Optimal reaction conditions for pyridine synthesis in riser reactor☆
Shuaishuai Zhou,Zelong Liu,Xiao Yan,Qin Di,Mengxi Liu,Chunxi Lu,*,Guangzhou Jin,*
1State Key Laboratory of Heavy Oil Processing,China University of Petroleum,Beijing 102249,China
2Department of Chemical Engineering,Beijing Institute of Petro-Chemical Technology,Beijing 102617,China
Keywords:Pyridine synthesis Riser reactor Optimal operating condition
ABSTRACT Pyridine has been generally synthesized by aldehydes and ammonia in a turbulent fluidized-bed reactor.In this paper,a novel riser reactor was proposed for pyridine synthesis.Experiment result showed that the yield of pyridine and 3-picoline decreased,but the selectivity of pyridine over 3-picoline increased compared to turbulent fluidized-bed reactor.Based on experimental data,a modified kinetic model was used for the determination of optimal operating condition for riser reactor.The optimal operating condition of riser reactor given by this modified model was as follows:The reaction temperature of 755 K,catalyst to feedstock ratio(CTFR)of 87,residence timeof3.8sandinitialacetaldehydesconcentrationof0.0029mol·L−1(acetaldehydestoformaldehydesratioby mole(ATFR)of 0.65 and ammonia to aldehydes ratio by mole(ATAR)of 0.9,water contention of 63wt%(formaldehyde solution)).
1.Introduction
Pyridine and 3-picoline are im portant chemicals and have been widely used for synthesizing of pharma and agrochemicals for the bene fits of high chemical reactivity as well as biological activity[1].Traditionally,pyridine bases were isolated from coal tar with high sulfur content.In 1920s,large demand of pyridine and its bases encouraged development of new technologies for pyridine synthesis,in which the one involving ammonia-aldehyde reaction gave the best yield.Currently,most of the pyridine bases have been massively produced based on Chichibabin condensation process[2].Major development of pyridine synthesis focused on the following three fields:catalyst design[3–17];catalyst deactivation and regeneration[18–21];reactor[4,6,7,11,22].We strongly suggest that the reader to read Suresh Kumar Reddy's work[1]for the interest in first and second fields development.As for reactor types,Goe et al.[7]pointed out that reactor designs varied within the basic categories of fixed-bed and fluidized-bed forms.Fixed bed reactor is robust,and the flow pattern is near plug flow,which has less axial back-mixing and means high selectivity of pyridine over 3-picoline in pyridine synthesis reaction.Ramachandra et al.[11]synthesized pyridinebases byammonia and aldehydes aswellasmethanol over W-ZSM-5 catalysts in a tubular,Pyrex reactor with 20-mm internal diameter,and the yield by weight was 61.5%and 16.8%for pyridine and 3-picolinerespectively at a reaction temperature of 693 K.Reddy et al.[20]synthesized pyridine bases by ammonia and aldehydes over HZSM-5catalystsinatubular,Pyrexreactorwith20-mminternaldiameter,and the yield by mole was 49.5%and 12.3%for pyridine and 3-picoline respectively at a reaction temperature of 673 K.Ramachandra's and Reddy's work verified that fixed bed reactor had high selectivity.However,there exist two problems for large scale fixed bed reactor for pyridine synthesis.The first one is temperature difference along radial direction,whichowingtopoorheatremovalperformance.Themainreactionsin pyridinesynthesis processare highly exothermic,whichindicated that efficient heat removal ability of reactor was required.Obviously, fixed-bedreactorisnottheoptimalchoice.Thesecondproblem is that the catalysts inside the fixed-bed reactor need replaced frequently for deactivation,which would cause shut down of factory and result in huge economic lose.To overcome the heat removal and catalyst in-situ regeneration problem in fixed-bed reactor, fluidized-bed reactor was come up for pyridine synthesis.Francis et al.[12]pointed out that fluidized-bed reactor is convenient for heating and favors continuous operation.Experimental results by Francis et al.[12]in a turbulent fluidized-bed reactor for pyridine synthesis in 1957 showed that the total yield of pyridine bases was higher(35wt%and 27wt%for pyridine and 3-picoline respectively).Similar results were also obtained in experiment carried out by Feitler et al.[4]and Goe et al.[7].Goe et al.[7]pointed out that since the obvious advantage of turbulent fluidizedbed reactor,until 1993,handful of commercial scale pyridine synthesis units worldwide all incorporate fluidized-bed reactor.Recently,Xin et al.[22]estimated the performance of GDM-70 catalyst in a turbulent fluidized-bed reactorunder the operating condition of reaction temperature of 723 K,space velocity of 0.3h−1,and the ratio of ammonia toaldehydes and formaldehydes to acetaldehydes of unity and found that the total yield for pyridine and 3-picoline reached 72%and selectivity of pyridineover3-picolinewas2.22.Xin'stechnology was commercialized ina 25 kt·a−1industrialunits of China.Usually,a fluidized-bed reactor is the favorite choice for the benefits of excellent heat transfer capability,great specific surface area of catalyst,in-situ regeneration and recycle of the catalyst particles[1].
Though turbulent fluidized-bed reactor has been widelyadopted for its advantages of heat removal and catalyst in-situ regeneration,the back-mixing makes the selectivity of pyridine over 3-picoline become low.In present work,we attempted to use riser reactor for pyridine bases synthesis at the consideration of the following benefits:
(1)The flow pattern in riser reactor is near plug flow,which can favor the selectivity of pyridine over 3-picoline.
(2)Riser reactor has higher specific surface area,which favors heat removal.
(3)The catalyst is easy to be conveyed and regenerated in process using riser reactor.
Tostudy pyridine synthesis performance inriser reactor,we synthesized pyridine by ammonia,formaldehydes and acetaldehydes over GDM-70 catalyst in a riser reactor.
2.Experiment Apparatus
Fig.1 shows the schematic diagram of the experiment apparatus(Beijing Huiersanji Green Chem-Tech co.,Ltd.),which consisted of a riser reactor of 14 mm ID and 3500 mm in height and a regenerator of 500 mm ID.There was a cyclone separator in the disengager for catalysts and gas products separation.In this experiment the cyclone separator was replaced by a ceramic filter.The experimental process is illustrated in Fig.1.
Formaldehydes and acetaldehydes feedstock flow were pumped and measured by a electronic balance(EP20K,Changshu Yiou Instrument and Meter Plant,China)and were preheated to 373 K.Then they were mixed with ammonia,which was measured by a glass rotameter(LZB-4F,Tianjin FDS DODD Instrument Co.,Ltd.,China).The feedstock mixture was introduced into the riser reactor by a nozzle,mixing with hot regenerated catalysts and vaporizing.The vapor and catalysts flowed upwards in the riser reactor and were separated by a filter.Then the gas phase products were lique fied by two condensers and collected by a flask.The products were analyzed by a gas phase chromatograph(GC7890II,Shanghai Tianmei Keji Instrument Co.,Ltd.,China).The spent catalysts were first stripped by steam and then introduced into the regenerator.The regenerated catalysts were recycled to the riser through regeneration pipe.
The experimental operating conditions were that the reaction temperature ranged from 723 K to 798 K,residence time ranged from 2.0 s to 9.6 s,initial acetaldehydes concentration ranged from 0.00265 mol·L−1to 0.00448 mol·L−1,and CTFR ranged from 11 to 87.The absolute pressure at the entrance of the riser reactor was 178000 Pa in this experiment.The catalyst used in this experiment was GDM-70,which was developed by Dalian Institute of Chemical Physics,Chinese Academy of Science.The particle density of the catalysts is 2400 kg·m−3,and the mean diameter is 70 μm.
3.Calculation of Reaction Temperature
The temperature of the riser is measured in five axial positions as illustrated in Fig.2.Thereaction temperature was the average of the temperatures registered at points 2,3 and 4(these three points had small variance in temperature).

