soot particles aggregates in the lubricating oil can increase the viscosity of lubricating oil and the size of particles[8], which are detrimental to the circulation of lubricating oil and can even lead to increased engine wear.Penchaliah, et al.[9]analyzed the tribological behavior of soot in lubricating oils using a pin-on-disc tribometer under different conditions. It was found that soot could generate abrasion and oil starvation wear. Green, et al.[10]reported that carbon black (acting as a soot simulator)leads to significant levels of abrasive wear when the content of carbon black in the lubricating oil is higher than 3%. Liu, et al.[11]reported that the bio-fuel soot could cause abrasive wear and corrosive wear. Previous research by this author indicated that the physicochemical and aggregation properties of biodiesel soot were significantly different from those of petrochemical diesel soot[12]. As a result, the tribological behaviors of biodiesel soot (BDS)in lubricating oils are different from those of diesel soot.However, few reports have described the effect of biodiesel soot on the tribological behavior of lubricating oil.
In this paper, a four-ball tribometer was used to investigate the effect of BDS on the tribological performance of liquid paraffin (LP, acting as a lubricating base oil simulator). The tribological mechanism of BDS in LP was also studied. The aim is to lay a foundation for reducing the wear of BDS in lubricating oil, as well as for providing an experimental basis for the extensive use of biodiesel in engines.
2 Experimental
2.1 Materials and samples preparation
Biodiesel was made from soybean oil via transesterification with methanol over the self-made green biomass ash catalyst[13]. Table 1 shows the physical and chemical properties of biodiesel. Biodiesel soot (BDS) was collected using a self-made soot trap from the combustion of biodiesel[12]. The collection of BDS comprised the following steps. Biodiesel was burned out in a porcelain crucible at normal temperature and atmospheric pressure. A glass slide (soot capture slide) was held above the flame of biodiesel until a soot layer with a few micrometers in thickness was deposited[14]. This soot layer was then scraped off from the slide and was very carefully cleaned and collected in a glass bottle. Finally the collected soot was dried under a vacuum condition at 120 °C for 3 h and was ground to become a fine granular powder. LP was purchased from the Hengshui Diyi Petroleum Chemical Co., Ltd. in China, with its physical and chemical properties shown in Table 1. The individual oil samples were composed of: LP + 1% of BDS, LP + 2% of BDS, LP +3% of BDS, LP + 4% of BDS, and LP + 5% of BDS, respectively. The oil samples were stirred using a glass rod for 15 min. Finally, the oil samples were prepared under continuous magnetic stirring for 1 h to reduce experimental deviation.

Table 1 Physical and chemical properties of biodiesel and liquid paraffin
2.2 Tribological tests and characterization
Tribological tests were carried out on an MRS-10D fourball tribometer produced by the Jinan Shunmao Testing Machine Co., Ltd. in China. The balls (φ12.7 mm with a HRC of 65 in hardness, and a roughness average of 0.012 μm) were made of GCr15 steel.
The primary particle morphology and internal structure of BDS were analyzed using a field-emission transmission electron microscope (FETEM; JEM-2100F). The X-ray diffractometry (XRD, D/MAX2500V) was used to evaluate the phase of BDS. The chemical states of BDS were characterized via the X-ray photoelectron spectroscopy (XPS; ESCALAB250). After friction, the rotational and stationary balls were washed with acetone.The morphology of the wear zones and the elemental composition on the surfaces were analyzed via the 3D laser scanning microscopy (VK-X100, Keyence) and the scanning electron microscopy coupled with the energy dispersive spectroscopy (SEM/EDS, SU8010,Hitachi). The chemical states of the worn surfaces were investigated using XPS.
3 Results and Discussion
3.1 Characterization of biodiesel soot
Figure 1 shows a FETEM image of BDS. The chain-like aggregates of BDS were composed of spherical nanoparticles and the average diameter of BDS was 35 nm determined by Nano Measurer analysis and calculations based on Figure 1(a). Figure 1(b) shows that the graphitic layers in BDS were not continuous and were surrounded by an amorphous phase[15]. These results were confirmed via the XRD spectra(Figure 2) by the strong (002) and (101) diffraction peaks of Graphite-2H at 24.9º and 43.5º, respectively.
The XPS spectra of BDS are shown in Figure 3. As shown in Figure 3(a), the C1s spectrum consisted of two main peaks. One was a strong peak at 284.8 eV, which corresponded to C-C group; the other was a small peak at 286.9 eV, which denoted the existence of C-O-C group and C-O-H group. As shown in Figure 3(b), the O1s peak at 531.9 eV was ascribed to C-O-C group. The O1s peak at 533.8 eV belonged to C-O-H group, suggesting that the oxygen-containing functional groups in BDS were C-O-C group and C-O-H group, making it possible for BDS to adsorb on friction surfaces. Interestingly, BDS did not contain C=O group, which was possibly attributed to the C=O group in biodiesel which could be evolved as CO2or CO during combustion because the biodiesel sample contained more oxygen than the conventional diesel[12,16].

