Screening potential mitochondria-targeting compounds from traditional Chinese medicines using a mitochondria-based centrifugal ultra filtration/liquid chromatography/mass spectrometry method
Xing-Xin Yng,Yu-Zhen Zhou,Feng Xu,Jie Yu,Gegentn,Ming-Ying Shng,Xun Wng,Sho-Qing Ci,*
aDepartment of Natural Medicines,School of Pharmaceutical Sciences,Peking University,38 Xueyuan Road,Beijing 100191,PR China
bDepartment of Chemical Biology,School of Pharmaceutical Sciences,Peking University,38 Xueyuan Road,Beijing 100191,PR China
cCollege of Pharmaceutical Science,Yunnan University of Traditional Chinese Medicine,1076 Yuhua Road,Kunming 650500,Yunnan Province,PR China
Keywords:Mitochondria Bioactive constituents Traditional Chinese medicines Centrifugal ultra filtration Liquid chromatography/mass spectrometry
ABSTRACT Mitochondria regulate numerous crucial cell processes,including energy production,apoptotic cell death,oxidative stress,calcium homeostasis and lipid metabolism.Here,we applied an efficient mitochondria-based centrifugal ultra filtration/liquid chromatography/mass spectrometry(LC/MS)method,also known as screening method for mitochondria-targeted bioactive constituents(SM-MBC).This method allowed searching natural mitochondria-targeting compounds from traditional Chinese medicines(TCMs),including Puerariae Radix(PR)and Chuanxiong Radix(CR).A total of 23 active compounds were successfully discovered from the two TCMs extracts.Among these 23 hit compounds,17 were identified by LC/MS,12 of which were novel mitochondria-targeting compounds.Among these,6 active compounds were analyzed in vitro for pharmacological tests and found able to affect mitochondrial functions.We also investigated the effects of the hit compounds on HepG2 cell proliferation and on loss of cardiomyocyte viability induced by hypoxia/reoxygenation injury.The results obtained are useful for in-depth understanding of mechanisms underlying TCMs therapeutic effects at mitochondria level and for developing novel potential drugs using TCMs as lead compounds.Finally,we showed that SM-MBC was an efficient protocol for the rapid screening of mitochondria-targeting constituents from complex samples such as PR and CR extracts.
1.Introduction
Mitochondria play a crucial role in maintaining cellular life and are involved in various significant bio-functions and metabolic pathways,including calcium homeostasis,thermogenesis,gluconeogenesis,citric acid cycle,β-oxidation of fatty acids,urea cycle,electron transport chain and oxidative phosphorylation that end with ATP generation[1].The integrity of mitochondrial function is fundamental to cellular homeostasis.Consequently,mitochondria dysfunctions will contribute to many diseases with a great diversity of clinical appearances,such as neurodegenerative disorders,ischemiareperfusion injury,diabetes,aging,inherited mitochondrial diseases and most importantly,cancer[2].As mitochondria are key modulators of cellular homeostasis,mitochondrially-targeted compounds that remedy mitochondrial dysfunctions represent an attractive approach for the treatment of human disorders[3–5].
Traditional Chinese medicines(TCMs)have a long history of clinical testing and reliable therapeutic efficacy,which have gained attention as excellent sources of bioactive compounds for the discovery of new drugs[6].A rapidly growing number of literatures proposed that many TCMs could regulate mitochondrial functions to perform their pharmaceutical efficacy[7].For example,extracts of Puerariae Radix(PR)and components of Chuanxiong Radix(CR)have protective effects on mitochondria[8,9].However,bioactive constituents binding to mitochondria in TCMs are almost unrecognized so far,hampering the enhancement of our comprehension about therapeutic principles of TCMs,and the development of new potential drugs from TCMs.Disclosing the presence of bioactive substances targeting to mitochondria in TCMs has thus become of utmost importance.
The traditional activity-guided isolation procedure for complex mixtures is a labor-intensive,time-consuming,and expensive process,and it is often invalid for the direct search of bioactive constituents from complex samples [10].High-throughput screening[11]and high-content screening methods[12]have also been used for the efficient identification of mitochondria-targeting compounds,but they cannot be applied for direct screening of multiple ligands from complex agents simultaneously.In our previous study,we developed an efficient mitochondria-based centrifugal ultra filtration/liquid chromatography/mass spectrometry method,which is called screening method for mitochondria-targeted bioactive constituents(SM-MBC),and our method is compatible with the searching of mitochondria-targeting compounds from complex samples[7].It was confirmed that this method simultaneously possessed excellent recognition,separation and identification abilities,and displayed advantages as efficient use of labor,a simple procedure,and low time and sample requirements.Thus,SM-MBC,preventing unnecessary resource utilization in downstream isolation of inactive compounds from extracts used in the screening process,can be performed to the direct screening of mitochondria-targeting compounds in complex matrix such as TCMs.