Fig.1.Schematic diagram of experiment apparatus.1.carbon dioxide detector;2,8,11.condenser;3.regenerator;4,5.valve;6.stripper;7.separator;9.riser;10.bottom of riser;12.pipe condenser;13.gas–liquid separator;14.liquid separation tower;15.piston pump;16.heater;17.glass rotameter;18.electronic balance;19.product collector;20.refrigerator;

Fig.2.Position of thermometers.
The initial acetaldehydes concentration CA0was calculated by Eqs.(1)–(4).

where nA0is the mole flow rate of acetaldehydes.V is the volumetric flow rate of both aldehydes flow and ammonia flow at reaction conditions at the bottom of the riser.

where nis the mole flow rate of both aldehydes flow and ammonia flow.

where xA0is the mole fraction of acetaldehydes in aldehydes flow,and nmis the mole flow rate of ammonia flow.

where ηafis acetaldehydes to formaldehydes ratio by mole(ATFR),and ηamis ammonia to aldehydes ratio by mole(ATAR),which were obtained by analyzing the composition of both aldehydes flow and ammonia flow before experiment.
4.Calculation of Residence Time
Reddyetal.[20]suggested that the volume change in a micro reactor can be neglected,therefore the gas velocity wasassumed to beconstant alongaxial direction in the riser reactor.The residence timet wascalculated by Eq.(5).

where h is the height of riser.viis super ficial gas velocity in the riser reactor and was calculated by Eq.(6).

where r is the radius of riser reactor.
5.Calculation of Produce Yield
In this experiment,the method for calculating pyridine yield ypand 3-picoline yield y3pwas shown in Eq.(7).

