Experimental study on the effects of drying methods on the stabilities of lignite☆
Yixin Zhang,Jixiang Dong,Fanhui Guo,Xiaokai Chen,Jianjun Wu,*,Zhenyong Miao
1National Engineering Research Center of Coal Preparation and Purification,China University of Mining and Technology,Xuzhou 221116,China
2School of Chemical Engineering and Technology,China University of Mining and Technology,Xuzhou 221116,China
Keywords:Lignite Mechanical thermal expression Stability Hydrophilicity Readsorption Spontaneous combustion
ABSTRACT The drying processes are always applied prior to the transportation or utilization of lignite,and result in notable changes in the stabilities of lignite.In this paper,the study on the effects of nitrogen and MTE drying process on the physico-chemical properties and stabilities of Zhaotong lignite was carried out.The briquettes produced by MTE drying in this study were 150 mm in dimension,and so had a much larger particle size than nitrogendried samples.Nitrogen adsorption,mercury intrusion porosimetry and scanning electron microscopy all suggested that drying was accompanied by the transformation of larger pores into smaller ones.Compared to nitrogen drying,the pore structures could be stabilized by the MTE process.The soluble salts were removed during MTE drying which resulted in the decrease in ash and the concentrations of some of the major metals.The removal of water enhanced the hydrophilicity of nitrogen dried samples,but did not affect the hydrophilicity of MTE dried samples.The moisture holding capacity of MTE dried samples reduced faster than nitrogen dried samples with the decrease of residual moisture content.The moisture readsorption processes of MTE dried samples were strongly inhibited due to the much larger particle size of sample produced by MTE drying than nitrogen drying.The susceptibility to spontaneous combustion,indicated by cross point temperature and self-heating tests,of nitrogen and MTE dried samples increased with the decrease of residual moisture content.The MTE dried samples are more liable to spontaneous combustion than nitrogen dried samples with the same residual moisture and particle size.However,the larger particle size of the MTE product made it more stable with respect to spontaneous combustion and also moisture readsorption.
1.Introduction
In China,with the continuous increase of energy demand,lignite attracts increasingly more attentions because of its large deposit[1–8].Due to the high moisture content(30%–60%(wet basis))in lignite,drying technologies are always applied for lignite preparation prior to transportation or utilization processes[9–11].However,the dried lignite is not stable,in that it can easily absorb moisture from air,is highly reactive toward oxygen at ambient temperature and is highly susceptible to spontaneous combustion,which seriously hampers the long distance transportation and storage of lignite[12–14].So,it is important to understand the effects of drying methods on the stabilities of lignite.
The stability of dried lignite with respect to readsorption of moisture,self-heating rate in air and spontaneous combustion is significantly affected by the physico-chemical properties of dried lignite.The effects of drying processes on the physico-chemical changes of lignite have been widely reported.The predominant chemical changes of lignite are reported to be the destruction of the functional groups(including carboxyl,hydroxyl,and carbonyl)which are significantly affected by the drying temperature[9].It is reported that phenolic groups,carbonyl groups,free carboxylic acid groups,aliphatic hydrogen O–Hand C–O groups gradually break down with the increasing drying temperature from 150°C to 250°C[15,16].Aromatic ring stretch and aromatic carbon remained relatively unchanged up to 250°C[17].The decomposition of oxygen functional groups of lignite resulted in the reduction of water adsorption[18,19].The carboxyl and hydroxyl groups are the preferential sites of moisture adsorption when compared with other functional groups[18,20,21].It is reported that the adsorption of water vapor increases in proportion to the square root of the carboxyl group concentration[20].The distribution of oxygen containing functional groups is also a major factor affecting spontaneous combustion[14].But the oxygen containing functional groups would remain stable unless lignite is dried at relatively high temperature.Little obvious destruction of the oxygen containing functional groups was reported under 150°C.
The pore structures of dried lignite also play important roles by controlling mass and energy transport to active sites during moisture readsorption and low temperature oxidation[21–23].The pore structures of lignite are significantly changed on the removal of water due to its gel-like porous structures[9,24].Deevi and Suuberg[25]and Li et al.[26]reported that the irreversible changes of pore structure of lignite took place during drying which was associated mainly with collapse of macroporosity and transitional porosity.Androutsopoulos and Linardos[27]observed a considerable particle contraction of Greek lignite on the removal of water under vacuum,which resulted in a minor decrease in the volume of macropores and partly mesopores and a marked increase in the surface area.During the mechanical thermal expression(MTE)process,the pores of lignite areal most completely filled by water[28].The significant collapse of macropores due to the dewatering and the applied mechanical pressure contributes to the reduction of pore volume and the increase of mesopores and micropores[29,30].The changes of pore structures of lignite are considerably different during different drying processes,but the effects of these changes on the stability of dried lignite are still poorly understood.
It is reported that,with the increase in particle size of dried lignite,the readsorption of moisture increased while the spontaneous combustion susceptibility decreased[13,31,32].But these studies mainly focused on the variations of particle size under 1 mm.It is known that the MTE products are briquettes[33],and significantly larger than products dried by evaporation drying processes,which are mainly in small particles.It is necessary to pay attention to the effect of large variations in particle size on the stabilities of dried lignite.In addition,the soluble mineral matter(such as aluminosilicate,alkalis and alkaline earth metals)could be removed with water during the MTE process,which would not occur in an evaporation drying process[29,30].The mineral matter in lignite also may act as promoters or inhibitors of its stabilities toward various disturbances[34–37].
