Definition of fine roots on the basis of the root anatomy,diameter,and branch orders of one-year old Fraxinus mandshurica seedlings
Xinzhu Du•Xing Wei
Abstract Fine roots are important in root absorption of nutrient and water,and in root turnover.Accurate definition of fine roots is a prerequisite to improved estimation of the physiological and ecological functions of forest ecosystems.Root development and physiological functions are reflections of root anatomical structure.In this study,the anatomical structures of different root orders were analyzed by examining paraffin sections of one-year old Fraxinus mandshurica seedlings.One-year-old F.mandshurica seedlings had over five root orders.The root anatomical structures of all orders showed more differences.First and second order roots consisted of four sections:the epidermis,cortex,pericycle,and vascular bundles.Fourth and fifth order roots were mainly composed of the skin and peripheral vascular bundles(including the xylem and phloem).Third order roots had root epidermal and cortical structures,but the quantity and integrity of the cortical cells were inferior to those of the first and second order roots,and superior to those of the fourth and fifth order roots.All the first and second order roots and some third order roots with discontinuous cork layer(<0.4 mm in diameter),but not the fourth and fifth order roots,were the fine roots of one-year old F.mandshurica seedlings.Although they had similar diameters,different portions of root systems had different anatomical structures and therefore,vary in capacity to absorb water and nutrients.Fine roots were accurately defined by root diameter,branch orders,and anatomical structural features of one-year old F.mandshurica seedlings.
Keywords Fine root·Root order·Fraxinus mandshurica ·Anatomical structure
Introduction
Tree fine roots are the main organs for absorbing nutrients and water;their production and turnover influence tree growth and material recycling of ecological systems(Valenzuela-Estrada et al.2008;Gordon and Ackson 2000;Gu et al.2015).Defining the fine roots of a plant is helpful for estimating the role of fine roots in carbon distribution in trees and ecosystem nutrient cycling(Wells and Eissenstat 2001;Norby and Jackson 2000;Tierney and Fahey 2002;Jagodzinski et al.2016).At present,the methods used to define fine roots are primarily the diameter-based method and order-based method.The diameter-based method names the fine roots according to root diameter,such as root diameter of less than 2 mm(Vogt and Persson 1991;Hendrick and Pregitzer 1993;Zhang and Wu 2001;Donnelly et al.2016;Jagodzinski et al.2016;Jagodzinski and Kalucka 2010,2011),less than 3 mm(Nadelhoffer et al.1985;Aber et al.1985),or less than 5 mm(Joslin and Henderson 1987).Fahey and Hughes(1994)thought that roots of less than 1 mm diameter yielded greater contribution to fine root biomass and root turnover than larger roots.The root orders method names the roots with tips as the first order,and their mother roots as the second order,and so on.They call the first two, first three,or even first five orders(Pregitzer et al.2002;Wang et al.2005;McCormak et al.2015)as fine roots.The lower order roots in the root apex have higher rates of absorption and respiration than the higher order roots(Pregitzer et al.1998),and their nitrogen(N)concentration and mycorrhizal infection rates are also higher(Pregitzer et al.2002;Eissenstat et al.2000).The production,death,and decomposition rates of the apex root are greater than those of the higher order roots(King et al.2002;Majdi 2001;Wells and Eissenstat 2003;Ruess et al.2003).
Heterogeneity differences in the morphology and function among the different orders of fine roots have attracted worldwide attention in ecological research on fine roots.Recent studies have shown great deviation between these two methods to estimate the root energy and substance flow(Hishi 2007).Therefore,before the examination of the demography and physiology of the branching fine root network,it is necessary to develop better understanding of functional modules in plant root systems according to their anatomical structures.
Numerous studies have confirmed that different root orders have different functions.Wells and Eissenstat(2003)reported that root functional heterogeneity was caused by root tissue and structural heterogeneity.Anatomical observation has long been considered a reliable method for estimating the physiological functions of individual roots(McCrady and Comerford 1998;Eissenstat and Achor 1999;Taylor and Peterson 2000).Anatomical structures of the root system directly reflect root development,which has a close relationship with physiological function(Macfall et al.1991).Many issues need further clarification to accurately estimate the contributions of fine roots to tree growth,such as:(1)Do anatomical structures differ among root orders?(2)If so,what are the different traits?(3)What do these differences mean for roots?And(4)Which order or diameter class could be defined as ‘‘ fine roots’’that function in absorption?
