Lesson Eighty four Ventricular arrhythmias originating from papillary muscles in the right ventricle
Patient characteristics
Patients in this study consisted of eight consecutive patients with frequent premature ventricular complexes(PVCs)or both PVCs and ventricular tachycardia(VT)who had been referred for catheter ablation and whose arrhythmia was mapped to one of the right ventricular(RV)papillary muscles (PAPs).The control group consisted of 10 consecutive patients who were referred for ablation of symptomatic idiopathic arrhythmias originating in the right ventricle.Patients with RV PAP arrhythmias were predominantly men (seven of eight),while patients in the control group were all women(10/10).Four patients in the RV PAP group and one patient in the control group had an impaired left ventricular function before the procedure.Mean right ventricular ejection fraction was 0.53%±0.08%in the PAP group and 0.57%±0.10%in the control group.MRI did not reveal evidence of right ventricular dysplasia in any of the patients.One patient in the study group had nonsustained VT provoked by ventricular burst pacing,and four patients had inducible VT with burst pacing.Isoproterenol triggered nonsustained VT in one patient and increased the PVC frequency in two patients.In the control group,three of 10 patients had nonsustained VT triggered by isoproterenol infusion.During VT,entrainment could not be demonstrated.
Twelve-lead electrocardiogram
Twelve-lead electrocardiograms(ECGs)of the PVCs and VTs were analyzed for bundle branch block morphology,axis,QRS width,presence of notching in V1to V6(Figure 1),R-wave pattern in V1(rS,QS),and the transition point from predominantly negative S-wave to predominantly positive R-wave deflection in the precordium.An early transition was defined as transition in lead V4or earlier (Figure 1,left panel),and late transition was defined as a transition in V5or V6(Figure 1,right panel).Notches were defined as deflections in the QRS complex aside from1a triphasic pattern(Figure 1).
Pleomorphic PVCs were present in three of the eight RV PAP patients and in three of 10 patients in the control group.A total of 15 VAs were mapped to the RV PAPs.All RV PAP arrhythmias had an rS or QS pattern in lead V1and displayed a left bundle branch block morphology.The mean QRS width during ventricular ectopy was greater in the PAP group(163±21 ms)than in the control group(141±22 ms).In the PAP group,10 PVC morphologies had an inferior axis,and five had a superior axis.In the control group,all arrhythmias had an inferior axis.Notching in the precordial leads was present in 11 of 15 PVC morphologies in the RV PAP group and in five of 13 PVC morphologies in the control group.Late precordial transition (>V4)was present in patients with posterior or anterior RV PAP arrhythmias.This corresponds to the more apical insertion of both anterior and posterior papillary muscles. Early precordial transition(≤V4)and an inferior axis occurred more often in arrhythmias originating in the septal RV PAP owing to2the more basal insertion of the septal papillary muscle.
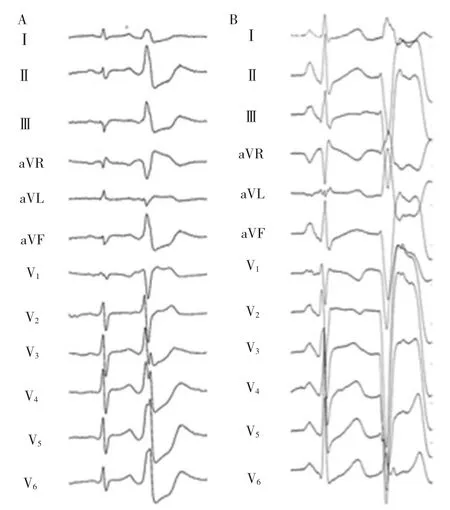
Figure1 A:A 12-lead ECG of PVCs originating in a septal papillary muscle.Note the inferior axis,the early transition in lead V3,and the presence of notching.B:A 12-lead ECG of frequent PVCs originating in a posterior PAP.Note the superior axis and the late precordial transition in V5.
Mapping and Ablation(Figures 2 and 3)
Severalmultipolar electrode catheters were introduced into the right ventricle,right atrium,and His position.Programmed right ventricular stimulation was performed using up to four extrastimuli to assess for inducible VT.Programmed stimulation was repeated during infusion of isoproterenol at 2-20 μg/min.An electroanatomical mapping system(CARTO)was used to guide mapping.Activation mapping was performed during ventricular ectopy or VT.In the setting of infrequent ventricular ectopy,pace mapping was used to identify the site of origin.
