Altitudinal differentiation of Quercus robur in Bosnia and Herzegovina
D.Ballian •M.Memisěvić •F.Bogunić •I.J.Diaz-Maroto
Abstract Morphological variation based on eight measured and four derived traits was studied to establish whether there was significant variation between populations and to identify the influence of the altitude on morphological differentiation among 44 natural stands of common oak(Quercus robur L.)in Bosnia and Herzegovina,ranging from 82 to 860 m.The results point to significant intra-and inter-population differences.Elevation-related variation is less pronounced and determined largely by microclimatic factors.The results could provide management strategies for species reintroduction in the study area.There are no differences in leaf morphology between the different branches of individual trees.However,there are important variations between the altitudinal groups and closely linked to environmental factors in all traits.An important recommendation is to use seeds from oak with attention to their altitudinal origin.
Keywords Common oak·Conservation·Ecology·Management·Morphology ·Renewal
Introduction
Over the last few decades attention has increasingly focused on evolutionary responses to the ability of longlived species to adapt to climate change(Lindner et al.2009).Stressful environments,such as mountain ecosystems,provide an ideal experimental setting for studying adaptation in such species(Lesica and McCune 2004).In these areas,sharp changes in abiotic factors occur over short distances,leading to major changes in the selection pressures acting on plant life-history traits(Vitasse et al.2010).Altitudinal gradients provide an opportunity for analysing the variation of functional traits in response to environmental factors and their implications for the capacity of populations to respond to changing environments(Körner 2007).Along an altitudinal gradient,the divergence between populations may be influenced by differences in the selective pressures imposed by different environments,neutral evolutionary processes or both(Still et al.2005).Morphological and physiological adjustments allow individuals to persist in the stressful environment.For instance,some plants have distinctive morphological features such as including dwarfness or densely pubescent leaves.Abiotic factors and evolutionary processes affecting bud phenology have been widely studied,but little is known of the factors affecting morphological or physiological leaf traits(Premoli and Brewer 2007).
Common oak(Quercus roburL.)is an important species in Europe in different ecological conditions(Stoyanova and Zhiyanski 2004;Kavgaci et al.2010).Fukarek(1959)proposed an infra-specific classification system,citing the following taxa:var.robur,var.cuneifolia(Vukot.)Beck.,var.australis(Heuff.)Simk.,var.latilobaLasch,var.crassiusculaBorbas,and var.fastigiata(Lamk)Spach.However Šilić(2005)refers to only two subspecies in the Dinaric hinterland and the Sava valley region.As a result of past human actions there are presently remnant forests with low economic importance(Memisěvić2008).A survey of forested areas reveals areas of 1,130,183 and 841,303 ha for high and coppice forests,respectively.High forest comprises beech(Fagus sylvaticaL.), fir(Abies albaL.)and spruce(Picea abies(L.)Karst.),Austrian(Pinus nigraJ.F.Arnold)and Scotspine(PinussylvestrisL.),and sessileoak(Quercus petraea(Matt.)Liebl.).The area covered byQuercus roburisestimatedat31.7%(10,260 ha).However,varioussources indicate that common oak once covered much larger areas which were felled over a period of 80 years,1830–1912.About 3,600,000 mature oaks were felled to produce staves for manufacturing barrels in France,and 375,000 m3of cordwood from trees tumps(Memisěvić2008),hence most current oak forests are from natural regeneration(Diaz-Maroto et al.2005).
Reducing the area of common oak is the result of many factors other than human impacts(Diaz-Maroto et al.2015).In upland regions,natural hybridization with sessile oak gives rise to numerous hybrid swarms and creates identification problems.These are processes known as dictating the very marked intra-and inter-population variations between both species(Aldrich and Cavender-Bares 2011)and leads to great polymorphism and variability in morphological traits.
Our aim was to determine if altitudinal variation promotes morphological discrimination among different natural populations of common oak in Bosnia and Herzegovina within an altitudinal gradient of almost 700 m,and to define the elevation of the studied stands.This study is of particular value for the renewal and conservation of the species using both in situ and ex situ methods.