where ypand y3pwere pyridine yield and 3-picoline yield respectively.Npand N3pare the mole number of pyridine and 3-picoline in product respectively.Narand Nfrare the mole number of acetaldehydes and formaldehydes in feedstock respectively.
6.Results and Discussion
The selectivity of pyridine over 3-picoline under most experiment conditions was above 3 and is higher than the selectivity in a fluidized-bed reactor,which was 2.22 in Xin's study[22].The total yield of pyridine and 3-picoline was at most 67%,indicating a lower yield than previous study[20,22].
The increase of selectivity mainly results from less back mixing in this riser reactor.Zhou[23]calculated the particle residence time of this reaction system,which ranges from 10.7 s to 24.5 s.Compared to the average residence time in the turbulent fluidized-bed reactor,which is around 1 h,the mean residence time of this riser reactor is dramatically reduced.The particle residence time is closely related to the catalysts activity.For this riser reactor,with a particle residence time of 10.7 s to 24.5 s,the catalysts activity is great than 0.99 by previous work[20].
In the other hand,for turbulent fluidized-bed reactor,the catalysts activity is around 0.95.Since the pyridine synthesis reactions with ammonia and aldehydes are parallel reactions,and the reactionrate of pyridine production is larger than 3-picoline production,an increase of catalysts activity will raise the selectivity of pyridine over 3-picoline.The experiment results in this paper also justified this phenomenon.
The highest pyridine bases production yield is 65%in this experiment,and the corresponding reaction conditions are reaction temperature of 773 K,super ficial gas velocity of 0.9 m·s−1,and the catalysts circulation rate of 20.6 kg·m−2·s−1.The pyridine bases production yield is about 10%lower than that in a commercial turbulent fluidized-bed reactor,probably because the contact of feed stocks and the catalysts in the riser was not as intensive as that in turbulent fluidized-bed reactor.This phenomenon suggests that the catalyst circulation flow rate may have a strong Influence on the production yield in the fast fluidized-bed reactor.
Table1listed few typical experimental operating conditions and corresponding reaction results.Table1indicated that the operating para meters such as reaction temperature-T,residence time-t,initial acetaldehydes concentration-CA0,CTFR-co,ATAR and ATFR determined the reaction results ypand y3p.For data 1 to data 3,we tried to control the residence time-t individually.However,from the results in Table 1,we can see that other operating parameters such as CA0,CTFR and ATAR were also changed.The reason was that variation of residence time means variation of feedstock flow rate.If we varied the flow rate of the feedstock but kept the reaction temperature to be constant,the energy balance of the reaction system would require the CTFR to be changed.In that case,it was impossible to change there sidence time individually.For data 4 to data 6,the reaction temperature was changed intentionally.However,Table 1 indicated that other operating parameters were also changed.For a hot-mode experimental apparatus,the system should obey the mass balance principle,energy balance principle and momentum balance principle,which made its hard to adjust operating parameters.As mentioned above,variation of one operating parameters may affect other operating parameters.
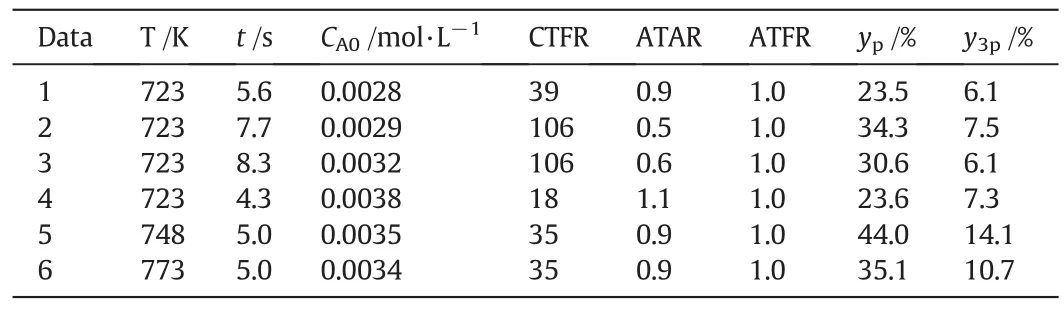
Table 1 Experimental operating conditions and corresponding reaction results
Since unable to obtain the optimal operating conditions for riser reactor by experiment,in present work,based on the experiment results,a modified kinetic model was suggested by the author to give instructions on determination of optimal operating condition of large scale riser reactors.
7.Kinetic Modeling
7.1.Analytical solution for relationship between produce yield and residence time
The main reactions involving in pyridine synthesis given by Reddy et al.[1,22]were as shown in Eqs.(8)–(11).

Reddy et al.[20]proposed a model based on the experiment data obtained in a fixed-bed reactor.The reaction scheme of the model is illustrated in Fig.3.
The reaction rate formulas for acetaldehydes(A),pyridine(D)and 3-picoline(E)were given by Reddy et al.[20],which were showed in Eqs.(12)–(14).

where k1,k2,k3and k4are the reaction rate constants for reactions8–11 respectively.a is the super ficial reactivity of catalyst and is unity for fresh catalyst.
In this paper,the model proposed by Reddy[20]was modified with the following assumptions.
(1)The reactivity of the catalyst was taken as unity during each experiment period(usually 1 h or less).
(2)Reaction step is the controlling step.Reddy et al.[17,20]suggested when catalysts were smaller than 600 μm,the internal diffusion resistance could be minimized.In the present work,catalysts diameter ranged from 40 μm to 210 μm,which indicated that the internal diffusion resistance could be neglected.The external diffusion resistance could also be neglected if the gas velocity were beyond 0.0069 m·s−1for fixed-bed reactor[17,20].In that case,if the gas–solid slip velocity was beyond 0.0069 m·s−1,the external diffusion resistance could be neglected in fluidized-bed reactor.The carryover velocity of single catalyst is 0.058 m·s−1,which is smaller than the real gas–solid slip velocity in a riser because of particles interaction and particle-wall friction.It was reasonable to assume that the slip velocity is beyond 0.0069 m·s−1,and the external diffusion resistance could be neglected in this experiment.In that case,reaction step is the controlling step of the process.
(3)The gas–solid flow inside the riser was considered as the plug flow,which was also suggested by other works[24–26].Axial back-mixing inside a riser could arise from friction between particles and the wall,and gas–solid slip velocity[27,28].Because particle circulation flow rate is small in present work,the wall affection and particle clustering was weak and neglected.Furthermore,gas and particles were assumed to move upwards with a constant gas–solid slip velocity close to the carryover velocity of single particle,so small a velocity that axial back mixing could be neglected.
(4)The temperature variation and pressure drop in riser reactor is neglected.
(5)The catalyst phase and the vapor phase had the same velocity in riser reactor.However,previous works indicated that there exists slip velocity between particle phase and gas phase[27,28].For the simplification of the model,we assumed that the gas and solid has the same velocity,but the external diffusion was neglected,which took the Influence of unknown gas–solid slip velocity into account.
As illustrated in Fig.4,the mass balance was carried out in a control volume of the riser.
Eq.(15)was obtained from mass balance:


Fig.3.Reaction scheme for pyridine synthesis.A:acetaldehyde B:ammonia C:formaldehyde D:pyridine E:3-picoline F:coke.
By solving Eqs.(2),(6)and(12),the following Equation was obtained:
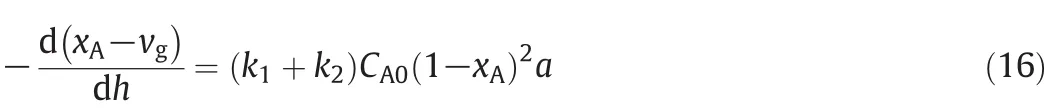
In this article,the value of vgwas assumed constant along axial direction of this riser reactor at a given residence time and equals to vi.Eq.(16)is simplified into Eq.(17).

Similarly,Eqs.(13)and(14)were also simplified as Eqs.(18)–(19).


Fig.4.Control volume of riser reactor.
To solve Eqs.(18)–(19),super ficial catalyst activity was assumed unity and do not change with residence time.Eq.(17)was changed into Eq.(20).

where bequalsto(k1+k2)CA0.Byintegrating Eq.(20)from 0tot and 0 to xA,Eq.(21)can be obtained.

Then Eq.(18)was rewritten as

By solving Eq.(22)with boundary condition:t=0,xD=0,Eq.(22)was changed into Eq.(23).

Similarly,Eq.(19)was solved and shown as Eq.(24).

We found it was impossible to find the primitive functions for Eqs.(23)and(24).In this paper,the value of Eqs.(23)–(24)was calculated by using numerical integration with quad method in Matlab.The corresponding error in this numerical integration is less than 10−6.
As illustrated in Eqs.(23)–(25),there are eight unknown parameters,the parameters of this modified model were obtained in two steps.Firstly,the Universal Global Optimization(UGO)method in 1st Opt was chosen to get the initial value of ki0.The initial value of Eiwas assumed to be same as that in Reddy's study[20].Then the first step results were set as the initial value of parameters,and least squares method was selected to determine the final value of these parameters.The final value of these parameters is listed in Table 2.
The average deviation was 17%and 21%for pyridine and 3-picoline respectively,indicating a reasonable prediction of Reddy's[20]model.
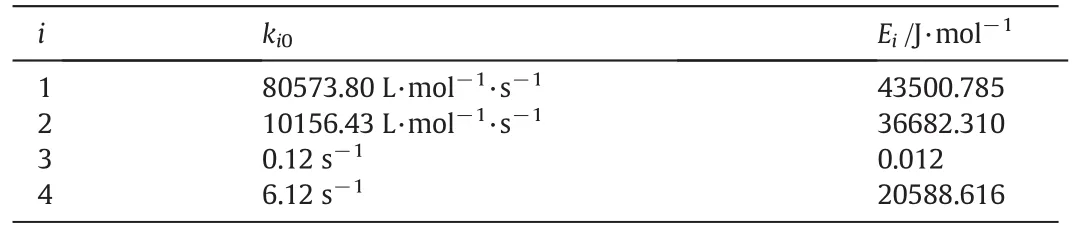
Table 2 Values of parameters ki0and Ei
7.2.Super ficial catalyst activity modification
The unsatisfactory prediction of the yields probably arose from the assumption that in the riser reactor the super ficial catalyst activity was unity.Results from previous works[6,17]indicated that super ficial catalyst activity was significantly affected by reaction temperature and CA0.
Fig.5 illustrated the Influence of CTFR(defined as mass flowrate of recycled catalyst divided by mass flow rate of feedstock)on the produce yields of pyridine and 3-picoline in our experiment.A linearly increasing evolution of both pyridine yield and 3-picoline yield with CTFR can be observed.In present study,the solid holdup in the riser reactor is ranged from 2%to 4%,indicating a super dilute catalyst phase.In that case,higher CTFR brought higher contact opportunity between reactants and catalyst,which guaranteed higher super ficial catalyst activity.
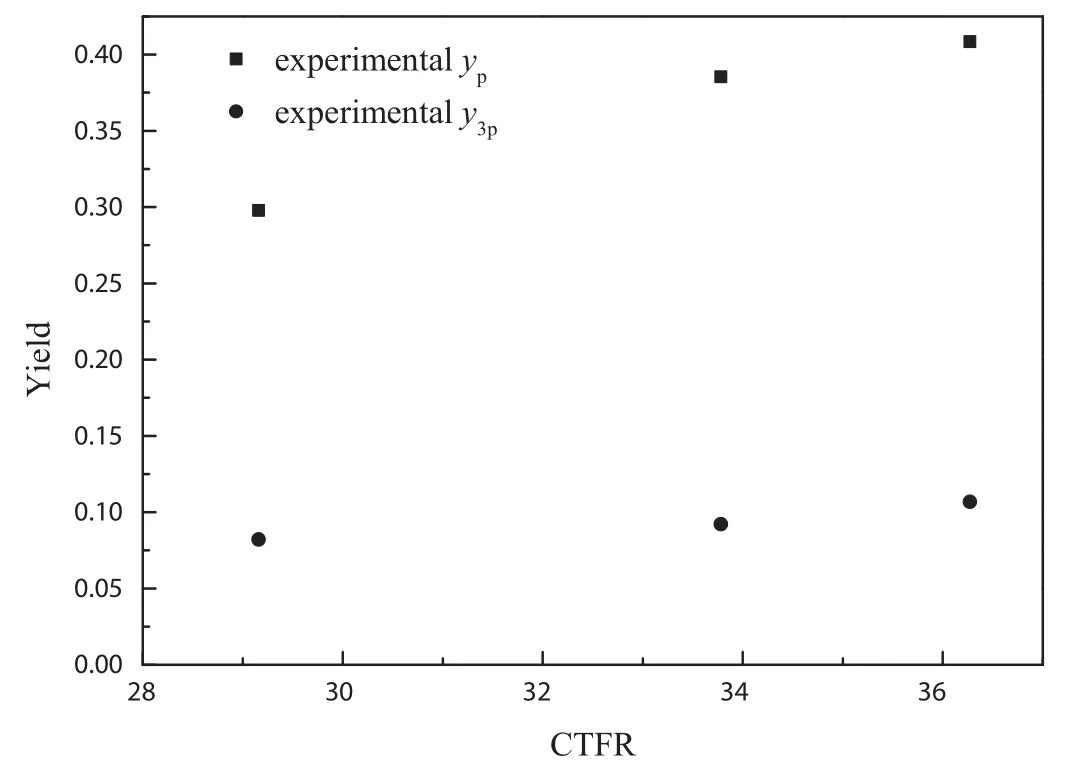
Fig.5.Influence of CTFR on produce yield by experiment.Residence time:3.44 s,CA0:0.00327 mol·L–1,reaction temperature:798.15 K.
In present work,the Influence of CTFR,reactiontemperature and CA0on superficial catalyst activity was expressed as Eq.(26).