In the present study,we applied SM-MBC for the direct screening of natural mitochondria-targeting compounds from TCMs(i.e.PR and CR).Bioactive molecules selectively linked to mitochondria were isolated by centrifugal ultra filtration(CU).The isolated fractions were then collected and injected into liquid chromatography/mass spectrometry(LC/MS)for rapid isolation and identification.Our screening results were meaningful for indepth understanding of mechanisms underlying TCMs action,but also for the development of mitochondrial modulators from TCMs.Moreover,SM-MBC was shown to be an efficient method for the rapid screening of mitochondria-targeting compounds from complex samples.
2.Materials and methods
2.1.Chemicals and materials
Puerarin(lot no.G-008–131221),daidzein(lot no.D-016–131102)and daidzin(lot no.D-013–131111)were purchased from Chengdu Herbpurify Co.,Ltd.(Chengdu,China).3′-methoxypuerarin(lot no.131217),3'-hydroxypuerarin(lot no.131416),senkyunolide A(lot no.140914),ligustilide(lot no.131204)and levistolide A(lot no.130910)were purchased from Chengdu Pufeide Biological Technology Co.,Ltd.(Chengdu,China).Formononetin(lot no.GMED-0013)was provided by Hong Kong Jockey Club Institute of Chinese Medicine(Hongkong,China).Dulbecco's Modified Eagle's Medium(DMEM)and Bicinchoninic Acid(BCA)protein determination kit were obtained from M&C Gene Technology(Beijing)Ltd.(Beijing,China).DMEM/F12 and fetal bovine serum(FBS)were purchased from Gibco(Grand Island,NY,USA).WST-8 Kit (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,monosodium salt)was purchased from Neuronbc®(Beijing,China).Nycodenz was purchased from Axis-shield PoC AS(Oslo,Norway).HPLC grade methanol and acetonitrile were provided by Fisher Scientific(Fairlawn,NJ,USA).Deionized water was purified using a Milli-Q Water Purification System(Millipore,Billerica,MA,USA).Calycosin was isolated from Astragali Radix by our group.The structure was verified by LC/MS and nuclear magnetic resonance(NMR),and the purity was found to be > 98%by HPLC–DAD(based on the percentage of total peak area).All other reagents used were of analytical reagent grade or higher.
PR(purchase date 2014/04)and CR(purchase date 2009/12)were purchased from the Tianheng Drug Store(Beijing,China)and the Hebei Anguo Yaoxing Pharmaceutical Co.(Anguo,Hebei,China),respectively.All the samples were authenticated by Professor Shao-Qing Cai and the voucher specimens of PR(No.7546)and CR(No.6332)have been deposited in the Herbarium of Pharmacognosy,School of Pharmaceutical Sciences,Peking University(Beijing,China).
2.2.Experimental animals
Experimental protocols involving conscious animals were performed in according with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and approved by the Biomedical Ethical Committee of Peking University(No.SYXK2011-0039).Healthy male Sprague-Dawley rats(300±50 g)provided by the Department of Laboratory Animal Science,Peking University Health Science Center(Beijing,China)were used in this study.Rats were bred under an environmentally controlled room with ad libitum access to food and tap water.In addition,neonatal Sprague-Dawley rats(<24h)provided by the Vital River Laboratories(Beijing,China)were used for the primary culture of cardiomyocytes.All efforts were made to minimize the number of the animals used and their suffering.
2.3.Preparation of analytical solutions
Working solutions of PR(450 mg/mL)and CR(600 mg/mL)were prepared by dissolving the freeze-drying powder of PR and CR extract(the preparation methods are described in the Supplementary material)in dimethyl sulfoxide(DMSO).For the pharmacological test,analytical solutions of standard compounds from PR and CR extracts,including perarin,daidzein,daidzin,formononetin,ligustilide and levistolide A were prepared in DMSO and diluted with physiological saline to the demanded concentration.All solutions were stored at 4°C in the dark.