Materials and methods
Quercus roburforests covered large areas in Bosnia and Herzegovina and had an important economic role.Unfortunately,mainly due to human activities,the forests have declined sharply.This decline is compounded by its polymorphism and variability of morphological features,indicative of introgressive hybridization of the partial reproductive isolation between oak species(Diaz-Maroto and Vila-Lameiro 2007).
During our study,the intra-population and inter-population variations of certain morphological traits of the foliage were analysed.Herbarium material was collected in the summer and autumn of 2007 and 2008(July to October)at every location where a group of at least 10 naturally occurring oaks was found.The material was collected from 44 populations,both from self-seeded and planted oaks(Fig.1;Table 1).
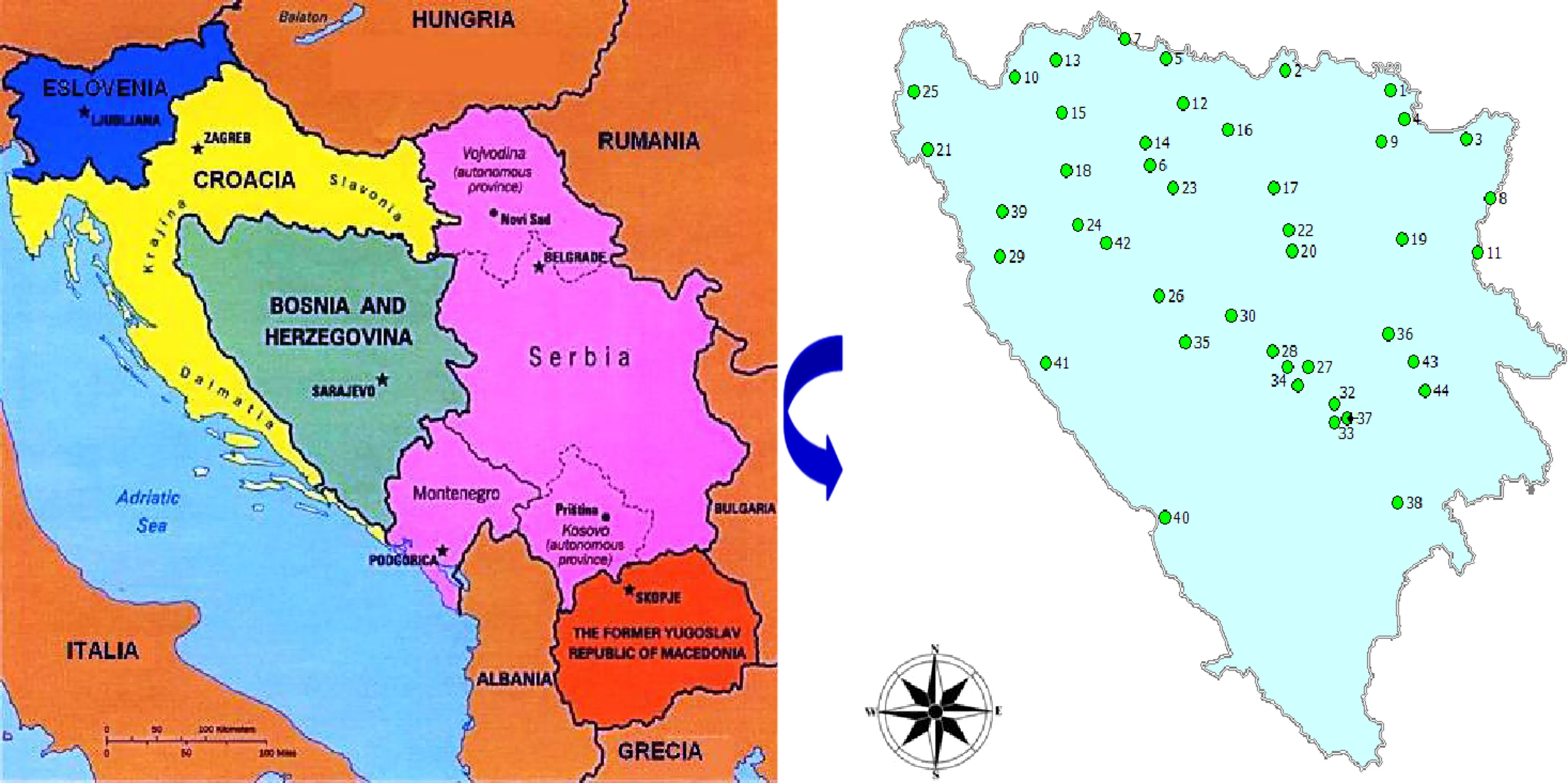
Fig.1 General map of the Balkans location and specific map with the populations of Quercus robur in Bosnia and Herzegovina
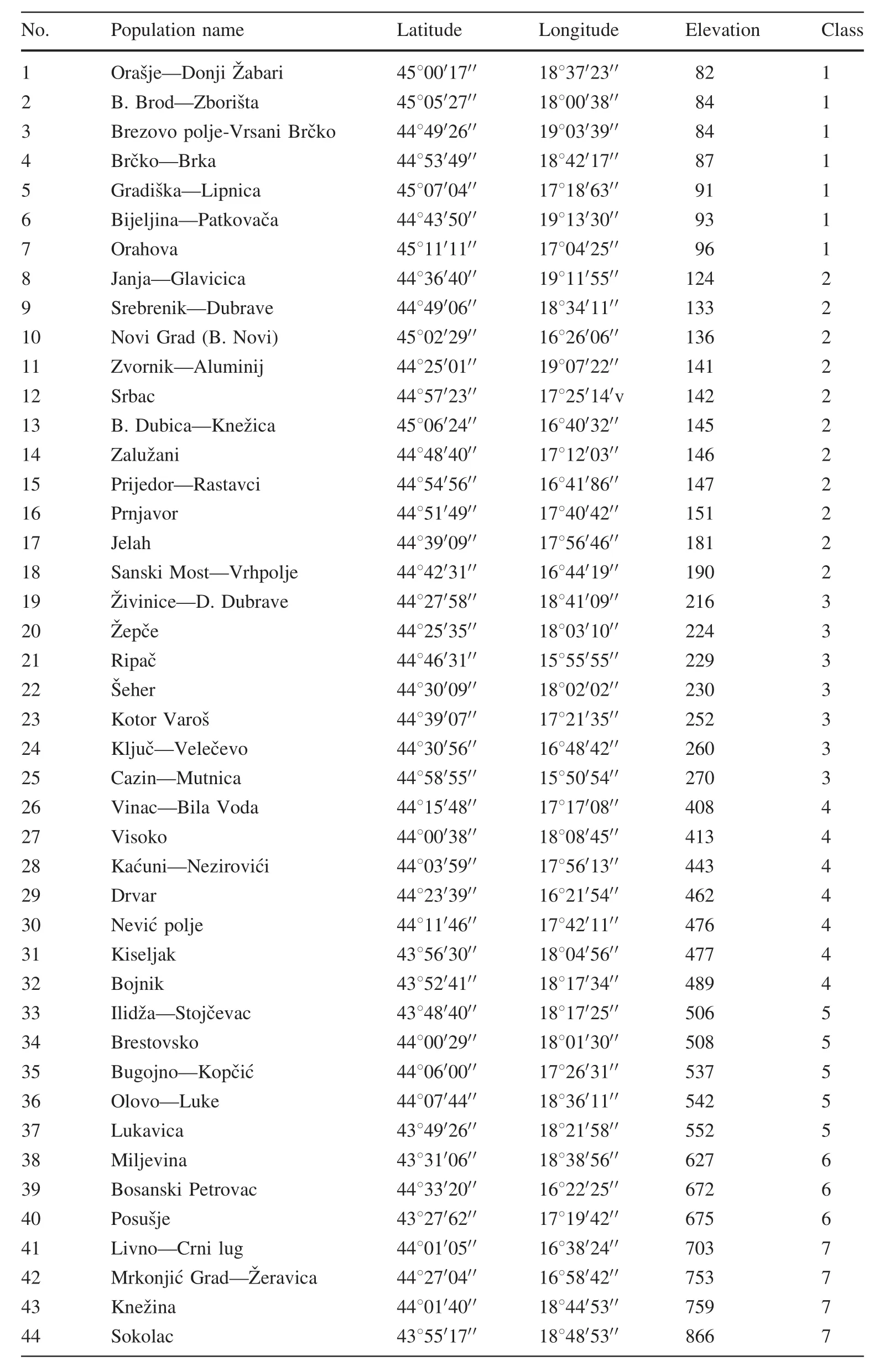
Table 1 Geographical characteristics and altitudinal classes of populations of Quercus robur in Bosnia and Herzegovina
Leaves were gathered from fertile shoots of adult trees growing in isolation or on forest margins.Previous studies have shown that such material is the most suitable for morphological metric analysis and a good description of stands under study(Bašićet al.2007;Ballian et al.2010a).Care was taken to ensure that the populations were from a variety of ecological and geological conditions and,when possible,that they belonged to different phytocoenosis(Stoyanova and Zhiyanski 2004;Kavgaci et al.2010).