The character is tics of dried lignite are significantly affected by different drying methods.However,a full understanding of the effect of drying process on the stability characteristics of lignite dried by different methods has not been achieved.In this study,the lignite obtained from Zhaotong(Yunnan,China)was dried by nitrogen and MTE drying process.The hydrophilicity,moisture holding capacity,moisture readsorption and spontaneous combustion susceptibility of dried lignite were tested.The effects of the changes in the characteristics of lignite during drying processes on its stability characteristics were discussed.
2.Experimental
2.1.Coal sample and preparation
The characteristics of lignite obtained from Zhaotong,Yunnan,China,are given in Table 1.The proximate analysis was carried out following the ISO 11722,ISO 1171 and ISO 562 methods.The ultimate analysis was carried out following the ISO 625,ISO 333 and ISO 334methods.The errors are less than±0.5%.

Table 1 Characteristics of the raw coal sample
The raw lignite was crushed and sieved into different size fractions,then dried under nitrogen at 150°C.The MTE drying was carried out at 150°Cund era pressure of10MPa as in the literature[33].The briquette produced by MTE drying(150 mm in diameter in this study)was crushedandsievedintodifferentsizefractions.The samples with different residual moisture contents were obtained by controlling drying time.The MTE dried sample with 20%residual moisture content was obtained by 2 h drying,and the removal of water was not observed after 1 h 50 min.It is believed that 20%is approaching the minimum residual moisture content which can be achieved by the MTE drying process.All the tests of MTE dried samples are with theresidual moisture content above 20%.The samples were named by drying method and residual moisture content.For example,Nitrogen-20%and MTE-20%represented sample prepared by nitrogen drying with 20%residual moisture and MTE drying with 20%residual moisture,respectively.
2.2.Functional group analysis
The functional groups on the coal surface were analyzed by Fourier transform infrared spectroscopy(FTIR)[1].The FTIR spectra were recorded by a Nexus 470 spectrometer(Nicolet)in the 450–4000 cm−1wavenumber range with 32 scans per sample at a resolution of 4 cm−1.The pellet prepared from a mixture of 1 mg coal and 100 mg KBr was used for the test.The coal sample was dried under nitrogen at 105°C before test.
2.3.Pore structure analysis
A BELSORP-max ver 2.1 gas adsorption instrument(Bel)was used to analyze the micropore and mesopore structures of samples by nitrogen adsorption method.The sample was degassed under vacuum at 105 °C for 4 h and then placed in the instrument for test.The Barrett–Joyner–Halenda(BJH)model was used to calculate the pore volume of mesopores between pore diameters of 2 nm and 50 nm.
An AUTOPORE IV mercury porosimeter(Micromeritics)capable of applying pressures between 3.4 kPa and 207 MPa was used to analyze the pore volume and the distribution of macro pores and of the largerdiameter mesopores between pore diameters of 50 to 60000 nm(macropores)and6 to 50 nm(mesopores),based on mercury intrusion porosimetry(MIP)method.The instrument cannot intrude mercury into pores smaller than 6 nm in diameter.Prior to MIP test,the sample was dried under nitrogen at 105°C.
2.4.Surface morphology
Sample morphology was investigated by scanning electron microscopy(SEM),using a Quanta FEG 250(FEI)instrument.
2.5.Coal ash analysis
A S8 Tiger X-ray fluorescence(XRF)spectrometer(Bruker)was employed to quantify major elements in ash of dried lignite sample.The ash was prepared by low temperature ashing by using a PR300 plasma ashing reactor(Yamato).
2.6.Contact angle test
The contact angle was measured by the sessile drop method[38].The test was carried out by using a DSA100 goniometer(Kruss).The sample was pressed into a pellet for the measurement,and then the droplet of distilled water was deposited on the pellet.The images were captured,and the contact angle was calculated using the built-in imaging software.The measurement was carried out 5 times for every sample,and the average value was used.
2.7.Moisture holding capacity and moisture readsorption tests
The moisture holding capacity(MHC)and moisture read sorption tests were conducted using a BPS-250CL humidity chamber(Yiheng Co.,Ltd.).In each test,approximately 5 g sample was put into a glass dish with lid,and then the open glass dishes were positioned in the humidity chamber under a constant relative humidity of 96%at a temperature of30°C[30].The moisture holding capacity test was not terminated until the equilibrium of adsorption was reached.During moisture read sorption test,the weights of sample were recorded at set intervals.The measurement was carried out with three separate aliquots of each sample,and the average value was used.
2.8.Cross point temperature test
The cross point temperature(CPT)test of the sample was carried out by the wire basket method[39].The sample was placed in a 5 cm cubic wire basket made of a 0.05mmstainless steel mesh.Then,the wire basket wa sheated from30°Cat ther ate of1°C·min−1wit hair in a furnace.The temperatures of the furnace and the center of the sample in the wire basket were monitored by thermocouples and recorded by a computer.The test was terminated when the temperature of the sample center exceeded the temperature of the furnace.The CPT is defined as the temperature of the point at which the temperature of the sample center exceeded the temperature of the furnace,which was used to indicate the susceptibility of a coal to spontaneous combustion[40].The measurement was carried out with three separate aliquots of each sample,and the average value was used.