2.4.Preparation of mitochondrial suspension
Mitochondrial suspension was prepared as previously described[7].Brie fly,hearts isolated from Sprague-Dawley rats were quickly placed into ice-cold isolation buffer(buffer A:210 mM mannitol,70mM sucrose,10 mM Tris base,1 mM EDTA and 0.5 mM EGTA,pH 7.4)to remove blood,minced into 1mm3pieces,and homogenized by using a Dounce glass homogenizer(Kimble/Kontes,Vineland,NJ,USA)with buffer B(buffer A plus a mixture of 1 mM PMSF and protease inhibitor cocktail).After centrifugation at 1000×g for 10 min,the supernatant was collected and centrifuged at 10,000×g for 10min to obtain crude mitochondria.Nycodenz was dissolved to 34%,30%,25%,23%and 20%(w/v)with buffer B.The crude mitochondria were resuspended in 1.3 mL of 25%Nycodenz and layered on a discontinuous Nycodenz gradient of 0.5 mL of 34%and 0.9 mL of 30%,which was topped off with 0.9 mL of 23%and finally 0.4 mL of 20%.The gradient was centrifuged at 52,000×g for 90 min in a Beckman SW 60 Ti rotor(Beckman Coulter,Inc.,Fullerton,CA,USA).The band at the 25%/30%interface was collected,diluted with the same volume buffer A and then centrifuged at 10,000×g for 10 min.The pellet was washed with buffer B and collected as purified mitochondria that were resuspended with buffer A to obtain a 1.0 g/L of mitochondrial suspension.Mitochondrial protein concentration was determined using BCA method.Furthermore,a part of the mitochondrial suspension was persistently heated for 2 h in boiling water to produce denatured mitochondria without their original biological functions.
2.5.Screening process with SM-MBC

Fig.1.Schematic representation of the analytical procedure used for the screening of mitochondria-targeting compounds from TCMs extracts by SM-MBC.
Screening process using SM-MBC was based on previously reported method(Fig.1)[7].Brie fly,5μL PR working solution and CR working solution were separately incubated with 200μL of the mitochondrial suspension at 37°C for 60 min(90 min for the CR working solution),and then were filtered through a 10 kDa molecular weight cutoff ultra filtration membrane(Microcon YM-10,Millipore Co.,Bedford,MA,USA)by centrifugation at 14,000×g for 25 min at 4°C.Pellets entrapped in the membranes were washed three times with 200μL ammonium acetate buffer(50 mM,pH 7.5)through centrifugation at 14,000×g at 4°C for 25 min.Afterward,bound molecules were released from mitochondria by adding 400μL of 80%aqueous methanol followed by centrifugation at 14,000×g for 25 min at room temperature.Purified ultrafiltrates were dried under a stream of nitrogen and reconstituted with 100μL of 80%methanol.Samples containing screened compounds were analyzed by LC/MS(The conditions of LC/MS analysis are described in the Supplementary material).Control experiments for nonspecific binding were performed in a similar way with denatured mitochondria.LC/MS peaks obtained with and without denatured mitochondria were compared.When the percentage of discrepancy of peak area between experiment and control(ΔP)was >20%,this increase in peak area indicated a specific binding that was deemed as the mitochondria-targeting compounds.ΔP value was calculated using the formula:

where Peand Pcare the peak areas in the experiment and control,respectively.
2.6.Determination of mitochondrial permeability transition pore opening in isolated myocardial mitochondria
The opening of the mitochondrial permeability transition pore(mPTP)was determined by Ca2+-induced swelling of isolated myocardial mitochondria and was performed to investigate the interaction between the hit compounds and the mitochondria.The mPTP opening results in mitochondrial swelling,which will induce a decrease of absorbance at 520 nm(A520).This absorbance fluctuation provides a convenient and frequently used determination of the mPTP opening[7,13].Brie fly,isolated cardiac crude mitochondria(0.25 g/L)were resuspended by swelling buffer(120 mM KCl,20 mM MOPS,10 mM Tris-HCl,5 mM KH2PO4,pH 7.4),and then preincubated with tested compounds for 3min at room temperature followed by the addition of 250μM CaCl2to induce mPTP opening for 15 min.Afterward,the A520was immediately measured with UV–VIS Spectrophotometer(Beijing Purkinje General Instrument Co.,Ltd.,Beijing,China).The experiment was performed five times.
2.7.Evaluation of cancerous cell proliferation
For cancerous cell proliferation assay,aliquots of HepG2 cells(2×104cells/well)were seeded in 96-well plate,and cultivated in DMEM supplemented with 10%FBS at 37°C in a humidified atmosphere of 5%CO2.Confluent beating cells were then treated with tested compounds at different concentrations,and imaged using the CloneSelect Imager system(Genetix,UK)after 0,24,48,72 and 96 h treatment.The proliferation curves were drawn with the cell confluence(%)at the indicated time points to evaluate the inhibition effects of compounds on cell proliferation.The assay was performed five times.