Fraxinus mandshuricaRupr.(Manchurian ash)is a common forest tree in northeast China.Because of its huge branch root systems,it has been used as a model species to study the physiological and ecological functions of root branch orders.The examination of fine-root systems of this species in plantations has revealed different anatomical structures,primary(non-woody)or secondary structure(Guo et al.2008).However,for one-year old seedlings,which have been used as a typical root system study model in boreal climates,the fine roots could not be clearly identified.In this study,we observed the anatomical traits among the root branch orders in one-year oldF.mandshuricaseedlings.This study aimed to(1)reveal the differences in morphological and anatomical traits among different branch order roots of one-year oldF.mandshuricaseedlings and(2)determine the role of fine roots in one-year oldF.mandshuricaseedlings.
Materials and methods
Seedling culture and root sampling
The experiment was performed in July 2013 in green house at Northeast Forestry University(NEFU).The seeds were obtained from 26 year-oldF.mandshuricatreesat Mao’ershan Experimental Forestry Center of NEFU,Heilongjiang Province.After a whole winter of wet sand and low temperature(4°C)storage,the seeds were grown in plastic pails(diameter=40 cm)in a greenhouse in NEFU.To avoid entanglement of roots,only two seed were separately grown in each plastic pail.The soil in the pail was natural soil dug from the vicinity of NEFU.The average day and night temperatures in the greenhouse were 30 and 20 °C,respectively,and relative humidity was>85%.The average light in the greenhouse was 14 h d-1.Seedlings were watered twice in the morning and evening each day.After three months of culture,the average height of all seedlings was about 16 cm.Four seedlings were selected to cut from the root collar.Five root branches(including all orders)were collected from each seedling root system.Our order designation strictly followed Strahler’s stream ordering system(described in detail by Pregitzer et al.2002).In total,20 root segments of each order were examined.
Anatomical assessments
Root samples were gently washed in deionized water and immediately fixed in formalin-aceto-alcohol(FAA)solution(90 mL 50%ethanol,5 mL 100%glacial acetic acid,5 mL 37%methanol)and stored in a refrigerator(4°C).Examination of root anatomy followed the procedure described by Guo et al.(2008).After dissection,individual root segments were stained with safranin-fast green,dehydrated in alcohol solution,embedded in paraffin,and prepared as 8-μm-thick sections(Guo et al.2008).These sections were measured for anatomical features.The diameter of each root order,vascular bundle diameter,and the ratio of vascular bundle diameter(V)to root diameter(R)(V/R)were recorded or calculated.The sections were photographed using a compound microscope(BH1,Olympus).For each root segment, five cross-sections were observed and measured.
Morphological analyses
Differences in the diameter,vascular diameter,and V/R between each root order were compared using one-way analysis of variance(ANOVA).Next,the differences in these indicators were determined using least significant difference(LSD)analysis at significance level of α=0.05.All statistical analyses were performed using SPSS software(2010,V.19.0;SPSS Inc.,Cary,NC).Diagrams were constructed using Sigma Plot software(Version 10;Syst at Software Inc.,USA).
Results and analysis
Variations in root diameter,vascular bundle diameter,and V/R by branch orders
Root diameter,vascular diameter,and V/R ratio increased with increasing root order(Fig.1).The average and vascular diameters of fourth order roots were significantly greater than those of the first three orders.They were 1.8 times and 3.4 times wider,respectively,compared to those of the third-order roots.The average diameter and vascular bundle diameter of the fifth order roots were the largest(Fig.1).The V/R of fourth order roots was significantly greater than that of the third order roots.The V/R of fifth order roots was greatest,but was statistically similar to that of the fourth order roots.