where a′is the modified super ficial catalyst activity;co is CTFR.un1and un2are parameters,and a0is the super ficial activity of regenerated catalyst and equals to unity.F(T)and F(CA0)are the Influence of reaction temperature and CA0on super ficial catalyst activity respectively,which are given by Eqs.(27)–(28).
Francis et al.[12]found out that at reaction temperature under 623K,largepartofreactantspassthroughwithoutreacting,whileatreaction temperature above 823 K,the catalyst inactivated more rapidly and resulted in many side reactions.Reddy et al.[17]proposed that at high temperatures,the catalyst surface becomes dehydroxylated and Bronsted centers are transformed into their Lewis counterparts,resulting in a drop in overall conversion and selectivity.
Francis's and Reddy's study indicated that with increasing reaction temperature,the super ficial catalyst reactivity firstly increases and then decreases.In the present work,the relationship is given as Eq.(27).

Liepinya et al.[21]pointed out higher initial aldehydes concentration resulted in higher coke yield,suggesting lower catalyst reactivity.The relationship between super ficial catalyst activity and CA0was expressed by Eq.(28).

In the present work,a′was taken as the value of experimental produce yield divided by calculated produce yield.The value of parameters un1-un8is listed in Table 3.

Table 3 Values of parameters un1-un8
The average deviation was 16%and 20%for pyridine and 3-picoline respectively.Fig.6 is thecomparison between model prediction andexperiment data of produce yield,indicating this modified model agreed well with experimental data.
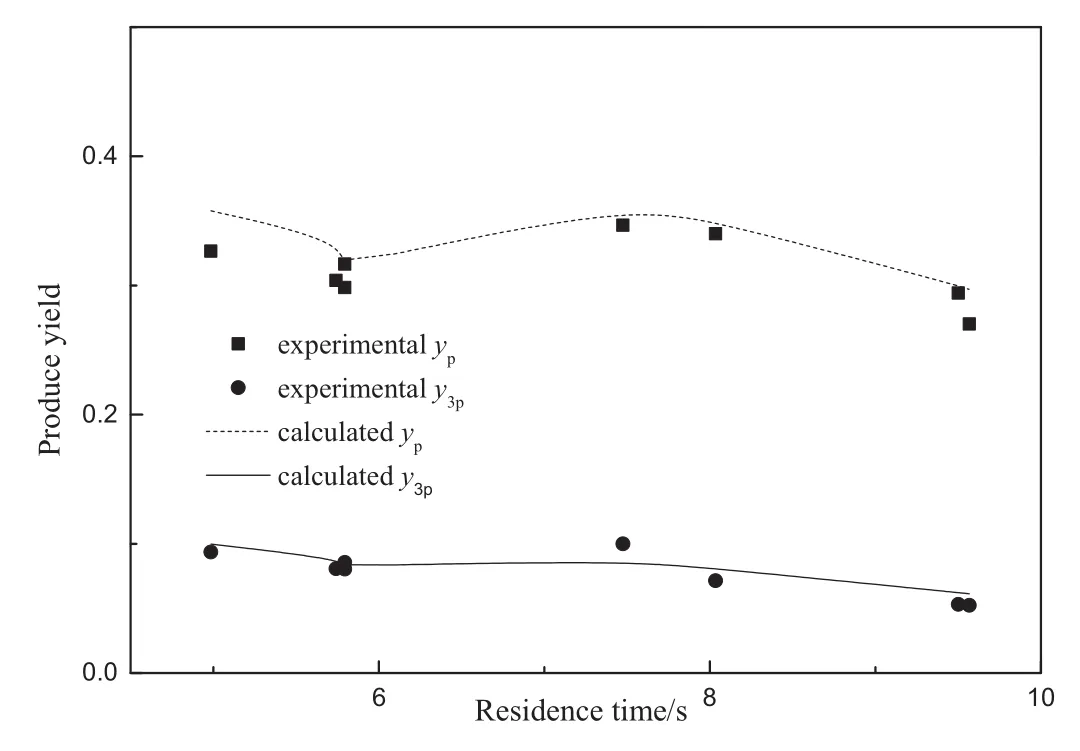
Fig.6.Comparisonbetween themodelprediction andexperiment results.Temperatureof 748.15 K,CA0of 0.0027–0.0037 mol·L–1,CTFR of 17–87.
8.Influence of Operating Condition on Produce Yield
8.1.Influence of CA0on produce yield
The Influence of CA0on produce yield by this modified model is shown in Fig.7.With the increase of CA0,both pyridine yield and 3-picoline yield firstincreased,reached a maximum atCA0of 0.0029 mol·L−1,after which,the produce yield decreased.When temperature and pressure were fixed,CA0was determined by ATFR,ATAR as well as water contention in formaldehyde solution.In present work,Eqs.(1.1)to(1.4)determinedthatCA0waspositivelyrelatedwith ATFR and ATAR.Fig.8 illustrated the variation of CA0with ATAR and ATFR.