2.9.Self-heating test
The self-heating test was carried out foll owing the procedures of the adiabatic test[41–43].The coals ample was charged into a vacuum flask reaction vessel,and then placed in an oven to stabilize at 40°C,with nitrogen passing through it.As soon as the sample temperature had stabilized,the gas flow was switched to air with a constant flow rate of 50 ml·min−1,the oven was set to track the sample center temperature rise due to oxidation.The gas was heated to oven temperature before entering the vacuum flask reaction vessel by being passed through a 10 m copper pipe which was placed in the oven.The test was terminated when the temperature of the sample center exceeded 100°C.The temperatures of the sample center and the oven were monitored by thermocouples and recorded by a computer for later analysis.The average rate of temperature increase over the time taken for the temperature to rise from 40 °C to 70 °C,expressed in °C·h−1,is defined as R70[41].The measure men twas carried out with threeseparate aliquots of each sample,and the average value was used.
3.Results and Discussion
3.1.Physico-chemical properties of dried lignite
Fig.1 shows the FTIR spectra of nitrogen and MTE dried samples.Because drying under nitrogen was used in the sample preparation for FTIR test,the only spectrum of a nitrogen dried sample included in Fig.1 is that for the sample dried to zero moisture content.The decomposition of functional groups is determined mainly by the highest temperature to which the sample is subjected[15–17].Due to the same drying temperature during nitrogen and MTE drying process,there is no obvious difference between the FTIR spectra of nitrogen and MTE dried samples as shown in Fig.1,indicating no obvious difference in functional group distributions on the surfaces of nitrogen and MTE dried samples.

Fig.2.Pore volume of samples(by MIP and briquette dimensions methods)as a function of residual moisture content(particle size 0.5–1 mm).(The error is±0.006 cm3·g−1,based on the difference between three tests of Nitrogen-0%.)
The variations of the pore volume of samples as afunction of residual moisture content are shown in Fig.2(by MIP method)and Fig.3(by nitrogen adsorption method).Due to the gel-like porous structure of lignite,the pore structures of samples were irreversibly changed with the removal of water.But it was difficult to accurately identify the variations of pore volumes of samples,due to the further drying applied in the sample preparation for MIP and nitrogen adsorption tests.As shown in Figs.2 and 3,pore volumes measured by MIP decreased significantly and the pore volumes measured by nitrogen adsorption method increased significantly with the decrease of residual moisture content of MTE dried samples,while only relatively slight variations in the pore volumes of nitrogen dried samples were observed.It is reported that the pore structure could be stabilized by the MTE process,which could effectively inhibit the further shrinkage of the MTE samples during the further drying for the preparation of pore structure tests[44,45].The structure of nitrogen-dried samples is not strengthened in this way,and shrinks severely during total drying;the pore volume depends only on the final(zero)moisture content and not on previous drying history.The much severer shrinkage of the nitrogen dried samples compared to the MTE dried samples during the further drying necessary before pore structure measurements by the methods used resulted in the MIP pore volumes of nitrogen dried samples being significantly lower than the pore volumes of MTE dried samples at the same residual moisture content,as shown in Fig.2.The opposite trends of pore volume variations are shown in Fig.2(including macropores and mesopores)and Fig.3(including mesopores),indicating the transformation of larger pores to smaller ones which is proved by the pore size distribution of samples,as shown in Fig.4.Because the same or similar drying method