The anatomical characteristics of different root orders
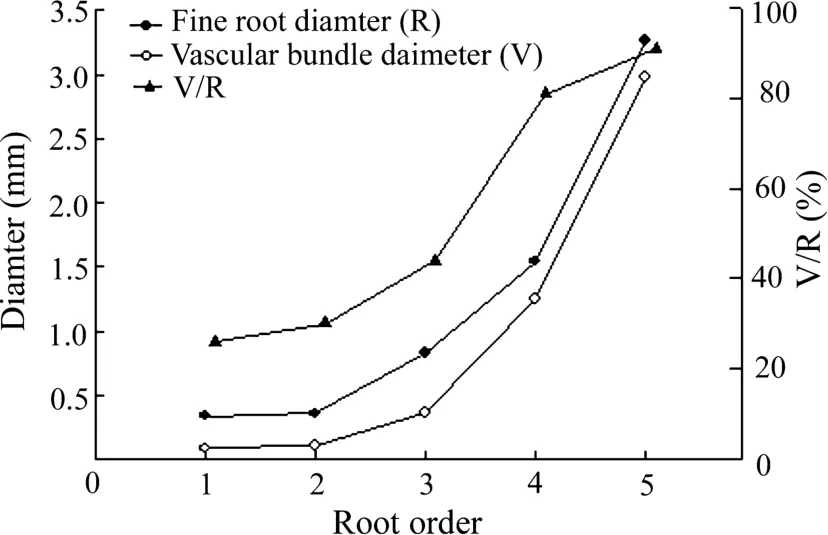
Fig.1 Vascular bundle diameter(V),root diameter(R),and V/R among the first five orders of the roots of one-year old Fraxinus mandshurica seedlings
The epidermis is the outermost layer of fine roots.The main function is protection and absorption.First and second order roots ofF.mandshuricahad one layer of epidermis.Parts of the epidermal cells extended outward to form root hairs,which easily fell off in the first two order roots(Fig.2a,b).A few third order roots also had epidermis,but no root hairs were present.Epidermal cells were flat around the cortex(Fig.2c,d).Fourth and fifth order roots did not have epidermis.The outermost layer was periderm surrounding the continuous phellem(Fig.2e,f).Periderm thickness was not uniform:some portions of the periderm were very thin,but still wrapped around the phloem(Fig.2e,f).
The cortex consisted of several layers of parenchymal cells within the epidermis,including the exodermises,cortical parenchyma,and endodermis from the outside to inside.The cortex status was similar between first(Fig.2a)and second order roots(Fig.2b).One layer of rectangular exodermises(the radial wall was the longer side)was observed around the cortical parenchyma cells.Two kinds of exodermal cells were observed:passage cells and nonpassage cells(Fig.2g).The arrangement of passage cells was adjacent or alternating with non-passage cells.Cortical parenchyma,characterized by irregular circular or polygonal parenchyma cells,was the largest component of the cortex.Several layers of different-sized cortical parenchyma were present.The diameter of the first two layers of cortical parenchyma cells near the exodermis was larger than that of the inner cells.The innermost cortex was endodermis,in which cells were closely and neatly arranged.The radial wall of the exodermis was filled with phellem,forming the Casparian strip in the first two order roots.Two different phenomena were observed in the third order roots.One kind of roots had no typical Casparian strip,but instead had a layer of exodermis filled with phellem in all cell walls.The other kind of third order root had a Casparian strip,similar to that in the first two order roots,which had fewer passage cells than that in the first two order roots.The fourth and fifth order roots had fewer cortex cells.
Pericycle is the inner layer closest to the endodermis.Pericycle cells can be differentiated into meristem cells.In first and second order roots,only one successive pericycle layer was observed,and the cells were regular and neatly arranged(Fig.2a,b).However,in third,fourth,and fifth order roots,no pericycle layer replaced the phellem layer.Some of the third order roots had a non-successive phellem layer(Fig.2c),and some others had a successive phellem layer.All fourth and fifth order roots had a successive periderm located on the outermost side(Fig.2e,f).The core of the roots was vascular.According to the number of primary xylem bundles,the roots ofF.mandshuricacan be considered as diarch roots,triarch roots,and tetrarch roots(Fig.2h–j).All three kinds of roots were present in the first order(Table 1).