2.8.Assessment of cellular viability in cardiomyocyte model of hypoxia/reoxygenation(H/R)injury
Cellular viability was measured using the cell counting kit(CCK)-8 assay,according to the manufacturer's recommendations.Confluent beating cardiomyocytes from neonatal rats primarily cultured for 4 days were exposed to hypoxia for 2 h,and then were incubated with tested compounds.Subsequently treated cells were reoxygenated for 1 h.After experimental treatment,10μL of WST-8 solution was added to each well,and cardiomyocytes were incubated for additional 2h at 37°C.The absorbance of each well at 450 nm was determined using a SpectraMax M5 microplate reader(Molecular Devices,Sunnyvale,CA,USA).The percentage of cellular viability was calculated by the following formula[14]:%cell viability=(mean absorbance in test wells-mean absorbance in blank wells)/(mean absorbance in negative control well-mean absorbance in blank wells)×100.The primary culture procedure for cardiomyocytes and the preparation of H/R injury model are reported in the Supplementary material.The assay was performed in triplicate.
2.9.Data treatment
All data are presented as mean±SD.For statistical analysis,one-way analysis of variance(ANOVA,Dunnett's method)was performed using SPSS software(13.0 for Windows;SPSS,Chicago,IL,USA).P<0.05(two-tailed)was considered significant.
3.Results and discussion
3.1.Principle of SM-MBC
SM-MBC was previously developed by combining CU and LC/MS techniques to perform the direct search for mitochondria-targeting compounds in complex samples as TCMs compounds[7].In this method,active constituents against specific targets in mitochondria could be specifically recognized and isolated.Structural characteristics of corresponding constituents were identified by LC/MS(Fig.1).SM-MBC showed the recognition,separation and identification capabilities that were sufficiently validated using positive and negative controls,avoiding the use of unnecessary resources on downstream isolation of compounds of little or no value from extracts used in the screening process.Thus,SM-MBC can be efficiently applied for the identification of mitochondriatargeting compounds in complex preparations.

Fig.2.Screening of mitochondria-targeting compounds from PR extract using SM-MBC.Compared with the control,including denatured mitochondria,(red line),HPLC chromatograms of screened PR extract(A,0–45 min;B,45–85 min)exhibited 11 peaks(P1–P11),which were enhanced because of specific binding to mitochondria(black line).The conditions for analyzing the PR extract are presented in the Supplementary material.
3.2.Effect of screening conditions
The developed SM-MBC was used to identify natural mitochondria-targeting compounds from PR and CR extracts.In order to acquire the best screening performance for active compounds from the extracts and investigate the major in fluencing factors in SM-MBC,the effects of several screening conditions were investigated,such as mitochondrial concentration,sample concentration and incubation time.
First,different mitochondrial concentrations(0.25,0.50 and 1.0 g/L)were used for screening the active compounds present in PR and CR extracts.The number of active compounds selected from the two extracts remarkably increased with the increase of mitochondrial concentration(Figs.S1 and S2),which was consistent with the previously-reported screening of other extracts[7].Therefore,mitochondrial concentration in fluences SM-MBC sensitivity.Higher mitochondrial concentrations were related to higher sensitivity,which could select more active compounds from TCMs extracts.However,higher mitochondrial concentrations(>1.0 g/L)will block the ultra filtration membrane,and 1.0 g/L was the optimum mitochondrial concentration for SM-MBC,enabling the screening of the maximum number of active compounds from TCMs extracts.
Next,three concentrations of PR(2.625,5.250 and 10.50 g/L)and CR(3.875,7.750 and 15.50 g/L)samples were separately used for the screening of active compounds.The number of active compounds found from PR and CR extracts increased with higher sample concentrations(Figs.S3 and S4).In particular,10.50 and 15.50g/L,the saturated concentrations for the two samples,were respectively found to be the optimal concentrations for the screening of PR and CR extracts.In addition,we previously confirmed that 7.5 and 2.5 g/L were the optimum for the screening of mitochondria-targeting compounds from other two TCMs extracts[7].Hence,sample concentration may affect the identification of mitochondria-targeting compounds.The screening of active compounds for different TCMs extracts showed a sizable discrepancy in optimal sample concentration,which may be attributed to the differences of chemical constituents in various TCMs.Hence,the sample concentration should be optimized for each TCM extract analyzed by SM-MBC.
Finally,the effect of different incubation times(30,60 and 90 min)was investigated.The number of active compounds found in PR and CR extracts increased with the incubation time(Figs.S5 and S6),but identical results were obtained at 60 and 90 min for the PR extract.Thus,60 min incubation time was sufficient for the screening of the PR extract and 90 min for the screening of the CR extract.Furthermore,we previously found that 60 or 90 min was the optimal incubation time for the screening of active compounds from other two TCMs extracts[7].Thus,the optimal incubation time for the screening of active compounds from TCMs extracts by SM-MBC might be in the range of 60–90 min,which should be optimized to obtain the best screening performance.