was applied in the sample preparation for pore structure tests, no obvious difference was observed in the pore size distribution of nitrogen dried samples with various residual moisture contents, so that only the pore size distribution ofcompletely dried sample is given in Fig. 4. Compared to MTE dried samples, the smaller volume of macro pores and the larger volumes of mesopores and micropores of Nitrogen-0% indicated that severe shrinkage and collapse of larger pores occurred. The variations of the pore size distribution of MTE dried samples as a function of the residual moisture content indicate that the macropores gradually collapsed and were transformed to mesopores with the removal of water
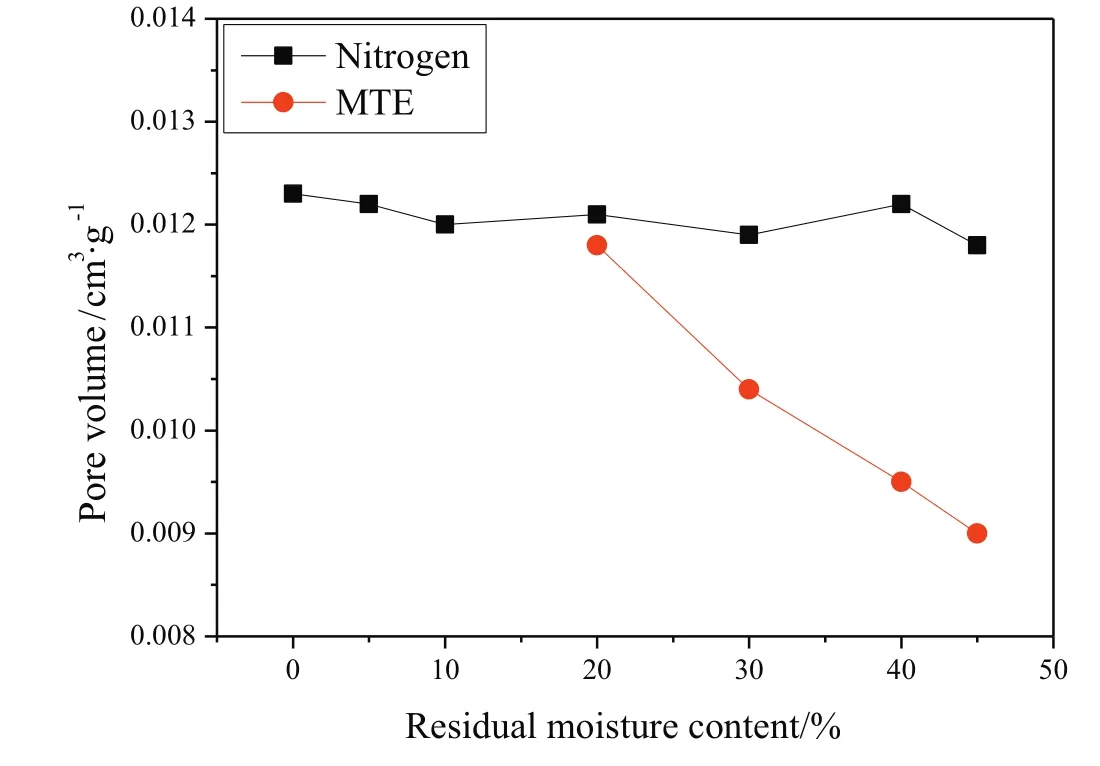
Fig.3.Pore volume(by nitrogen adsorption method)of samples as a function of residual moisture content(particle size 0.5–1 mm).(The error is±0.0005 cm3·g−1,based on the difference between three tests of Nitrogen-0%.)
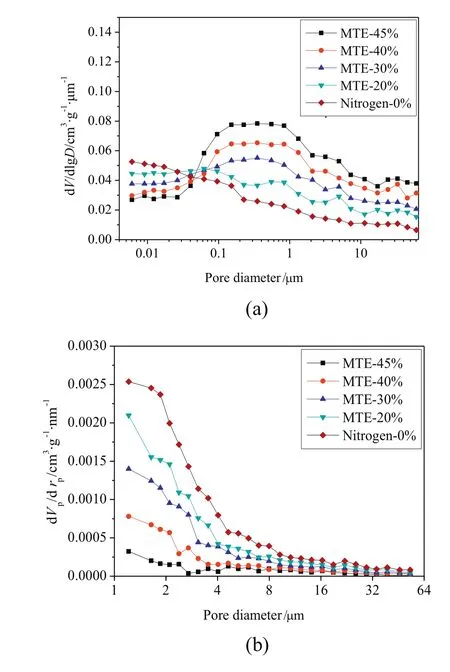
Fig.4.Pore size distribution of samples:(a)by MIP methods;(b)by nitrogen adsorption method(particle size 0.5–1 mm).
The surface morphology characteristics of samples are in agreement with the results of pore structuretests.As shown in Fig.5(a),the surface of Nitrogen-0%was highly rough and irregular,with few large pores,so that the surface morphology reflected the small concentration of large pores and the large concentration of smaller pores suggested by the MIP and N2adsorption results.As shown in Fig.5(b)–(e),with the increase in drying intensity by the MTE process,the coarse pore networks decreased and transformed into fine pore networks,indicating the decrease of macropores and increase of smaller pores.
Table 2 illustrates the ash contents and main components of the mineral matter in nitrogen and MTE dried samples.During nitrogen drying process,the mineral matter in coal samples could not be removed,the contents of mineral matter in coal samples with different drying intensities would keep constant.It is reported that the soluble salts could be removed from lignite with the removal of water during the MTE process resulting in the reduction of mineral matter contents in samples[29,46].The ash content and the concentrations of some of the main inorganic components decreased with the decrease of the residual moisture content of MTE dried samples.
3.2.Hydrophilicity,moisture holding capacity andmoisture readsorption of dried lignite
Water can be more easily spread on a hydrophilic surface than a hydrophobic surface. Contact angle is an important parameter for characterizing the hydrophilicity of coal surface. The contact angle of a hydrophilic surface is smaller than a hydrophobic surface. The contact angles of nitrogen and MTE dried samples as a function of residualmoisture content are shown in Fig. 6. The contact angles of nitrogen dried samples decreased with the decrease of residual moisture content, which indicates that the hydrophobic surface was transformed to hydrophilic with the removal of water. But for MTE samples, the contact angles almost kept constant. Wang et al. [47] found that contact angle is closely related to the distribution of oxygen functional groups whichare the preferential sites of moisture adsorption. During nitrogen drying process, more and more oxygen functional groups on the surfaces of lignite were exposed with the gradually removal of water resulted in the enhancement of attractions between lignite surface and the droplet. The removal of water by nitrogen drying led to the decrease of contact angle and increase of hydrophilicity of sample. The pores of lignite remained almost completely occupied by water during the MTE process indicating the surfaces of lignite were covered by water and no more oxygen functional groups on the surfaces of lignite were exposed. It is deduced to be the reason of that the contact angles of MTE dried samples almost kept constant and no increases in hydrophilicity were observed.