Fig.7.Influence of CA0on produce yield by model prediction.Residence time of 4 s,reaction temperature of 748.15 K,CTFR of 30.
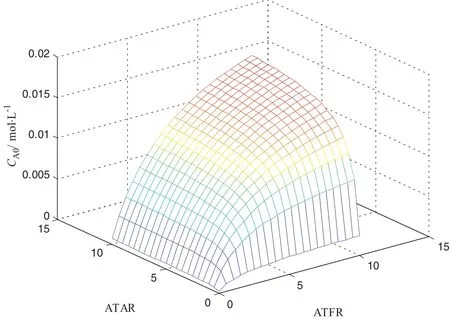
Fig.8.Relationship between CA0and CTFR,ATAR.
Fig.9wastheInfluenceofATARonprodu e tonarrow down the value difference of other operating parameters,the selected data still could not be used to illustrate the Influence of ATAR on produce yield.To study the Influence of ATAR and ATFR on produce yield,the modified model was used in present work.Figs.10 and 11 were the Influence of ATAR and ATFR on produce yield respectively by modified model.Fig.10 showed that the produce yield first increased,reached maximum at ATAR of 0.9 and then decreased with the increase of ATAR.Fig.12 showed that the produce yield first increased and then decreased with the increase of ATFR.A ATFR of 0.65 gave the best produce yield at given conditions in Fig.12.By comparison between Figs.10 and 12,the conclusion that ATFR had more Influence on produce yield than ATAR could be obtained.
8.2.Influence of residence time on produce yield
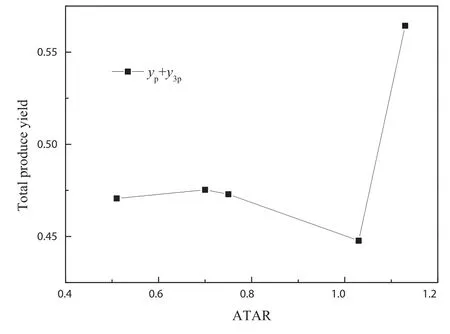
Fig.9.Influence of ATAR on produce yield by experiment.Residence time of 4.8–5.2 s,reaction temperature of 773 K,catalyst to feedstock ratio from 39–63.
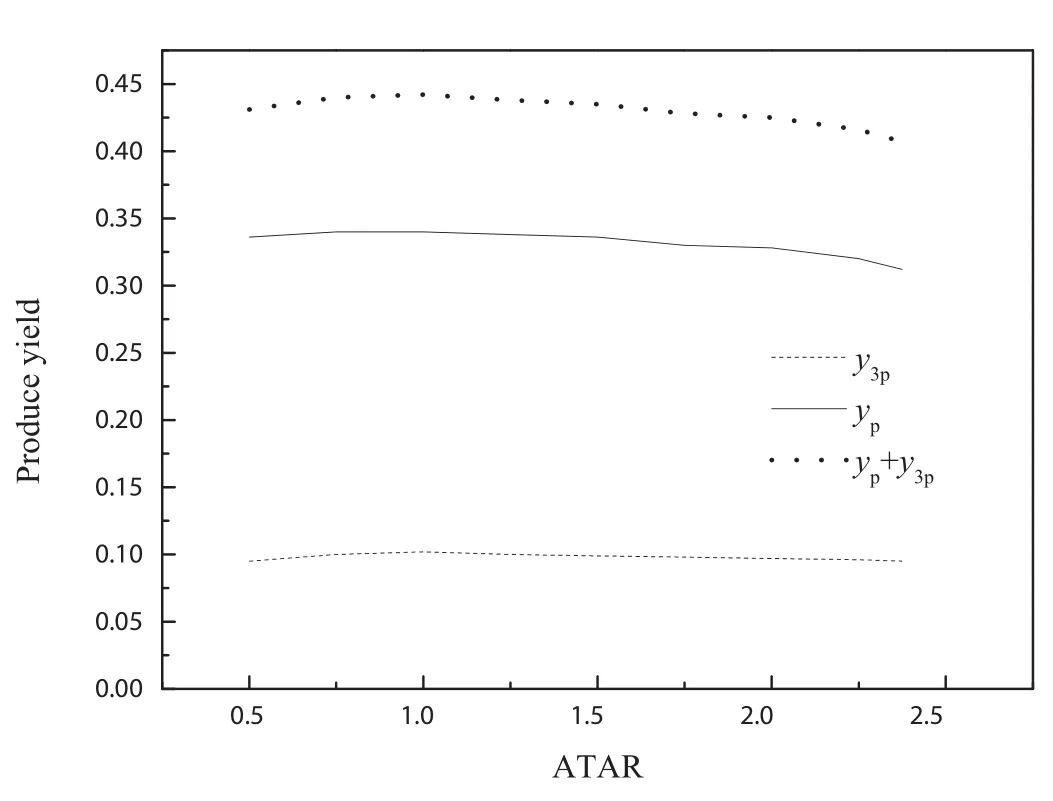
Fig.10.Influence of ATAR on produce yield by model prediction.Residence time of 4 s,reaction temperature of 748.15 K,CTFR o f 30,ATFR of 0.6,water mass content of 0.63.
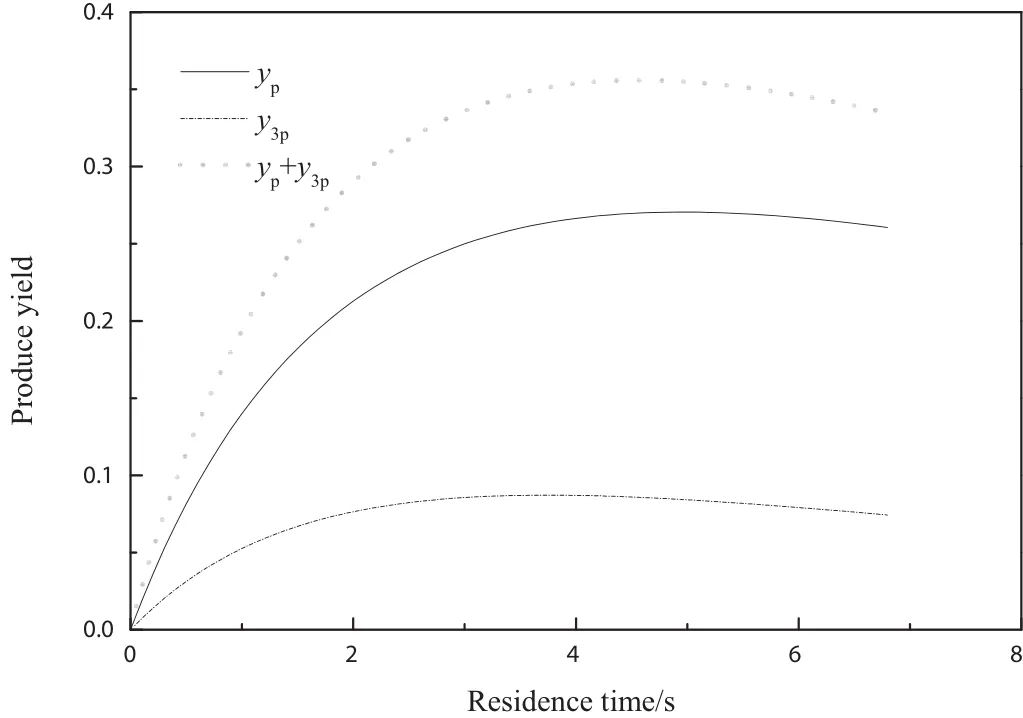
Fig.11.Influence of residence time on produce yield by model prediction.Temperature of 723.15 K,CA0of 0.0036 mol·L–1,CTFR of 30.
Residence time is important for determination of reactor volume.Baylis et al.