Figure 1 FETEM images of BDS

Figure 2 XRD pattern of BDS

Figure 3 XPS spectra of BDS
3.2 Effect of biodiesel soot on viscosity of liquid paraffin
Figure 4 shows the variation of dynamic viscosity of LP contaminated with different concentration of BDS at 20 °C. The dynamic viscosity was measured on an NDJ-5S rotary viscometer, manufactured by the Ningbo Weide Instrument Co., Ltd. in China. The viscosity change in LP caused by soot contamination can directly reflect the degree of soot aggregation[17]. The dynamic viscosity of LP showed the significant increase with an increasing BDS content. The results indicated that the content of BDS aggregates in LP increased with an increasing BDS concentration[18].
3.3 Effect of biodiesel soot on the tribological behavior
Figure 5 shows the effect of BDS content on the maximum non-seizure load (PBvalue) of LP. The PBvalue of LP presented an increasing trend with an increasing content of BDS. When the content of BDS exceeded 3%, the PBvalue of LP remained stable at 560 N. The increased PBvalue can be attributed to the dynamic viscosity of LP because it increases with an increasing content of BDS, which is beneficial to the improvement on the bearing capacity of the oil film[19].

Figure 4 Variation of dynamic viscosity with BDS content in contaminated LP
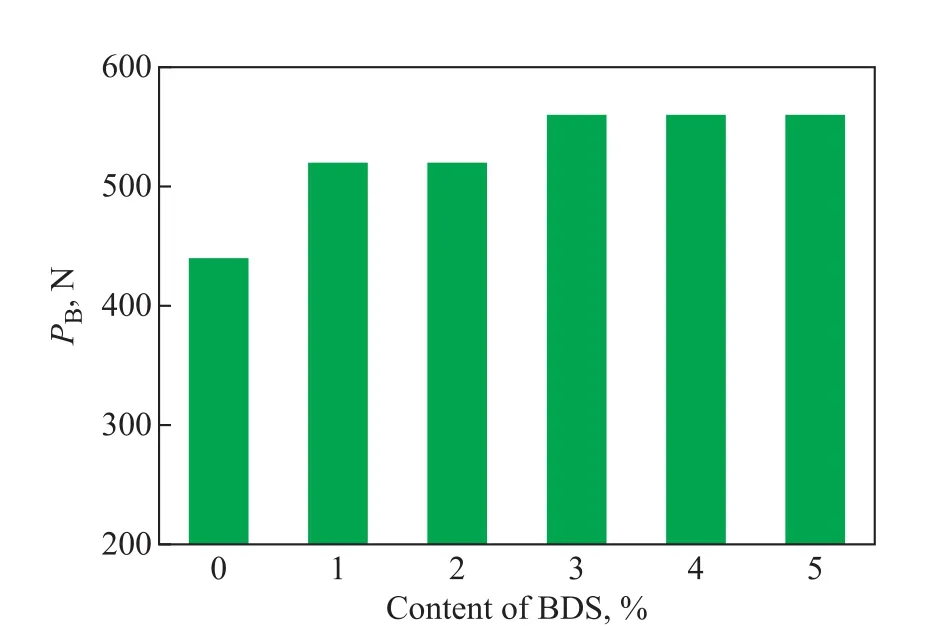
Figure 5 Effect of BDS content on the maximum nonseizure load of LP
Figure 6 shows the effects of BDS content on the average friction coefficient and average wear scar diameter(AWSD) of LP. The tribo-tests were performed on a fourball tribometer operating at a load of 196 N, a rotary speed of 1 450 r/min, and room temperature (ca. 20 °C)for 30 min. As shown in Figure 6(a), with an increasing content of BDS, the average friction coefficient of LP at first decreased. However, when the content of BDS exceeded 1%, the average friction coefficient of LP showed an increasing trend. This indicated that a small amount of BDS in LP was beneficial to the improvement on the friction-reducing performance of LP. However,with an increasing content of BDS, more BDS aggregates accumulated at the entraining edge of the tribocontact resulting in partial starvation. Thus, a high content of BDS could degrade the friction-reduction properties of LP[20].As shown in Figure 6(b), with an increasing content of BDS, the AWSD of LP increased significantly. It indicated that the number of BDS aggregates in LP increased with an increasing content of BDS, which resulted in an increased abrasive wear on the friction surfaces. Hence,BDS could reduce the wear resistance of LP.