Fig.2 Fertile shoots with leaves of common oak with detail of the polymorphism from leaves(Trinajstić1988)
Thirty leaves were collected from each tree(Fig.2),always on the south-facing parts of the crown.Before collecting,there was a first selection of leaves of equal size in which traits were measured and marked for further analysis,both with respect to leaves of each tree and all the trees of same population(Trinajstić1988).The leaves were dried between sheets of newspaper,the standard herbarium procedure,after which ten leaves from each tree were measured.
Leaf measurements followed by Franjić(1994),as modified by Bašićet al.(2007)and Ballian et al.(2010a),for the following(Fig.3):(a)length of leaf blade(K1);(b)length of petiole(K2);(c)distance between widest part of blade and base of blade(right half)(K3);(d)width of right half of blade(K4);(e)width of left half of blade(K5);(f)width between inter-lobe and central vein(K6);(g)size of auricles at base of blade(K7),all in mm;and,(h)number of lobes on right side of blade(K8),together with different derived traits(e.g.,overall width of leaf and average length of lobes).Some were taken from the method proposed by Kremer et al.(2002).
The following traits were determined:
K9—overall width of leaf blade in mm(K4+K5)
K10—overall length of leaf in mm(K1+K2)
K11—ratio between width and length of leaf(K9/K1)
K12—average length of lobes in mm(K1/K8)
To determine if there were differences between populations at different altitudes,the data were divided into the following groups:(1)0–100;(2)101–200;(3)201–300;(4)401–500;(5)501–600;(6)601–700;(7)701–900 m.It is important to note the absence of sampled populations between 300 and 400 m.
The measured morphological data were processed statistically using SPSS 15.0 software(SPSS Inc.2005)and first checked if the data were adjusted to a normal distribution or not.The elevation differentiations were analysed by means of:(1)Analysis of Variance(ANOVA)for the quantitative traits by groups based on elevation classes;(2)Discriminant analysis of the quantitative traits by groups based on elevation classes;(3)Duncan’s multiple range test;and,(4)Discriminant analysis taking into account the mean values of all analysed traits by population.