[29]pointed out that the residence time for vapor phase pyridine synthesis was within1 to about 60 sand the residence time for fixed-bed reactor should be within 1 to about 15 s.Feitler[4]reported that the preferred residence time for fluidized-bed reactor was around 3.5 s.Fig.11 illustrated the Influence of residence time on produce yield for riser reactor.With the increase of residence time,both pyridine and 3-picoline yields first increased,reached a maximum at 4.5 s and then slightly decreased.The optimal residence time for other reaction temperatures was listed in Table 4.As shown in Table 4,with increase of reaction temperature,the optimal residence time decreased,while the selectivity and total yield increased.
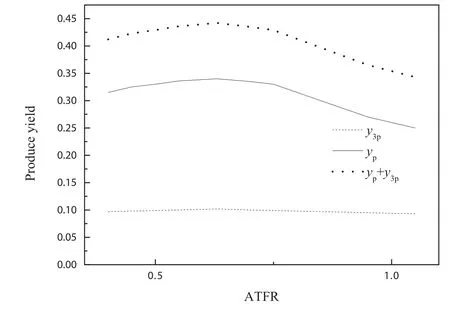
Fig.12.Influence of ATFR on produce yield by model prediction.Residence time of 4 s,reaction temperature of 748.15 K,CTFR o f 30,ATAR of 0.9,water mass content of 0.63.
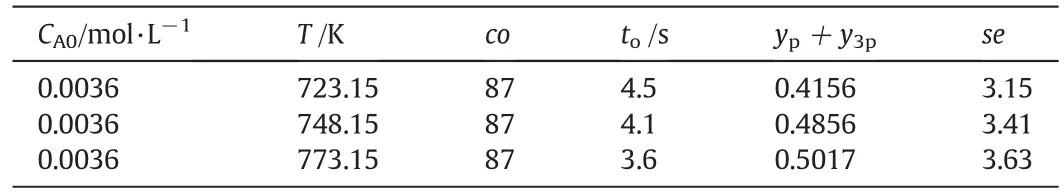
Table 4 Optimal reaction temperature at different reaction condition
8.3.Influence of reaction temperature on produce yield
Reactions(2.1)and(2.2)are highly exothermic,indicating lower reaction temperature would favor reactants conversion.On the other hand,the reaction rate is exponential positively connected with reaction temperature according to Arrhenius Equation,indicating high reaction temperature would bring high reaction rate and smaller reactor.In that case,a suitable reaction temperature is required to guarantee fast reaction rate without low reactants conversion.Feitler[4]found that the higher the reaction temperature the higher the selectivity of pyridine to 3-picoline,but with a lower total yield of the two.Similar phenomenon was also observed in this experiment(Fig.13).From Fig.13,we found that the produce yield almost kept constant from 740 K to 773 K.In present work,reaction temperature of 755 K was proposed for riser reactor.
Based on the Influence of operating parameters on produce yield,the optimal reaction condition for this riser reactor determined by this modified model was reaction temperature of 755 K,CTFR of 87,residence time of 3.8 s and initial acetaldehydes concentration of 0.0029 mol·L−1(ATAR of 0.9,ATFR of 0.65,water contention 63wt%in formaldehyde solution).