Fig.5.SEM images of samples:(a)Nitrogen-0%;(b)MTE-20%;(c)MTE-30%;(d)MTE-40%;(e)MTE-45%.
The moisture holding capacity decreased with the decrease of residual moisture content for both nitrogen and MTE dried samples(Fig.7).The irreversible changes of pore structure of lignite during nitrogen and MTE drying process are deduced to be the primary factor affecting the moisture holding capacity.As the lignite moisture content decreases, first the so-called bulk water,then the capillary water,thirdly the multilayer water and finally the monolayer water are lost[48,49].The collapse of macropores or the transformation of macro pores to smaller pores will prevent the water from existing in bulk form,and will also prevent capillary condensation and the for mation of multilayer water.If the pore volume remains sufficiently high to accommodate the moisture present,a reduction in pore volume would not result in a large decrease in moisture holding capacity.This can explain why the moisture holding capacity changed only a little in going from MTE-45%to MTE-40%,although the MIP pore volume decreased substantially.Also,the moisture holding capacity of an MTE dried sample was lower than that of the nitrogen dried sample with the same residual moisture content.This is probably due to the stabilization of the pore structure by the MTE process(Section 3.1),which makes it harder for the pores to expand during the moisture adsorption process than the less stable,gel-like pores of the nitrogen dried sample.Thus the nitrogen dried sample can adsorb more water at a given relative humidity(96%for moisture holding capacity)than the MTE dried sample.The moisture holding capacity of the MTE dried sample with residual moisture content 20%is 19.5%,which is even lower than the moistureholding capacity of the nitrogen dried sample with residual moisture content 5%.
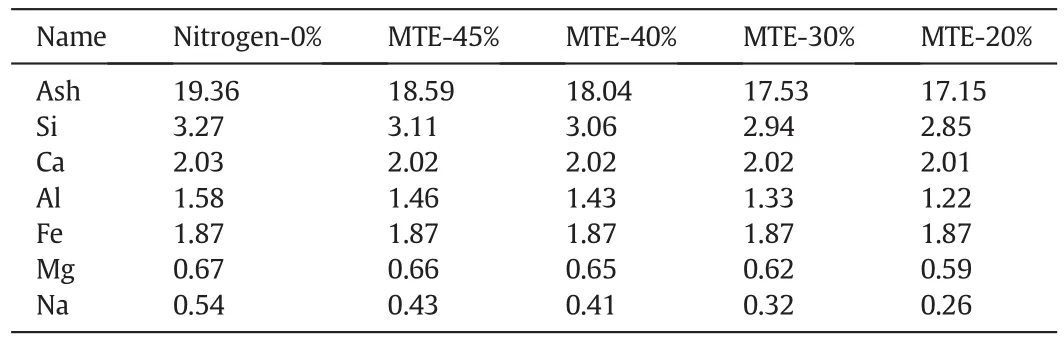
Table 2 Ash content and main components of the mineral matters in samples(wt%,db)
The moisture holding capacities of samples of different particle sizes are similar,as shown in Table 3.Since the test of moisture holding capacity was only completed when adsorption equilibrium was reached,the inhibition of gaseous transport caused by the increase of particle size did not affect the moisture holding capacities of samples,but affected the time for reaching the readsorption equilibrium.The moisture readsorption processes of nitrogen and MTE dried samples with various particle sizes are illustrated in Fig.8.For nitrogen and MTE dried samples with particle size <0.2 mm,0.2–0.5 mm,0.5–1 mm and 1–2 mm,the read sorption processes are similar,and the readsorption equilibrium was only slightly delayed with the increase of particle size.The read sorption equilibrium of the MTE sample with particle size 10–20 mm was significantly delayed.The briquette obtained by the MTE drying process is150 mm in diameter,so that it can be inferred that moisture readsorption would be strongly inhibited.

Fig.6.Contact angle of samples as a function of residual moisture content.(The errors are less than ±1.3°.)

Fig.7.MHC of samples as a function of residual moisture content.(The errors are less than±0.3%.)
Compared to nitrogen drying,the MTE drying showed the advantages of retaining the hydrophobicity of the lignite surface and giving a product with smaller moisture holding capacity and slower moisture readsorption rate.Some of the advantages were probably due to the larger particle size of the MTE dried product.