Radiofrequency energy wasdelivered via a 4-mm-tip catheter or a 3.5-mm-tip irrigated-tip catheter at sites with the earliest endocardial activation during the VA and/or at matching pace-mapping sites.Radiofrequency energy was delivered in a temperaturecontrolled mode with a target temperature of 55℃at a power of≤50 W.With the irrigated-tip catheter,radiofrequency energy was applied at a power of 30-35 W and a maximal temperature of 45℃.Programmed ventricular stimulation with and without isoproterenol infusion was repeated at the end of the procedure.
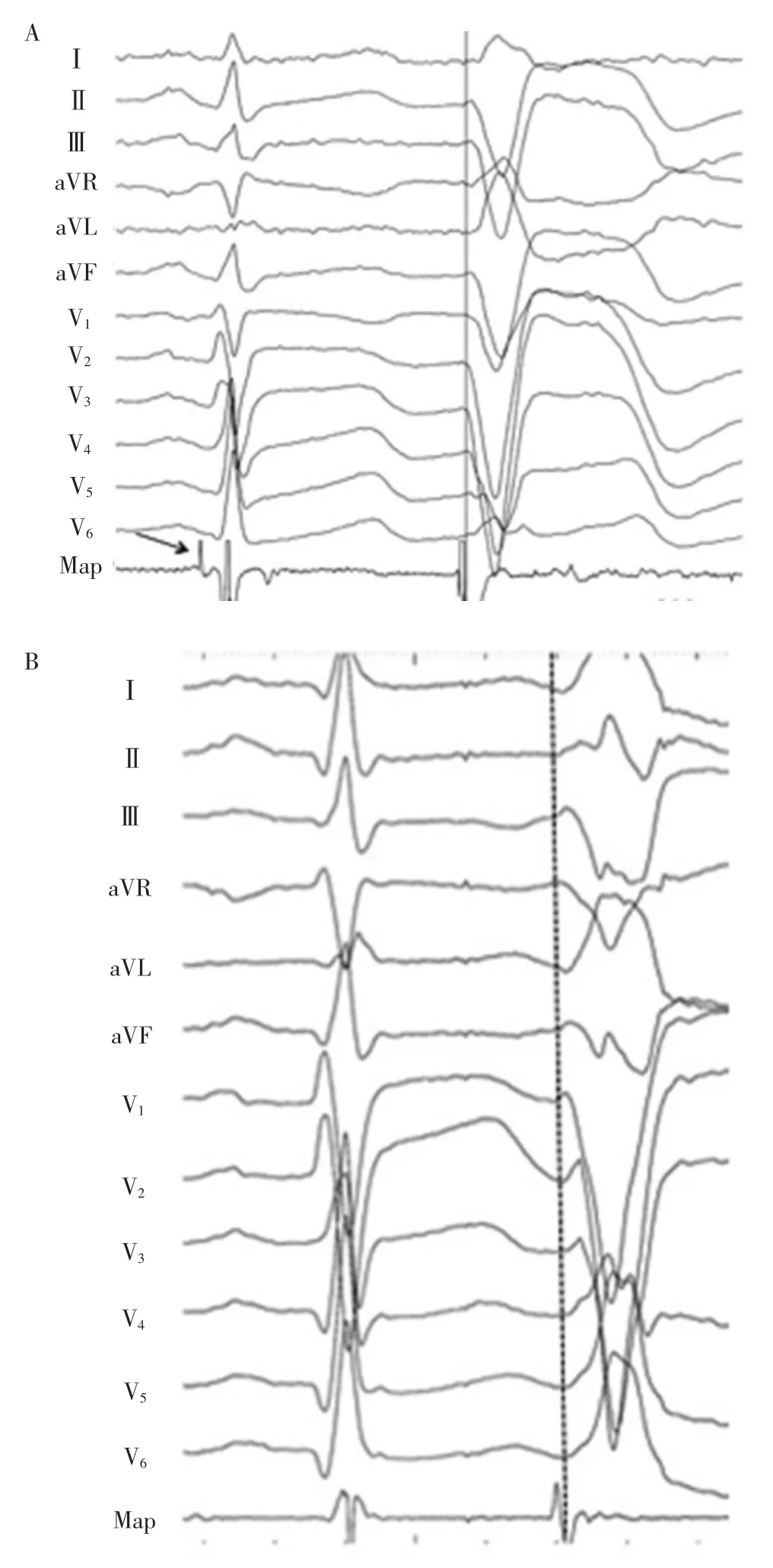
Figure 2 A:Site of origin of a PVC originating in a posterior papillary muscle.A Purkinje potential is present during sinus rhythm(arrow).The endocardial activation time was-20 ms at the successful ablation site.B:Site of origin of a papillary muscle PVC without a recorded Purkinje potential.