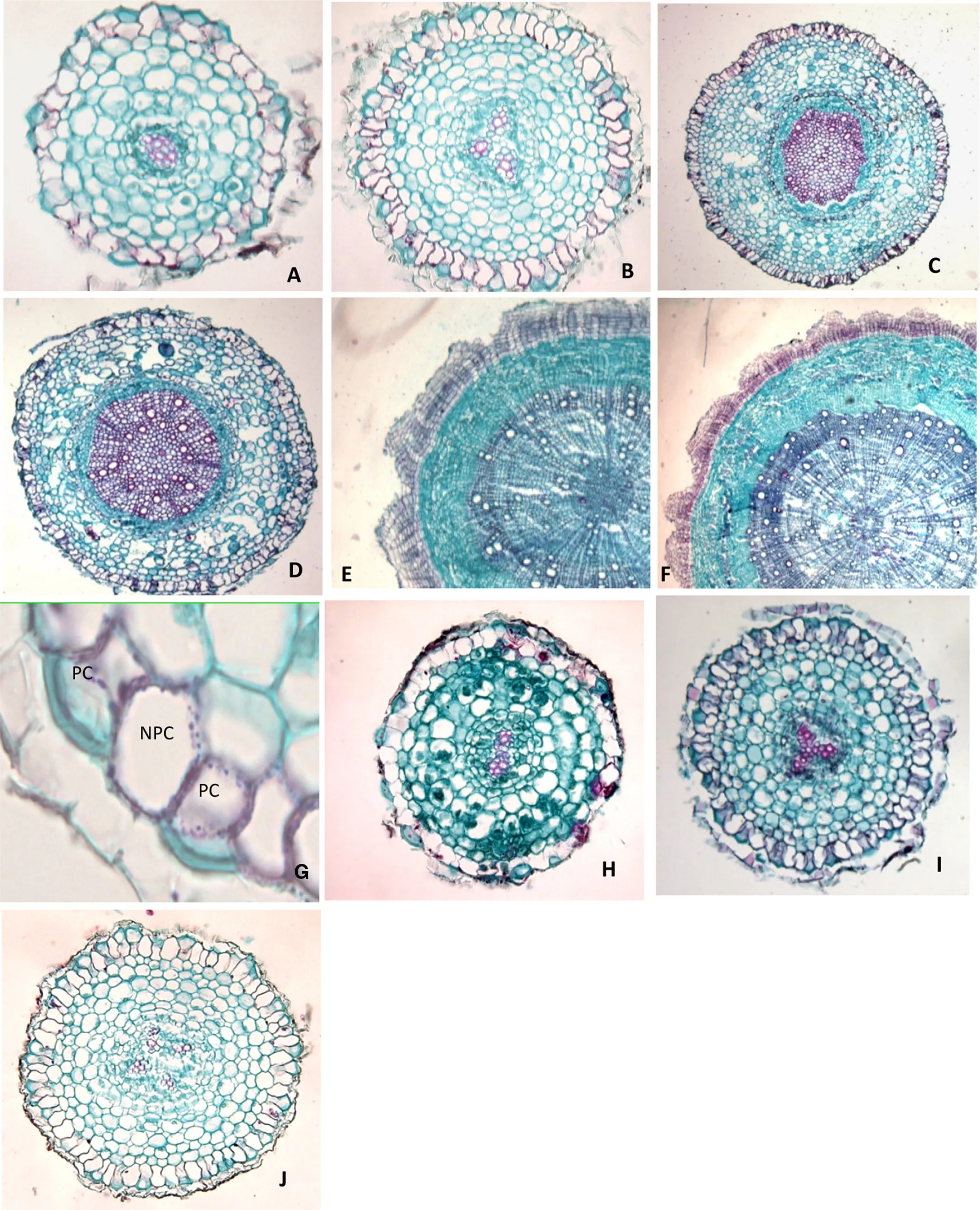
Fig.2 Anatomical structure of the first five order roots of Fraxinus mandshuricaone-year old seedlings.a first order root,b second order root,c,d third order root,e fourth order root,f fifth order root,g passage cells within the exodermis of the first order root,h diarch root,i triarch root,j tetrarch root.PC passage cell,NPC non passage cell.a,b,h,i,j:× 100 magnification;c–f:× 40;g:× 400)
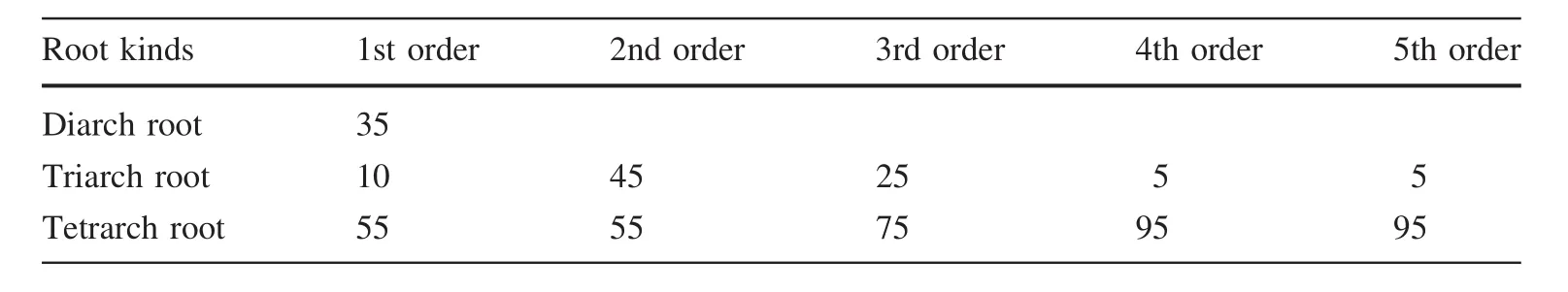
Table 1 Numbers of different kinds of roots among the root branch orders in F.mandshurica seedlings(n=20)(%)
The diarch roots only appeared among first order roots.Triarch and tetrarch roots were observed in second,third,fourth,and fifth order roots.The percentage of tetrarch roots increased with increasing root order(Table 1).All first and second order roots had primary structure(Table 2).All third,fourth,and fifth order roots had secondary growth; however,different structures were observed in third order roots.One of four roots with secondary growth in the third order had non-successive phellem and 75%had a successive phellem(Table 2).The diameter of the roots with primary growth was under 400 μm,including all first and second orders(the maximum diameter of first order roots was 351.42 μm,and that of second order roots was 389.76 μm),and the diameter of roots with secondary growth was over 1000 μm(Table 2).
Discussion
Anatomical traits and functional roles of root branches
Understanding the physiological and ecological functions of roots requires that the differences of the fine-root system at the anatomical,morphological,and architectural levels need to be considered(Hishi and Takeda 2005).Different parts of tree root systems have different functional roles but it is not clear how to distinguish roots of different functions within the branching fine-root systems(Guo et al.2008).Root anatomical traits determine the development of root systems.The root development level corresponds with root functions(Macfall et al.1991).Roots with primary development absorb nutrients and moisture from the soil through the channel cells.More passage cells and bigger cortical cells are beneficial for root absorption,as well as for mycorrhizal colonization(Peterson and Enstone 1997;Peterson et al.1999;Steudle and Peterson 1998).The heterogeneity of anatomical structures among root branches in one-year old seedlings ofF.mandshuricawas reported for plantations ofF.mandshuricaby Guo et al.