3.3.Screening for the potential mitochondria-targeting compounds in TCMs extracts
Some TCMs such as PR[15,16]and CR[17,18]have been suggested to possess a range of pharmaceutical effects,including anticancer,cardio-,hepatic-and neuro-protection.It has been reported that several substances in PR,such as puerarin[13],daidzin[19,20],daidzein[21,22],genistein[23,24]and formononetin[25,26],and one compound(ligustilide[27])in CR could remedy mitochondrial functions to exert their pharmacological efficacy.However,it is possible that other bioactive constituents present in PR and CR and affecting mitochondrial functions remain unclear,which refrains the interpretation of therapeutic principles of the two TCMs.PR and CR were thus selected to be analyzed in this study.For the first time,SM-MCB was used to identify mitochondria-targeting compounds from PR and CR extracts.Fig.2 shows the chromatogram of the PR sample analyzed by SM-MCB,with significant area enhancement of 11 peaks(P1–P11)compared with the control containing denatured mitochondria(ΔP> 20%,shown in Table 1),suggesting specific binding with mitochondria.The chemical structure corresponding to each peak was assigned by analyzing UV,MS and MSninformation obtained from LC/MS(Table 2)and comparing it with previously reported data[28–32]and standards.Active compounds were identified as nine isoflavones(P1–P6,P8–P11)and one phenylpropanoid(P7).

Table 1 LC/MS data and assignmentof the 11m itochondria-targeting compounds in the PRextract.
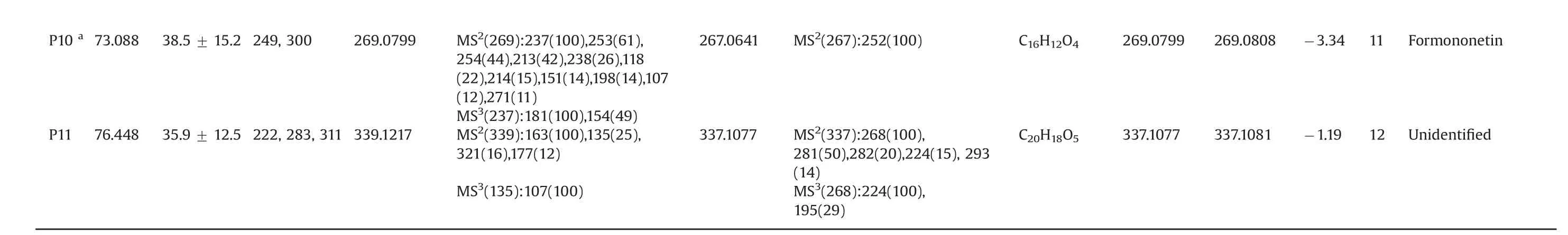
a Comparisonw ith standards.bΔP was calculatedusing the following formula:ΔP=(Pe–Pc)/Pe×100,where Pe and Pc are the peakareas in the experimentand control,respectively.Data wereobtained from 3 independent experiments and are expressed as the mean±SD.

Table 2 LC/MS data and assignmentof the 12mitochondria-targeting compounds in the CRextract.

Fig.3.Screening of mitochondria-targeting compounds from CR extract using SM-MBC.HPLC chromatograms of the CR extract(A,0–50 min;B,50–85 min)are displayed for the ultra filtrates,with active mitochondria(black line)and denatured mitochondria(red line)as the control.12 peaks(C1–C12)showed a significant area enhancement compared with the control.The conditions for detecting the CR extract are available in the Supplementary material.