The earliest endocardial activation time during VT orPVCswas23±8msbeforetheQRScomplexintheRV PAP patients and 28±4 ms in the control group.At the site of origin,there were matching pace maps compared with the targeted arrhythmia in the PAP group(a pace-mapmatchofatleast11/12leadswasfoundineight ofeightpatients)andinthenon-PAPgroup(apace-map matchofatleast11/12 leadswasfound in 10/10 patients).The site of origin of the arrhythmia was mapped to the right posterior papillary muscle (n=3),the anterior papillary muscle(n=4),or the septal muscle(n=8).A Purkinje potential at the effective ablation site was identified in two of eight patients in the RV PAP group andinnoneofthecontrolpatients.Themeanamplitudeof the ventricular electrogram at the site of origin of the arrhythmia during sinus rhythm was 2.1±1.4 mV in the RV PAPgroup and 2.9±2.4 mV in the control group.The mean power achieved was similar in the two groups,30.5±8.4 W and 30.4±5.8 W in the PAP and RVOT patients,respectively.The mean temperatures achieved during ablation were 35.5±7.3℃and 35.3±1.6℃in the PAP and RVOT groups and did not differ significantly.The mean impedance was higher in the PAP group(100.9±11.5 ohms)than in the control group (86.6±9.3ohms).Themeanablationtimewas8.9±2.3minutesin patients with single morphology papillary muscle ectopy and 16.9±9.1minutes in patients with two or more right papillary PVC/VT morphologies.The procedure time was longer in the PAP group as compared with in the control group(277±49vs.203±67minutes,respectively).
Real-time imaging
Intracardiac echocardiography was used during the procedures to identify the papillary muscles and to confirm catheter contact with the papillary muscle.Using intracardiac ultrasound the distinction of the site of origin was made between the septal PAP(insertion on the septum)and the anterior or posterior PAP(inserting in the RV free wall or apex).
Right-sided papillary muscle and arrhythmias
Prior studies described VAs originating in thepapillary muscles of the LV in patients with and without prior myocardial infarction.This study demonstrates that VAs also can arise in a RV PAP.Intracardiac echocardiography helped to identify the site of origin of arrhythmias originating from the right ventricle.Similar to arrhythmias originating from left-sided papillary muscles,adequate contact of the ablation catheter with the papillary muscle is important to assure a successful ablation.Structural abnormalities are often observed in patients with left-sided PAP arrhythmias.Scarring was occasionally observed even in the absence of prior myocardial infarction.
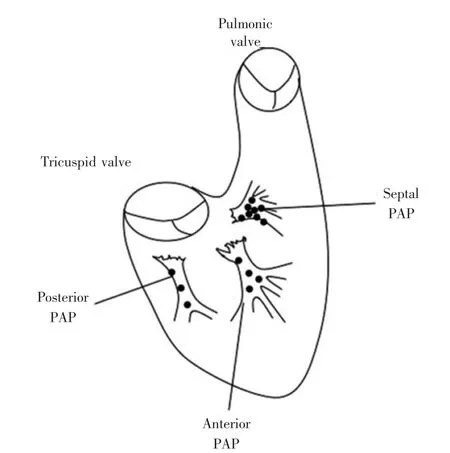
Figure 3 Schema of the right ventricle and the origin of PAP-related arrhythmias.
None of the patients in this series showed evidence of delayed enhancement on the preprocedural MRI.However,for the nulling of the MR signal,the signal from the healthy LV myocardium was used and right-sided abnormalities might have been missed owing to lower inversion times.The presence of a lower ejection fraction in half of the PAP patients,despite a similar baseline PVC burden in the control group,is intriguing butmighthave occurred by chance.Consistent with prior reports in patients with reduced ejection fraction and a high burden of PVCs,the LV ejection fraction improved after ablation in all patients with a reduced LV ejection fraction in this study.