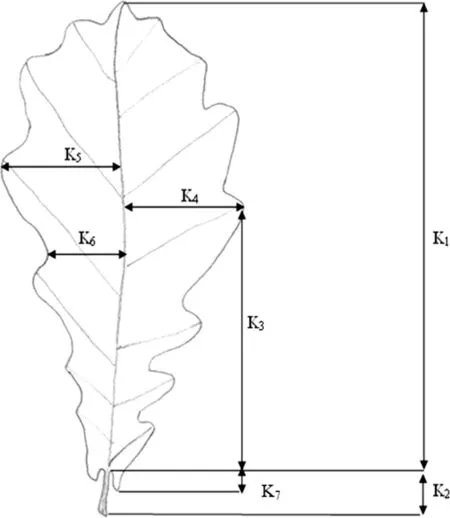
Fig.3 Measured leaf traits from 44 populations,both from selfseeded and planted(Franjić1994)
Results
Table S1(see ESM appendix)shows the descriptive statistics of the different traits measured.As since the data were adjusted to a normal distribution,an ANOVA to the different altitudinal classes was carried out,with the data divided into two groups(self-seeding and planted).The ANOVA revealed the existence of differences between groups for each of the traits analysed,with values of sig<0.05(probability 95%)and sig<0.01(99%).Conclusions concerning a possible grouping of elevation classes will be reached only after discriminant analysis and multiple ranges testing,since the data obtained reveal no discernible grouping trends(Table S2;see ESM appendix).Discriminant analysis,based on the elevation classes,revealed the presence of six functions of which the individual value is<1(Table 2).This shows no significant discrimination sets based on any functions(Fig.4).A further analysis was carried out using the average of the traits for all populations so as to eliminate the effect of marked intra-population variations which could have an impact on the discrimination value(Table 3;Fig.5).The results show that the individual values by functions 1 and 2 are superior than 1.The eigenvalue is equal to 3532 and 1365,functions 1 and 2,respectively.The presence of variance by discriminant function 1 may explain 61.6%,and by function 2,23.8%(Table 3).That is,there is a group differentiation by these functions.