(2008).For one-year oldF.mandshuricaseedlings,our study revealed that different branch orders were characterized by differences in anatomy.First and second order roots exhibited primary development with an intact cortex,a low stele proportion,and passage cells in both the exodermis and endodermis.First and second order roots served as absorptive roots.Third,fourth,and fifth order roots had secondary development.Successive secondary development,successive phellem layer,and no cortex were noted in the fourth and fifth orders,which had limited capacity for absorption.The impervious airtight phellem of roots with secondary development showed significant hindrance in material exchange between the cortex and vascular bundles,reducing the ability of these roots to absorb nutrients and water(Mckenzie and Peterson 1995).Eissenstat and Achor(1999)indicated that root systems maintain a balance between withstanding environmental stress and absorbing nutrients and water.Although continuous phellem reduces absorption capacity,it increases stress resistance to drought,disease,nematode infestation,or lack of moisture.Therefore,the root life is extended.Thus,the complete periderm structure of fourth and fifth order roots can help protect fine roots and strengthen thecapacity of fine roots to withstand stress and have a prolonged life.Individual fine roots might become stronger by suberization and cork formation,although they do not have high absorptive capacity or high stress tolerance.Fine roots absorb nutrients through non-suberized passage cells(Taylor and Peterson 2000),whereas suberization increases their tolerance to external stresses(Eissenstat and Achor 1999).When the mother roots(i.e.,higher fourth and fifth root orders)have a higher portion of stele and lower(or non-existent)portion of cortex,they have lower absorption capacity,but longer life and higher stress tolerance than the lower-order roots.