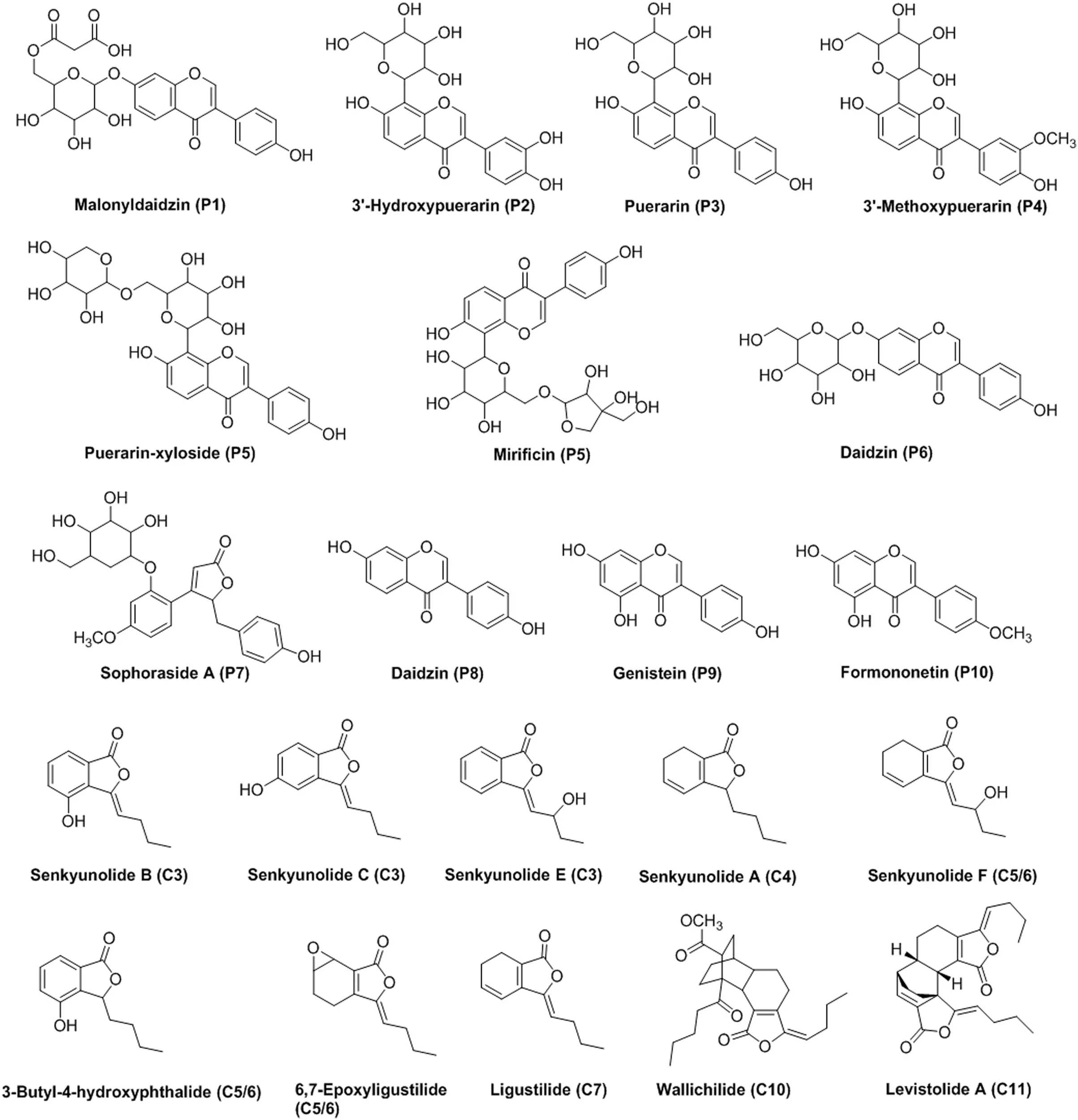
Fig.4.Chemical structures of the 17 assigned mitochondria-targeting compounds identified in PR and CR extracts.
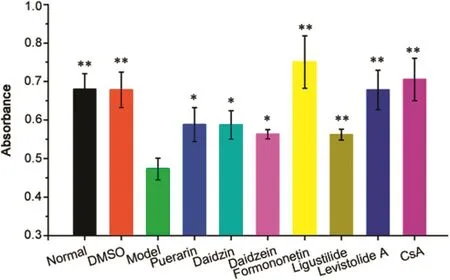
Fig.5.Effect of hit compounds on Ca2+-induced mPTP opening in suspensions of rat myocardial mitochondria.Myocardial mitochondria were preincubated with puerarin(75 μM),daidzin(140 μM),daidzein(100 μM),formononetin(100 μM),ligustilide(210 μM),levistolide A(100 μM)and cyclosporin A(10 μM).Data were obtained from 5 independent measurements and expressed as mean±SD.*P<0.05,**P<0.01,compared with model group.
CR extract was analyzed using the same method,and the obtained chromatograms(Fig.3)show remarkable enhancement of 12 peaks(C1–C12)compared with the control(ΔP > 20%,shown in Table 2),indicating specific binding with mitochondria.The characterization of the UV,MS and MSndata provided by LC/MS(Table 2)and comparison with previously reported data[33–37]and standards confirmed that the C1–C12 peaks were phthalides(C3–C7)and their dimmers(C10 and C11).
Totally,among these 23 hit compounds,the chemical structures of 17 were identified(Fig.4).These results further confirmed that SM-MBC could be used as an effective alternative for screening mitochondria-targeting compounds in complex mixtures such as TCMs extracts.The active mitochondria isolated from rat myocardium can recognize target molecules not only through affinity interactions between the molecules and targets on the mitochondrial environments,but also through the electrostatic interaction between them[7],which is helpful for decreasing interference from inactive impurities.