Figure 5 shows the groups are divided into two subgroups in terms of function 1.To the left of the zero line are:0–100,101–200,and 201–300 m,while to the right are the higher elevation classes,401–500,501–600,601–700,and 701–900 m.This means that there are differences between the values of the leaf traits from lower-elevation populations,0–300 m,and higher-populations,301–900 m.In terms of function 2,the elevation classes fall into two sub-groups,one belonging to groups 0–100,201–300 and601–700 m,and the other to 101–200,401–500,501–600 and 701–900 m.
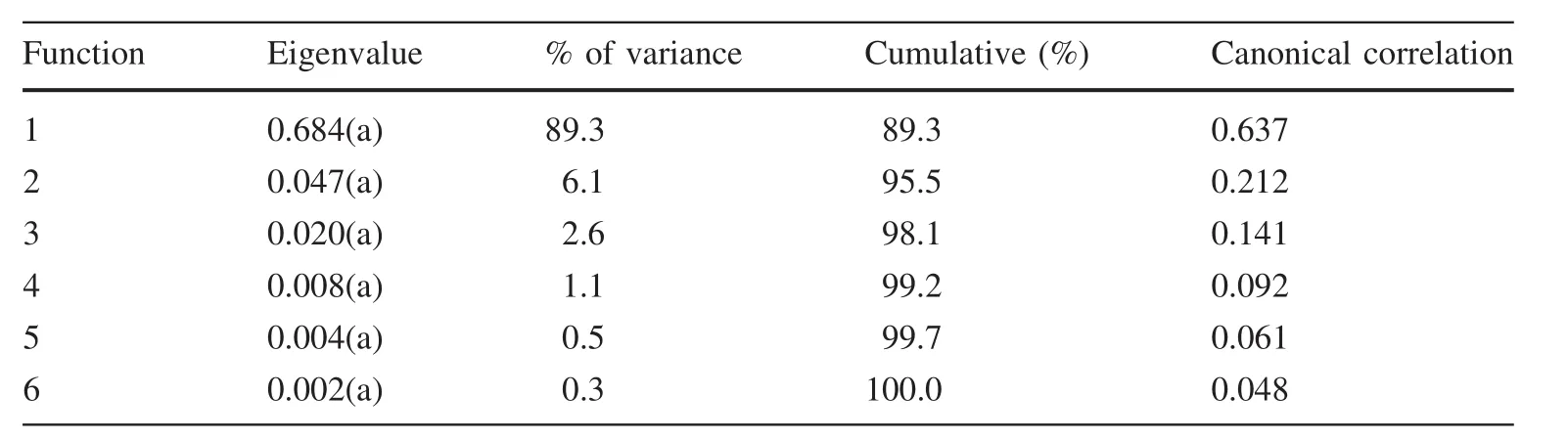
Table 2 Discriminant analysis of groups by altitudinal classes
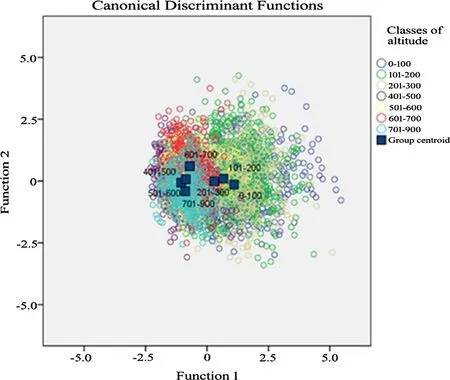
Fig.4 Discriminant analysis of groups by altitudinal classes
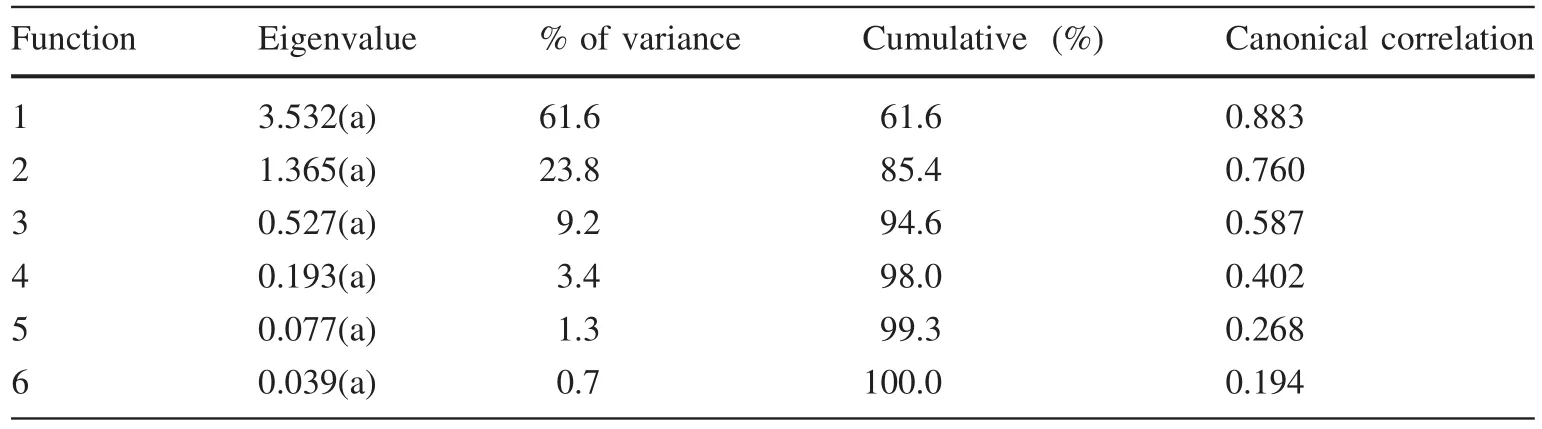
Table 3 Discriminant analysis based on the average values of population traits
The results obtained with Duncan’s test in Table S3(see ESM appendix),grouped populations according to traits and elevation,and show that by measuring only certain morphological traits(particularly K1,K4,K5,K8and K9),it is possible to differentiate populations.
Discussion
The distribution of forest communities depends mainly on geographic and climatic factors.According to the ANOVA results by groups on the basis of elevation(seven groups),our study recorded differences for each trait.In fact,there are variations of the morphological features of populations at lower and higher altitudes.Therefore,for the treatment of common oak wood,depending on altitude,seedlings and plantation material may be transferred within a range of 100 m for the first three classes,and somewhat higher for the remaining.It is possible that populations have been affected by changes in their ecological habitat and a marked anthropogenic impact in the past(Diaz-Maroto et al.2015).