3.3.Spontaneous combustion behavior of dried lignite
The spontaneous combustion behaviors of dried lignite were investigated by CPT and self-heating tests.Fig.9 shows the variation of CPT andR70of lignite samples with different residual moisture contents prepared by nitrogen and MTE drying processes.The CPT of samples decreased monotonically with the decrease of residual moisture content while the R70of samples increased,which suggests an increase in the spontaneous combustion susceptibility.The trends are in agreement with the literature[12,50,51].The effects of residual moisture content on the CPT and R70of samples can be divided into two major aspects:(1)the effect of moisture evaporation on the overall heat balance and(2)the effect of moisture on the rate of coal oxidation.As shown in Fig.10,the increase of the temperature in the sample center wasinitially slower than the increase of the oven temperature due to the necessarily non-instantaneous transfer of heat by conduction and convection to the sample center and the extraction of heat from the samples as the result of moisture evaporation at the initial stage of the CPT test process.The decrease of residual moisture content in the samples reduced the rate of water evaporation and the extraction of heat from the samples,which resulted in a decreasing difference between oven and sample center temperatures.During the self-heating test,the sample was heated by the energy released by the oxidation reactions between lignite and oxygen.The rate of water evaporation from the sample decreased with the decrease of residual moisture content,resulting in the decrease of energy consumption for water evaporation and the increase of energy available for the enhancement of sample temperature.The increase of sample temperature was accelerated with the decrease of residual moisture content.The time needed for the temperature in the sample center to exceed the oven temperature during CPT test(Fig.10)and for the sample temperature to increase from 40 °C to 70 °C(Fig.11)decreased with the decrease of residual moisture content,indicating the increase in the spontaneous combustion susceptibility.

Fig.8.Moisture readsorption of samples with various particle size fractions:a.Nitrogen-0%;b.MTE-20%completed dried by nitrogen drying.(The errors are less than±0.4%.)
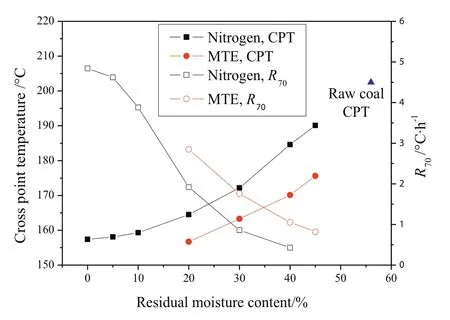
Fig.9.CPT and R70of samples as a function of residual moisture content(particle size 0.5–1 mm.)(The errors are:CPT,less than ±1.3 °C;R70,less than ±0.11 °C·h−1.)

Fig.10.Temperatures of the oven and sample with different residual moisture content as a function of time during CPT tests:(a)nitrogen dried sample;(b)MTE dried sample.
The oxidation of sample was also affected by residual moisture content during CPT and R70test processes.It is reported that there is a critical moisture content for eachcoal:below or above the critical moisture content,the low temperature oxidation rate is likely to be reduced[49,52,53].At the critical moisture content the maximum chem is or ption reactions which accelerated the oxidation could be achieved.If the moisture content is higher than the critical value,extra water forms multilayers at the surface of coal pores,increasing diffusion resistance for oxygen into the interior of the coal;while the moisture content is lower than the critical value,only a portion of the active sites have access to the monolayer water molecules which could be involved in the chemisorption reactions.Zhao et al.reported that the low temper atureoxidation of lignite with residual moisture content between6%and 13%was promoted[12].The temperature curves of nitrogen dried samples with5%and10%residual moisture content during CPT and R70tests are shown in Figs.10(a)and 11(a),respectively.The increases in temperature of samples with 10%residual moisture content are obviously faster than those of samples with 20%residual moisture content,while only slightly slower than those of the completely dried sample.The reason is deduced to be that the extra heat released by the chemisorption reactions partly compensated the heat for the evaporation of water and more heat was available for heating sample.The change in heating rate between the 10%and 5%samples is much less than would be expected from the change between the 20%and 10%samples.Thus the sample with5%initial moisture content heatedupmoreslowly than would beexpected from there sults for samples of higher moisture content,implying that the chemisorption reactions proceeded more slowly in the sample with 5%moisture content than in the sample with 10%moisture content.
The pore structures play an important role in the spontaneous combustion behavior of samples.It is reported that the transport of gaseous reactants and products,the key factors affecting spontaneous combustion,is mainly controlled by mesopores and pores with diameter less than 10 nm do not affect to the oxidation reaction rate at low temperature[23].The transformation of macropores to smaller pores with the removal of water restrained the transport of water vapor and oxygen which should result in the reduction of the spontaneous combustion susceptibility.However,this trend was not re flected by the CPT and R70of samples with increasing drying intensity due to the dominant effect of residual moisture content on the spontaneous combustion behavior.It also should be noticed that the CPT of MTE dried samples is lower and the R70of MTE dried samples is higher than those of nitrogen dried samples with the same residual moisture content.During the CPT and R70tests by the wire basket method,the further decrease of pore volume and the collapse of pore structure occurred with the further removal of water.If the pore volume of the N2-dried sample with 20%initial moisture was lower than that of MTE-20%this would help to explain the relative propensities to spontaneous combustion.Another possible explanation is that the greater rigidity of the MTE-dried lignite will prevent the pore structure from collapsing to the same extent during the CPT test as that of the N2-dried lignite,so that oxygen diffusion will be facilitated in the MTE-dried lignite.The differences of the evolution of pore structures during test processes were supposed to be an important reason of the higher spontaneous combustion susceptibility of the MTE dried sample than that of the nitrogen dried sample with the same residual moisture and particle size.