Fig.13.Influence of reaction temperature on produce yield by model prediction.
9.Conclusions
The vapor phase pyridine synthesis process was conducted in a riser reactor,and the produce yield of pyridine and 3-picoline,as well as selectivity of pyridine over 3-picoline were measured.The experiment was conducted under reaction temperature ranged from 723 K to 798 K,residence time ranged from 2.0 s to 9.6 s,initial acetaldehydes concentration ranged from 0.00265 mol·L−1to 0.00448 mol·L−1,and CTFR ranged from 11 to87.The experiment result showed that pyridine yield ranged from 16.00%to 50.51%,3-picoline yield ranged from 4%to 17%and selectivity ranged from 1.9 to 4.8,mostly above 2.5.Reddy's kinetic model[20]was modified by super ficial catalyst activity,which closely related to CTFR,reaction temperature and initial acetaldehydes concentration.Based on the modified model,the Influence of reaction temperature,residence time,initial acetaldehydes concentration and CTFR on produce yield was investigated.At last,the optimal operating condition for riser reactor was given,which was reaction temperature of 755 K,CTFR of 87,residence time of 3.8 s and initial acetaldehydes concentration of 0.0029 mol·L−1(ATAR of 0.9 and ATFR of 0.65,water content 63wt%).
The selectivity of pyridine over 3-picoline in riser reactor under optimal operating conditions was 3.35 and was higher than that in fluidized-bed reactor,which was 2.2.However,the produce yield in riser reactor was not as high as in fluidized-bed reactor(total yield around 60%for riser reactor and 72%for fluidized-bed reactor).
From the point of view of gas–solid fast fluidization,the multiphase hydrodynamics was not well considered.The modeling and calculations are somewhat simple due to many assumptions on multiphase flow and reaction.This limits the application of this model.Future work should be focused on the Influence of flow hydrodynamics on the production yield.
Nomenclature
a activity of catalysts,for fresh catalysts the value is unity
CA0concentration of acetaldehydes in feedstock,mol·L−1
k1reaction rate constant for reaction A to D,L·mol−1·s−1
k2reaction rate constant for reaction A to E,L·mol−1·s−1
k3reaction rate constant for reaction D to F,s−1
k4reaction rate constant for reaction E to F,s−1
nA0number of mol of acetaldehydes in feedstock,mol
P pressure of feedstock,Pa
R ideal gas constant 8.314,L·mol−1·K−1
rAreaction rate for acetaldehydes,mol·L−1·s−1
rDreaction rate for pyridine,mol·L−1·s−1
rEreaction rate for 3-picoline,mol·L−1·s−1
se selectivity of pyridine over 3-picoline
T reaction temperature,K
t residence time,s
V volumetric flow rate,m3·h−1
Vrvolume of riser,m3
xAyield of acetaldehydes
xDyield of pyridine
xEyield of 3-picoline
ηafacetaldehydes to formaldehydes ratio
ηamammonia to aldehydes ratio
 Chinese Journal of Chemical Engineering2018年7期
Chinese Journal of Chemical Engineering2018年7期
- Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Preparation of vapreotide-templated silver nanocages and their photothermal therapy efficacy☆
- DNA-assisted rational design of BaF2linear and erythrocyteshaped nanocrystals☆
- On the database-based strategy of candidate extractant generation for de-phenol process in coking wastewater treatment☆
- Hierarchical porous MgBO2(OH)microspheres:Hydrothermal synthesis,thermal decomposition,and application as adsorbents for Congo red removal☆
- Degradation analysis of A2/O combined with AgNO3+K2FeO4on coking wastewater