3.4.Effects of identified compounds on Ca2+-induced mPTP opening on isolated myocardial mitochondria
In order to evaluate the capability of identified compounds to regulate mitochondrial functions,a model of Ca2+-induced mPTP opening on isolated myocardial mitochondria was used.As indicated in Fig.5,250μM CaCl2,which can open the mPTP[13],induced a notable reduction at A520in cardiac mitochondrial suspensions.This effect was remarkably inhibited by cyclosporin A(CsA,10μM),which can specifically inhibit mPTP opening[13]with a reduction of A520.As for CsA,six hit compounds(P3,P6,P8,P10,C7 and C11)remarkably inhibited the Ca2+-induced reduction of A520,indicating that they refrained the Ca2+-induced mPTP opening(i.e.,mitochondrial protection)by directly interacting with the mitochondria.
These data indicated that the tested compounds were potential mitochondria-targeting compoundspotentially affecting mitochondrial functionality.In combination with the screening results provided by SM-MBC,the other 17 identified compounds were also potential mitochondria-targeting compounds,which merit further investigations.
Amongthese23 hitmitochondria-targetingcompounds,puerarin(P3)[13],daidzin(P6)[19,20],daidzein(P8)[21],and formononetin(P10)[25]can interact with mitochondria to exert their pharmacological action,whilst ligustilide(C7)[27]can affect mitochondrial functions,further validating the reliability of the screening results provided by SM-MBC.Nevertheless,whether the other 18 hits can also regulate mitochondrial functions remains unclear.Overall,all of the identified compounds might directly act on mitochondria to protect or damage mitochondrial functions,helping the elucidation of drug action mechanism and the identification of new drugs from TCMs.

Fig.6.(A)Effects of senkyunolide A on HepG2 cell confluence(%).(B)Cisplatin was used as positive control.Data were obtained from 5 independent measurements.*P<0.05,***P<0.001,compared with control group.
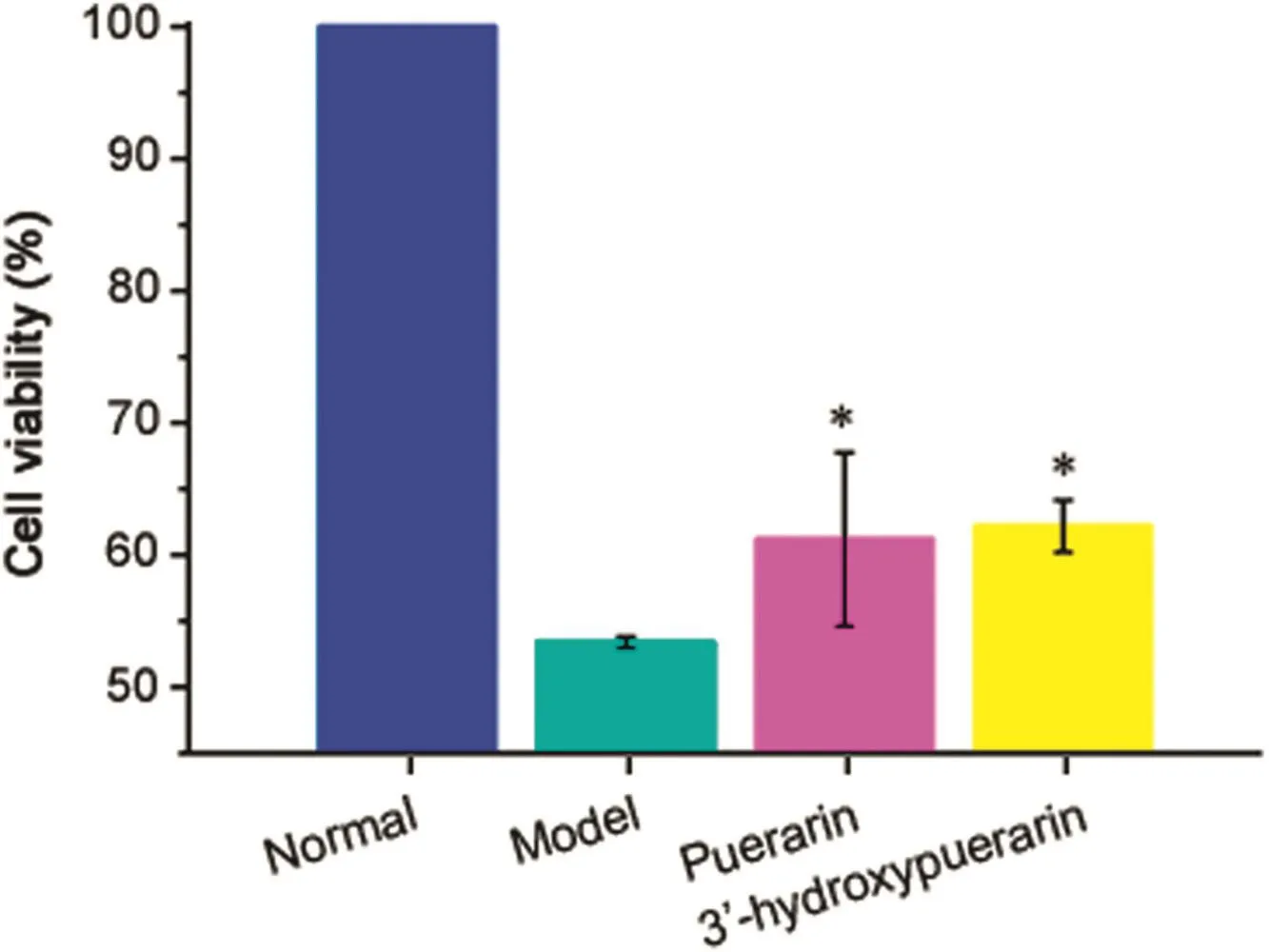
Fig.7.Effects of 3′-hydroxypuerarin on cellular viability in H/R-induced cardiomyocytes.Puerarin was used as positive control.Data were obtained from 3 independent determinations.*P<0.05,compared with model group.