Figure 6 Effect of BDS content on AWSD of LP
3.4 Tribological mechanism
LP+2% BDS and LP+5% BDS represent levels of low and high soot in lubricating oil, respectively[21]. Hence,the worn surfaces lubricated with LP, LP+2% BDS, and LP+5% BDS were selected to analyze the tribological mechanism. Figure 7 shows the SEM images and the corresponding worn surface profiles of wear scars of rotational ball lubricated with different oil samples. Many furrows could be observed on the surface of the rotational ball after being lubricated with LP in Figure 7(a). As shown in Figure 7(b) and 7(c), with an increasing content of BDS, the rotational ball displayed wider wear scars and more distinct furrows, which showed correspondence with the AWSD of the stationary ball in Figure 6(b).These results are confirmed by the worn surface profiles of wear scars of the rotational ball lubricated with different oil samples (as shown in Figure 7(d)). In the presence of LP, the furrows of the rotational ball were light and shallow. With an increasing content of BDS, the furrows of the rotational ball became wider and deeper[22].This fact supported the argument that the abrasive wear became more serious with the increase in the content of BDS[23].
EDS spectra of the worn surfaces of rotational balls lubricated with different oil samples are shown in Figure 8. Elements including C, O, Fe, and Cr were detected in the worn surfaces of rotational balls lubricated with different oil samples. The atomic content of C on the worn surfaces lubricated with LP, LP containing 2% of BDS, and LP containing 5% of BDS was 1.86%, 2.33%,and 3.20%, respectively. This fact means that C increased and O decreased on the worn surfaces with an increasing content of BDS. This indicated that more BDS aggregates were adsorbed on friction surfaces when the content of BDS increased, which was harmful to the formation of lubricating oil films[11].
The C1s, O1s and Fe2p spectra of the worn surfaces of rotational balls lubricated with different oil samples were recorded. The results are shown in Figure 9. Figure 9 (a―c)shows the C1s spectra of the worn surfaces. The (C-H, C-C),C-O, and (C=O, O-C-O) peaks, which were positioned at the binding energy of 284.7 eV, 285.9―286.1 eV, and 288.4―288.5 eV, respectively[24], were observed in these three samples. Table 2 shows the functional group contents of carbon on the worn surfaces. The content of C-O group increased obviously and the content of C=O,and O-C-O groups decreased in the presence of BDS in LP. This result was disclosed, because C-O group of BDS was adsorbed on the friction surfaces, suggesting that BDS could be adsorbed on rubbing surfaces. Figure 9 (df) shows O1s spectra of the worn surfaces. Metal oxide and (C-O, C=O, and -OH) peaks were positioned at the binding energy of 530.1―530.3 eV and 531.6―531.8 eV,respectively. Table 3 shows the functional group contents of oxygen on the worn surfaces. The content of C-O,C=O and -OH groups obviously increased in the presence of BDS in LP, which indicated that the oxygen-containing functional groups of BDS were adsorbed on the friction surfaces[25]. Figure 9 (g―i) shows the Fe2p spectra of the worn surfaces. Fe2p3/2and Fe2p1/2peaks were positioned at binding energies of 710.6―710.7 eV and 724.5―724.9 eV,respectively, which were in agreement with the existence of Fe2O3. The results indicated that iron atoms were subject to oxidative reactions on the friction surfaces[26-28].