Mechanism
RV PAP arrhythmias were not inducible by programmed atrial or ventricular stimulation.Sustained VT,if inducible,was provoked by isoproterenol or burst pacing,suggesting that the underlying mechanism is triggered activity.This is consistent with prior reports in patients with left-sided papillary muscle arrhythmias without prior infarction.Furthermore,VT could not be entrained or terminated by overdrive pacing,also supportingautomaticityinstead ofreentryasthe mechanism.Two of the eight patients in our study had a Purkinje potential at the successful site of ablation at the base of a papillary muscle,suggesting that the Purkinje fiber system might be involved in the arrhythmogenic substrate similar to left-sided papillary muscle arrhythmias.Failure to identify a Purkinje potential in the majority of the patients does not prove the absence of involvement of the Purkinje fiber system but may reflect the difficulty in achieving good contact with the arrhythmogenic papillary muscle.
词 汇
provoke v.对...挑衅,激起,诱导
isoproterenol n.异丙肾上腺素
ectopy n.异位
insertion n.插入,插入物,嵌饰
multipolar adj.多极的
extrastimuli n.期外刺激
impedance n.阻抗
distinction n.区分,荣誉,不同点,差别
scar n.&v.疤痕,疤,斑疤,痕,岩礁,创伤;结疤
null n.&v.零,零位;使...无效
inversion n.反向,倒转物,倒装
intrigue n.&v.阴谋,情节,私通;耍阴谋,吸引,使...困惑,私通
burden n.要旨,重负,载重吨,重担,低声部,负担
automaticity n.自动性
arrhythmogenic adj.致心律失常的
注 释
1.aside from指“除…之外,偏离,暂置不论”,常置于句首,如Aside from their scavenger function,macrophages also possess destabilizing and thrombogenic properties。除了清除功能外,巨噬细胞也有引发不稳定和促进血栓形成特性。
2.owing to指“由于……”,医学文献中常用,特别强调因果关系或内在联系,可置于句首、句中或句尾,如Owing to the different release kinetics of cTnI and cTnT,the magnitude of rise or fall in the level of these proteins has to be established for every cTn assay individually。由于cTnI和cTnT的不同释放动力学,每个cTn试剂盒都必须确立这些蛋白浓度升高或下降的量值。
参考译文
第84课 右心室乳头肌起源室性心律失常
患者特征
研究对象(PAP组)系因频发室性期前收缩(PVC)或PVC合并室性心动过速而来行射频导管消融手术(下称消融),同时标测到心律失常起源于右心室乳头肌(PAP)的8例连续患者。对照组为因右心室起源特发性心律失常来行消融的连续10例症状性患者。右心室PAP心律失常患者多为男性(7/8),而对照组全为女性(10/10)。术前右心室PAP组中4例和对照组中1例存在左心室功能障碍。PAP组平均射血分数(0.53±0.08)%,对照组为(0.57±0.10)%。所有患者 MRI均无右心室发育不良迹象。研究组心室猝发起搏诱发非持续性室性心动过速(VT)1例,猝发起搏诱发可诱发性VT 4例。异丙肾上腺素促发非持续性VT 1例,增加PVC频率2例。对照组10例中的3例滴注异丙肾上腺素促发非持续性VT。VT时不能拖带。