3.5.Effects of identified compounds on cell proliferation
The effects of hits on cell proliferation were investigated for evaluating their potential anti-cancer efficacy.Similar to cisplatin that is known to act against cancerous proliferation[38],senkyunolide A(C4)significantly inhibited HepG2 cell proliferation with a dose-dependent trend(P<0.05;Fig.6).Combining with the screening results,we can speculate that the inhibition of cell proliferation may be attributed to the direct interaction with mitochondria,affecting mitochondrial functionality.This point needs to be further investigated.
3.6.Effects of hits on cellular viability in hypoxia/reoxygenation(H/R)-induced cardiomyocytes
As expected,H/R notably decreased cell viability(~57%decrease in WST-8 reduction;P<0.01)in cardiomyocytes(Fig.7).This effect was remarkably inhibited by puerarin(P3;320μM),which can specifically inhibit mPTP opening to exert the effective cardioprotection[13,39].Analogously,3′-hydroxypuerarin(P2;140 μM)significantly prevented the loss of cardiomyocyte viability that resulted from H/R induction(Fig.7;P<0.05).This effect may be caused by direct binding with mitochondria that further remedies the mitochondrial dysfunctions induced by H/R.In combination with the results of senkyunolide A(C4),the developed screening method could be used as an effective alternative for discovering lead compounds from TCMs extracts.
4.Conclusion
We applied SM-MBC for identifying mitochondria-targeting compounds from TCMs extracts,including PR and CR samples,and 23 mitochondria-targeting compounds were successfully discovered from the two extracts,17 of which were identified by LC/MS.The direct mitochondria-bound ability of six compounds was validated by pharmacological tests in vitro.Moreover,the inhibition ability of senkyunolide A(C4)and 3′-hydroxypuerarin(P2)on HepG2 cell proliferation and on loss of H/R-induced cardiomyocyte viability were investigated.The results obtained will be helpful for in-depth understanding of therapeutic action of TCMs and developing novel potential mitochondrial modulators from TCMs.SM-MBC was further confirmed to be an efficient proposal for the rapid screening of mitochondria-targeting compounds from complex matrix.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Financial support from the National Natural Science Foundation of China(Grant 81660596,81673395 and 81373921),the Research Fund for the Doctoral Program of Higher Education of China(Grant 20130001110057)and the Application and Basis Research Project of Yunnan China(Grant 2017FF117-(013)and 2016FD050)is gratefully acknowledged.
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2018.06.001.
 Journal of Pharmaceutical Analysis2018年4期
Journal of Pharmaceutical Analysis2018年4期
- Journal of Pharmaceutical Analysis的其它文章
- Quantitation of tadala fil in human plasma using a sensitive and rapid LC-MS/MS method for a bioequivalence study
- Duplex microRNAs assay based on target-triggered universal reporter hybridization
- Extracellular synthesis of silver nanoparticles by Pseudomonas sp.THG-LS1.4 and their antimicrobial application
- Simultaneous determination of steroid drugs in the ointment via magnetic solid phase extraction followed by HPLC-UV
- Unusual retention behavior of omeprazole and its chiral impurities B and E on the amylose tris(3-chloro-5-methylphenylcarbamate)chiral stationary phase in polar organic mode
- Recent advances in screening of enzymes inhibitors based on capillary electrophoresis