The CPT and R70tests of Nitrogen-0%,MTE-20%,MTE-30%,MTE-40%and MTE-45%with the same status for the MIP test(completely dried under nitrogen at 105°C)were carried out for the further investigation of theeffectof pore structure on thespontaneous combustion behavior.The variations of CPT and R70as a function of pore volume(by MIP method)are shown in Fig.12.The CPT increased and R70decreased with the decrease of pore volume which accompanied the collapse of the pore structure and the transfer of macropores to smaller ones.The results provided direct evidence of the effect of the variation of pore structure during lignite drying processes on the spontaneous combustion susceptibility.
The mineral matter in lignite may act as promoters or inhibitors of its stability with respect to moisture adsorption,self-heating and spontaneous combustion[27–30].Wang and You[36]reported that the R70increased approximately linearly with the decrease of ash content.It is supposed that the mineral matter in coal may act as a heat sink that absorbed heat during the low temperature oxidation process and cover the coal surface resulting in the inhibition of oxygen diffusing from the internal and external surfaces.The removal during the MTE process of soluble aluminosilicate(re flected by the decreasein Si and Al contents with decreasing residual moisture content of the MTE dried samples)would have removed mineral sinhibiting the lowtemperature oxidation of the coal and hence may have contributed to the rise in R70for the MTE dried samples with decreasing residual moisture content.The presence of alkalis and alkaline-earth metal and pyrite could accelerate the spontaneous combustion[54].However,the contents of Na and Mg in the samples were low,and the contents of Fe and Ca in the samples did not vary greatly,as shown in Table 2.No reports have been found of the change in spontaneous combustion behavior with small decrease of alkalis and alkaline-earth metal and pyrite content.The removal of mineral matter during the MTE process was therefore believed to be a reason that the spontaneous combustion susceptibility of MTE dried sample washigher than that of the nitrogen dried samples with the same residual moisture content and particle size.
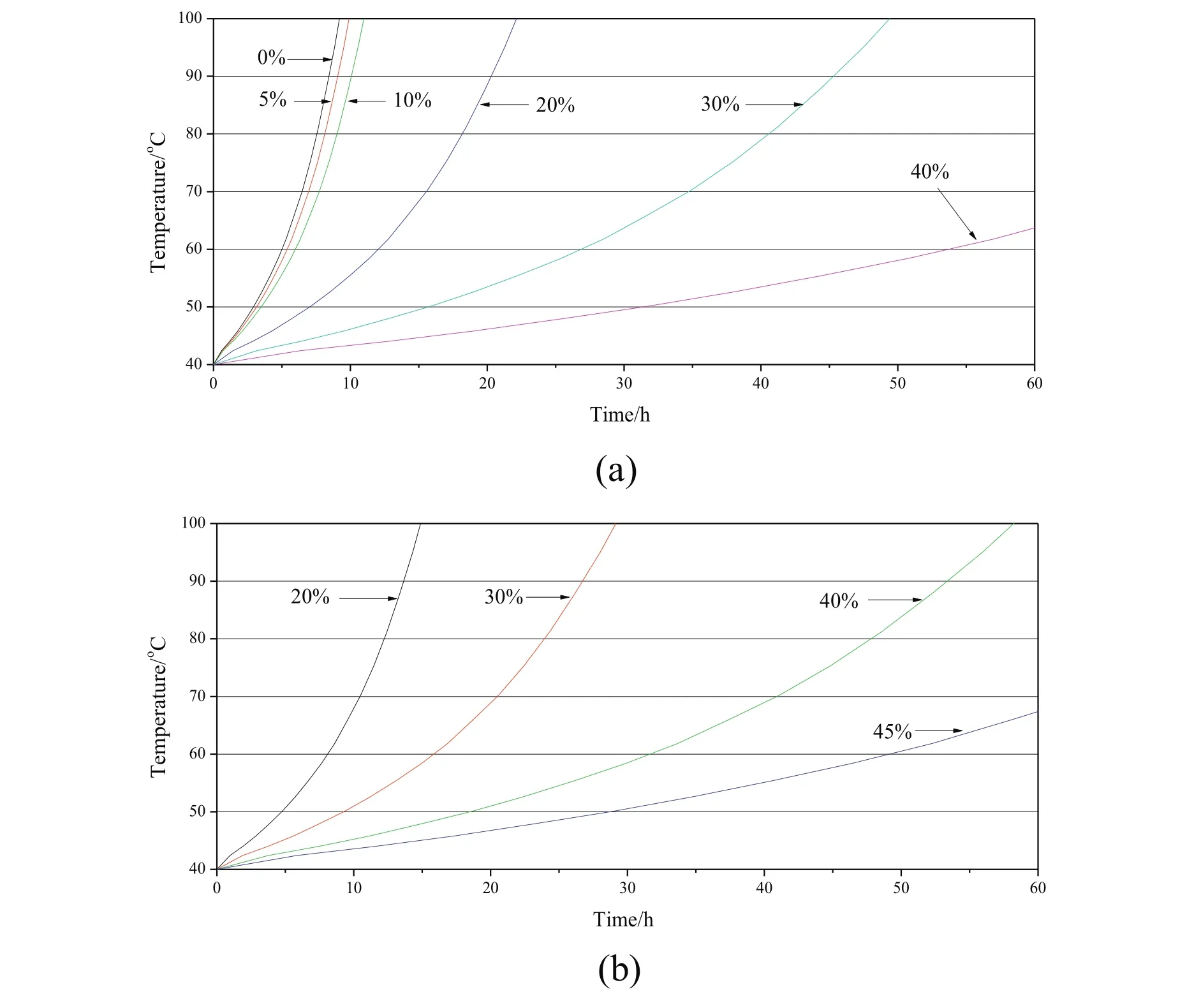
Fig.11.Temperatures of sample with different residual moisture content as a function of time during R70tests:(a)nitrogen dried sample;(b)MTE dried sample.