Figure 7 SEM images and the corresponding worn surface profiles of wear scars of rotational ball lubricated with different oil samples
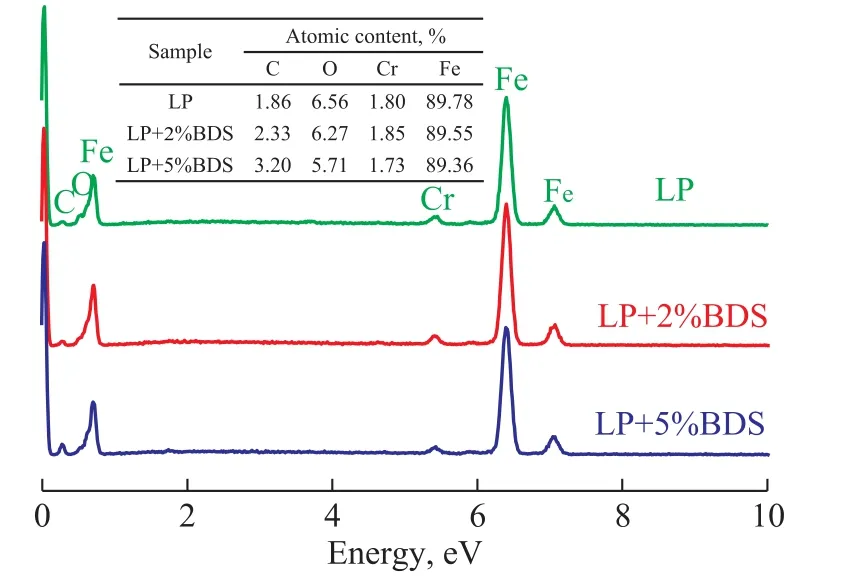
Figure 8 EDS spectra of the worn surfaces of rotational balls lubricated with different oil samples

Figure 9 C1s, O1s and Fe2p spectra of the worn surfaces of rotational ball lubricated with different oil samples
In general, the tribological mechanisms for BDS in LP could be divided into two cases. When the BDS content in LP was low, it could play an important role in reducing friction levels and could act as a friction modifier[11,29]. At high content of BDS, on one hand, more BDS aggregates were adsorbed on the interface between the rotational ball and the stationary ball[30], which impeded the effect of the lubricating oil film. On the other hand, the abrasive wear became more serious as a result of increase in BDS aggregates. Hence, it could lead to reduction in the friction reduction ability and wear resistance of LP.

Table 2 Functional group contents of carbon on the worn surfaces

Table 3 Functional group contents of oxygen on the worn surfaces
4 Conclusions
This research describes a study of the tribological behavior of BDS-contaminated LP. The chainlike aggregates of BDS were composed of spherical nanoparticles with an average diameter of 35 nm. The graphitic layers in BDS were not continuous and were surrounded by amorphous phases. The oxygen-containing functional groups in BDS were (C-O-C) and (C-O-H).
The dynamic viscosity of LP increased with an increasing BDS content. BDS exerted a strong effect on the PBvalue of LP. BDS could decrease the wear resistance of LP. The friction-reduction property of LP was improved, when the content of BDS was below 1%. However, when the content of BDS exceeded 1%, BDS would decrease the friction-reduction ability of LP.
The tribological mechanisms could be summarized as follows: At a low BDS content, BDS could act as a solid lubricant to lower the friction coefficient. However, at high BDS content, on one hand, more BDS aggregates were adsorbed on the friction surfaces to thereby influence the effect of the lubricating oil film. On the other hand,it could increase the BDS aggregation and would cause abrasive wear, which could lead to the friction reduction and anti-wear ability of LP.
Acknowledgments:This research was supported by the National Natural Science Foundation of China (Grant No.51675153) and the Major Science and Technology Special Project in Anhui (Grant No.17030901084), which are gratefully acknowledged. The authors thank Professor Hu Kunhong,Dr. Hu Enzhu and Mr. Shi Bin of Hefei University and Dr.Liu Tianxia of Beifang University of Nationalities for their assistance in the experimental analyses and discussion.
- 中国炼油与石油化工的其它文章
- Comparison of Lubricities of Two Novel Benzotriazole Derivatives Used as Additives in Water-Glycol Hydraulic Fluid
- Heat Transfer Investigation and Modeling of Heat Integrated Distillation Column
- Novel Control Structure Design of Differential Pressure Thermally Coupled Reactive Distillation for Methyl Acetate Hydrolysis
- Preparation of Sodium Cobalt Tetracarbonyl and Optimization of Process Conditions for Hydroesterification of Ethylene Oxide
- Bulk Ni-Mo Composites Prepared by Solid Reaction Method and Their Hydrodeoxygenation Performance
- Study on Synthesis of Mesoporous M-MCM-48 (M = Zr, Mg)and Its Activity for Isomerization of n-Heptane