Kjällgren and Kullman(1998,2002)studied altitudinal tree-limit records forPicea abiesandPinus sylvestris,in the Scandinavian Mountains,southern Sweden.These were compared with analogous data forBetula pubescensEhrh.ssp.tortuosa(Ledeb.).The large-scale geographical patterns of tree-limit altitudes were similar for all species,suggesting they are regionally controlled by the climate conditions,primarily summer temperatures and precipitation.These results agree with those obtained by Kohnle et al.(2014).Furthermore,differences between groupswere found for all traits analysed.This could be used in future work on regeneration and reforestation of common oak by altitudinal classes(Kavgaci et al.2010).
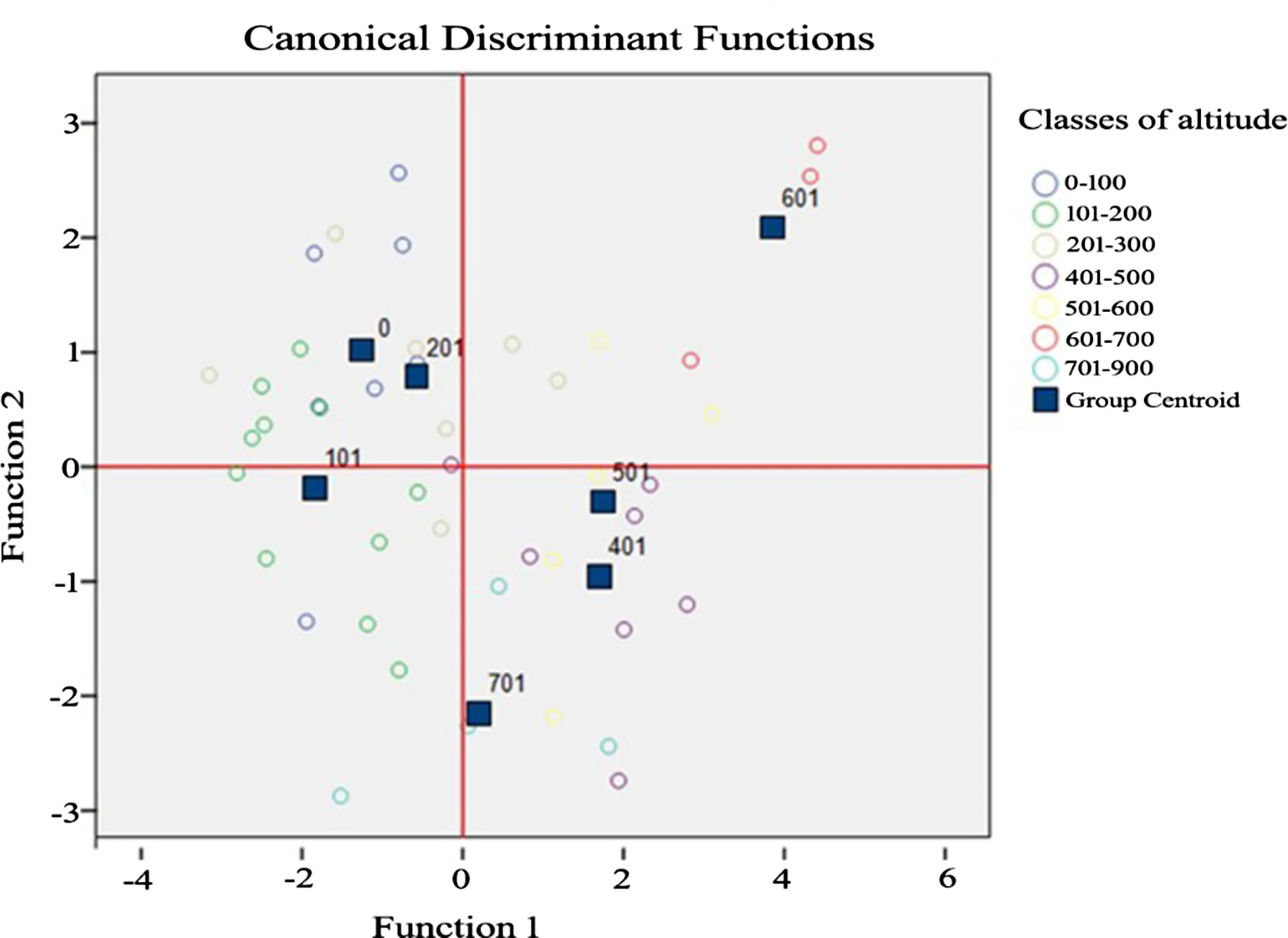
Fig.5 Discriminant analysis of groups by altitudinal classes based on average values of population traits
The discriminant analysis of all traits by population revealed the presence of differentiation into two groups by one of the functions.One group consisted of elevations between 0 and 200 m.The class from 201 to 300 m forms the boundary between the two sub-groups,while higher elevations,401–900 m,belong to a second group.That is,there are differences in the morphological characteristics of leaves between the populations located at different altitudes(Körner 2007;Premoli and Brewer 2007).