Fig.12.CPT and R70of samplesas afunction of porevolume(by MIPmethod)(particlesize 0.5–1 mm.)(The errors are:CPT,less than ±0.9°C;R70,less than ±0.11 °C·h−1.)
Thes pontaneous combustionbehavior of coal is al so affected by particle size[32].The CPT and R70of Nitrogen-20%and MTE-20%with different particle size fractions are listed in Table 3.The CPT and R70are almost in dependent of the particlesize for nitrogen and MTE dried samples with diameter below 1 mm.This is in agreement with the result published by Fei et al.[35].Above 1 mm,the CPT increased and the R70decreased with the increase of particle size,indicating that the samples with larger particle size were less prone to spontaneous combustion.It is reported that the oxidation rate of coal is independent of particle size if the diameter is below a critical value[49].At the critical particle size,the oxygen diffusing from the internal and external surfaces equals the maximum capacity of oxygen consumption for low temperature oxidation.Below the critical diameter,any further decrease in coal particle size did not promote the oxidation rate.Above the critical particle size,the increase of particle size led to less exposure of internal surface and reduced the external surface area per unit mass,so that the rate of reaction between coal and oxygen at the active sites on internal surfaces decreased[12,14,32,49].The self-heating process was significantly delayed with the increase of particle size.The spontaneous combustion susceptibility of MTE dried sample is higher than that of nitrogen dried sample with thes a meparticlesize and residual moisture content.But for the MTE process,whatever the feed particle size,the product is a briquette(150 mm in diameter in this study).The particle size of the MTE product is expected to be significantly larger than 20 mm(the maximum particle size for CPT and R70test in this study)which would result in the further increase of the CPT and decrease of R70.The CPT of MTE-20%with particle size 10–20 mm is 201.4°C,which is obviously higher than that of Nitrogen-20%.More than 50 h is needed for the temperature of MTE-20%with particle size 10–20 mm to increase from 40 °C to 70 °C,indicating a significantly delay in the self-heating process.The MTE process gave a dried product with less propensity for spontaneous combustion because a larger particle size was obtained.
4.Conclusions
The physico-chemical properties of lignite prepared by nitrogen and MTE drying process were analyzed. There is no obvious difference in functional group distribution between nitrogen and MTE dried samples, due to the decomposition of functional groups being dominated by temperature. The pore structures of samples were irreversibly changed with the removal of water for both nitrogen and MTE dried samples. The MTE drying resulted in smaller changes in pore structure than nitrogen drying. The pore structures of samples were stabilized by MTE drying which inhibited the further collapse of pore structures and the transfer of macro pores to smaller ones with the further removal of water. The soluble saltsw ere removed during MTE drying which resulted in the decrease in ash yield and the concentrations of some of the main inorganic components with the decrease in residual moisture content of MTE treated sample.
The stabilities are decreased with the removal of water,but there are notable differences in this regard between nitrogen and MTE dried samples.The removal of water enhanced the hydrophilicity of nitrogen dried samples,but did not affect the hydrophilicity of MTE dried samples.The moisture holding capacity of MTE dried samples was reduced faster than that of nitrogen dried samples with the decrease of residual moisture content.The moisture readsorption processes were strongly inhibited in MTE dried products due to their larger particle size compared to nitrogen dried products.The susceptibility to spontaneous combustion of both nitrogen and MTE samples increased with the decrease of residual moisture content.The MTE dried samples are more likely to combust spontaneously than nitrogen dried samples with the same residual moisture and particle size,due to the stabilization of pore structures and the removal of soluble salts by the MTE process.However,the MTE drying process leads to a larger-particle-size product,so that,as propensity for spontaneous combustion decreases with increasing particle size,in practical situations the MTE dried product will be less liable to spontaneous combustion.
 Chinese Journal of Chemical Engineering2018年7期
Chinese Journal of Chemical Engineering2018年7期
- Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Preparation of vapreotide-templated silver nanocages and their photothermal therapy efficacy☆
- DNA-assisted rational design of BaF2linear and erythrocyteshaped nanocrystals☆
- On the database-based strategy of candidate extractant generation for de-phenol process in coking wastewater treatment☆
- Hierarchical porous MgBO2(OH)microspheres:Hydrothermal synthesis,thermal decomposition,and application as adsorbents for Congo red removal☆
- Degradation analysis of A2/O combined with AgNO3+K2FeO4on coking wastewater