12导联心电图
对PVC和VT的12导联心电图的束支传导阻滞图形,心电轴,QRS 宽度,V1~V6导联的切迹(图 1),V1的 R 波形态(Rs,QS)以及胸导联从负向S波为主转为正向R波为主的移行点进行分析。移行点位于V4或以前的为早移行(图1A),位于V5V6的为晚移行(图1B)。切迹是指不考虑三相波图形的QRS波群中的反折(图1)。
右心室PAP组8例中的3例、对照组10例中的3例出现多形性PVC。共标测到15种室性心律失常源于右心室PAP。所有右心室PAP心律失常在V1表现为Rs或QS波,呈左束支传导阻滞型。室性异位心律QRS波群平均宽度PAP组(163±21)ms大于对照组(141±22)ms。PAP 组中 10种PVC形态表现为心电轴向下,5种心电轴向上。对照组所有心律失常心电轴向下。右心室PAP组15种PVC中的11种、对照组13种PVC中的5种胸导联存在切迹。右心室后或前乳头肌起源心律失常胸导联移行晚(>V4)。这与前和后乳头肌插入点更接近心尖相关。胸导联早移行与向下电轴更多见于右心室间隔乳头肌起源心律失常,这与间隔乳头肌插入点更近基底部有关。
标测与消融(图 2、3)
多根多极电极导管置于右心室、右心房和希氏束部位。采用多达四个期外刺激的程控右心室刺激诱发VT。滴注异丙肾上腺素2~20 μg/min期间重复程控刺激。电解剖标测系统(CARTO)用于标测。室性异位搏动或VT期间进行激动标测。室性异位搏动少见时采用起搏标测确定起源点。
采用头端4-mm的导管或3.5-mm的灌注导管,于室性心律失常时最早的心内膜激动点或匹配的起搏标测点进行消融。温控55℃、功率≤50W释放消融能量。灌注导管释放能量30~35W、最大温度45℃。消融结束时滴注或非滴注异丙肾上腺素下重复程控心室刺激。
VT或PVC时最早心内膜激动时间与QRS波群比较,RV PAP 组提前(23±8)ms,对照组提前(28±4)ms。在起源部位,起搏标测图与PAP组(全部8例起搏标测图匹配至少达11/12导联)和非PAP组(全部10例起搏标测图匹配至少达11/12导联)的目标心律失常相匹配。标测到心律失常起源点位于右后乳头肌3例、前乳头肌4例、间隔肌8例。右心室研究组8例中的2例有效消融靶点见到浦肯野电位,对照组10例均未见到浦肯野电位。窦性心律下心律失常起源点心室电图平均电位右心室 PAP组(2.1±1.4)mV、对照组(2.9±2.4)mV。两组消融达到的平均功率相近,PAP组与右心室流出道组分别为(30.5±8.4)W和(30.4±5.8)W。消融时达到的平均温度PAP组和右心室流出道组分别为(35.5±7.3)℃和(35.3±1.6)℃,无明显差异。平均阻抗PAP组(100.9±11.5)Ω高于对照组(86.6±9.3)Ω。平均消融时间单一形态乳头肌异位搏动(8.9±2.3)min,两种或以上形态右乳头肌PVC/VT(16.9±9.1)min。PAP 组手术时间(277±49)min大于对照组(203±67)min。
实时显像
术中心腔内心脏超声识别乳头肌并确定导管接触乳头肌。借助心腔内超声区分间隔乳头肌(插入点位于间隔)与前或后乳头肌起源点(插入点位于右心室游离壁或心尖)。
右侧乳头肌与心律失常
以前报道过室性心律失常起源于伴或不伴心肌梗死患者的左心室乳头肌。本研究证实室性心律失常也可源于右心室PAP。心腔内心脏超声有助于鉴别右心室心律失常的起源点。与左侧乳头肌起源心律失常相似,消融导管与乳头肌充分接触对于成功消融十分重要。左侧乳头肌心律失常患者结构异常常见,既往无心肌梗死者偶尔也可见到瘢痕形成。
本系列患者中术前MRI检查未见到延迟强化迹象。然而,MR信号的校零采用的是健康左心室心肌信号,右侧的异常可因较低的反转时间而遗漏。尽管对照组基线PVC负荷相近,PAP组患者半数存在较低的LVEF,这令人困惑,但也可能是偶然所致。与以前报道的LVEF下降和PVC负荷高的患者相一致,本研究中所有LVEF降低的患者,消融后LVEF均得到改善。
机制
右心室PAP心律失常不为心房或心室程控刺激所诱发。虽然异丙肾上腺素或猝发刺激可诱发持续性VT,提示基本机制为促发激动。这与既往报道的不伴心肌梗死的左侧乳头肌心律失常相一致。另外,VT不能被超速起搏拖带或中止,也支持自律性而非折返为其机制。本研究8例中的2例于成功消融点乳头肌基部见到浦肯野电位,提示浦肯野纤维涉及致心律失常基质,这与左侧乳头肌心律失常相似。未能在其余多数患者见到浦肯野电位不能证明未累及浦肯野纤维系统,但可反映难以达到良好接触致心律失常的乳头肌。
图1A:间隔乳头肌起源PVC 12导联心电图。电轴向下,早移行位于V3,并存在切迹。B:后乳头肌起源PVC 12导联心电图。电轴向上,胸导联晚移行位于V5
图2A:后乳头肌起源PVC的起源部位。窦性节律时出现浦肯野电位(箭头所示)。成功消融部位心内膜激动时间提前20ms。B:乳头肌PVC起源部位未记录到浦肯野电位
图3右心室和PAP相关心律失常起源的示意图