Duncan’s test results corroborated partly discriminant analysis except elevation classes 1–100,101–200 and 201–300 m,which formed separate groups,while classes between 400 and 900 m can be regarded as a single group,with the exception of the class between 601 and 700 m which was shown as a separate group for most traits.These results highlight the importance for the treatment of common oak in terms of altitude.
Research at the molecular and genetic levels anticipated a greater separation(Bordács et al.2002)between the Livno,Romanija and MrkonićGrad and the other populations(Ballian et al.2010b).
The elevation and the processes of historical migration on which several authors have written(Bordács et al.2002;Petit et al.2002;Slade et al.2008),have also noted the differentiation between population groups.It is worth noting here that the populations studied over the past 200 years have been under a constant negative selection pressure from over-felling and incorrect forest management(Memisěvić2008).The regulation of watercourses and the drainage of fields also had a major impact,and more recently,air quality,water and soil pollution levels(Diaz-Maroto and Vila-Lameiro 2008).Over the past decade,illicit felling was a serious problem causing the loss of the last old specimens of the species.This has led to a significant reduction in the number of trees in the populations,bringing common oak to the brink of extinction(Kavgaci et al.2010).The research carried out by Ducci(1991)in the Apennines provides a telling illustration of the historical anthropogenic impact on forests.The population groups analysed here must be seen as provisional and may not be the result of natural processes.Finally,an important consideration should be to revitalize oak stands and the widest possible gene bank networks be established in situ and ex situ in all ecological niches to maintain biodiversity.
Conclusions
Absence of differences to the variations in leaf morphology between the diverse branches of individual trees;also,there are important differences between groups closely linked to environmental factors,mainly elevation.Though,the phenotypic variation has a genetic basis.The study into individual inter-population variations may form the starting-point for further research into altitudinal variation,and the results could form a sound basis for the selection of seed stands,the renewal and preservation of genetic diversity as well as for distinguishing single populations.AsQuercus roburis a very valuable species and sites are suitable,it should be used for reforestation at available locations,with particular attention to the altitudinal origin of the seed.The results should be validated in the future by other methods,particularly through the establishment of common garden or reciprocal transplant studies.
AcknowledgementsWe are most grateful to our colleagues who helped collect these large quantities of herbarium material,and in particular Dr.Ned¯ad Bašić,Almir Dugonji,BSc.For.,and Goran Pejčinović,BSc.For.
 Journal of Forestry Research2018年5期
Journal of Forestry Research2018年5期
- Journal of Forestry Research的其它文章
- Environmental load of solid wood floor production from larch grown at different planting densities based on a life cycle assessment
- An overview of proven Climate Change Vulnerability Assessment tools for forests and forest-dependent communities across the globe:a literature analysis
- Assessing the vulnerability of a forest ecosystem to climate change and variability in the western Mediterranean sub-region of Turkey:future evaluation
- Effects of management regimes on carbon sequestration under the Natural Forest Protection Program in northeast China
- Mixed-effects modeling for tree height prediction models of Oriental beech in the Hyrcanian forests
- Estimation of a basal area growth model for individual trees in uneven-aged Caspian mixed species forests