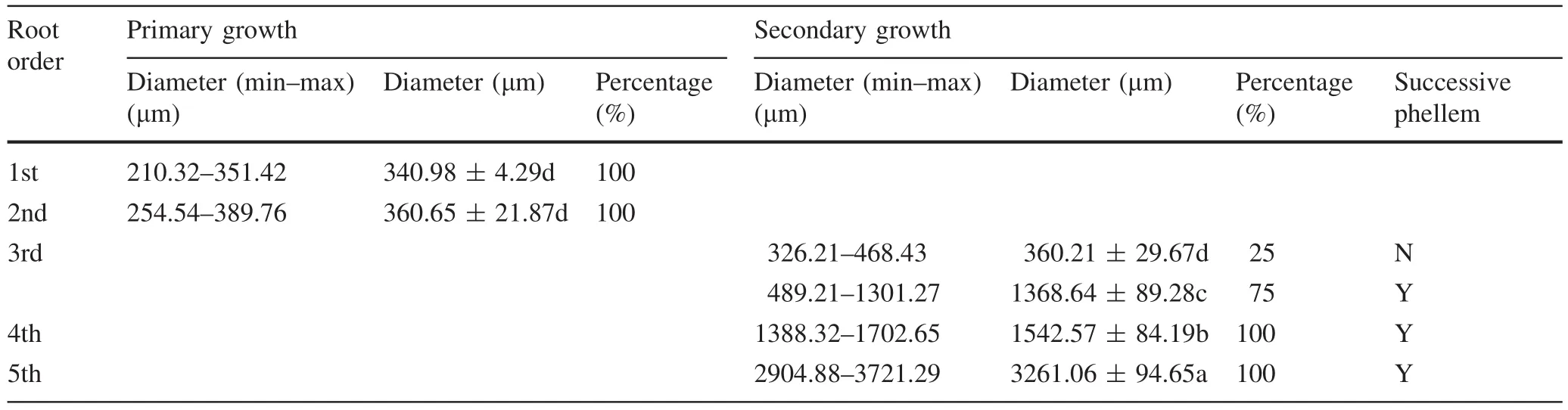
Table 2 Diameter and growth level of different kinds of roots among root branch orders in F.mandshurica seedlings(n=20)
Definition of fine roots
Studies linking anatomy,demography,and physiology to the branching fine root network can lead to better understanding of the functional modules of the plant root systems(Guo et al.2008).At present,two methods are used to define fine roots:diameter method(Joslin and Henderson 1987;Jagodzinski and Kalucka 2010;Gu et al.2014)and root order method(Fitter 1985;Pregitzer et al.2002;Wang et al.2005).Despite the differences in these methods,both agree that a primary function of fine roots is absorption.Thus, fine roots would be more accurately defined by our method,in combination with diameter,branch orders,and anatomical structural features(Macfall et al.1991).In this study,if roots of diameter<1 mm had been considered to be fine roots,then 2%of absorbing roots with unsuccessive phellem would have been incorrectly excluded from the fine root category.If diameters less than 3 mm had been considered as fine roots,55%of non-absorbing roots with successive phellem would have been incorrectly included in the fine root category.If first and second order roots had been considered as fine roots,25% of roots having absorption capacity with unsuccessive phellem in the third order would have been incorrectly excluded from the fine root category.If the first three root orders had been defined as fine roots,then 75%roots having no absorption capacity with successive phellem in the third order would have been incorrectly included in the fine root category.If the first five root orders had been defined as fine roots,75%of third order roots and all fourth and fifth order roots with complete phellem would have been incorrectly included among fine roots.Therefore,irrespective of the kinds of methods used, fine roots would have been either over-or underestimated.Even the diameter-based method or the order based method could not precisely and accurately distinguish fine roots.To accurately represent the important functionality of fine roots,it is better to use functional based methods that combine root diameter,root order and root anatomical structure in the categorization of fine roots.
In this study,we categorized all first and second order roots and a portion of third order roots(with unsuccessive phellem and diameter<0.4 mm)as fine roots.We did not categorize fourth or fifth order roots as fine roots.
AcknowledgementsThe authors are grateful to Jiachun Gu,Hailong Sun,and Lin Lüfor assistance in the field and laboratory studies.
 Journal of Forestry Research2018年5期
Journal of Forestry Research2018年5期
- Journal of Forestry Research的其它文章
- Environmental load of solid wood floor production from larch grown at different planting densities based on a life cycle assessment
- An overview of proven Climate Change Vulnerability Assessment tools for forests and forest-dependent communities across the globe:a literature analysis
- Assessing the vulnerability of a forest ecosystem to climate change and variability in the western Mediterranean sub-region of Turkey:future evaluation
- Effects of management regimes on carbon sequestration under the Natural Forest Protection Program in northeast China
- Mixed-effects modeling for tree height prediction models of Oriental beech in the Hyrcanian forests
- Estimation of a basal area growth model for individual trees in uneven-aged Caspian mixed species forests
