Fast adsorption of microcystin-LR by Fe(III)-modi fied powdered activated carbon*
DAI Guofei (戴国飞) , GAN Nanqin (甘南琴) SONG Lirong (宋立荣) FANG Shaowen (方少文) PENG Ningyan (彭宁彦)
1 Jiangxi Provincial Key Laboratory of Water Resources and Environment of Poyang Lake, Jiangxi Institute of Water Sciences,Nanchang 330029, China
2 Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
3 College of Life Science, Nanchang University, Nanchang 330031, China
Abstract Microcystins (MCs) are cyclic hepatotoxic peptides produced by the bloom-forming cyanobacterium Microcystis and present a public health hazard to humans and livestock. The removal of MCs from contaminated water with powdered activated carbon (PAC) has been employed as a simple and economic treatment strategy. In this study, PAC-Fe(III) was prepared and utilized for the fast and efficient removal of MCs from water. PAC-Fe(III) exhibited superior microcystin-LR (MC-LR) removal capacity and efficiency compared to the unmodi fied PAC. The MC-LR removal efficiency of PAC-Fe(III) increased with decreasing pH within the pH range of 4.3 to 9.6. PAC-Fe(III) could be reused for 3 times by methanol elution while the MC-LR removal efficiency was still over 70 percent. The removal efficiency was positively correlated to the ionic strength of water and negatively correlated to alkalinity. Natural organic matter(NOM) such as humic acid (HA) and salicylic acid (SA) generated low interference with MC-LR adsorption by PAC-Fe(III). The complexation reaction between Fe 3+ in PAC-Fe(III) and the functional groups of MCLR was suggested as the key mechanism of MC-LR removal by PAC-Fe(III). The results suggest that Femodi fied PAC is a promising material for the treatment of MC-contaminated waters.
Keyword: microcystin (MC); Fe; powdered activated carbon (PAC); microcystin removal
1 INTRODUCTION
Cyanobacterial blooms present a serious problem,threatening drinking water safety and human health worldwide (Song et al., 2007; Liu et al., 2011).Hepatotoxic microcystins (MCs) are generally formed by species of the genera Anabaena, Microcystis and Nostoc; in China, the most dominant genus is Microcystis. Exposure to MC-contaminated water may cause liver failure and cancer development in livestock and humans (Gan et al., 2010; Li et al.,2011). The World Health Organization (WHO) has established a safe guideline of 1.0 μg/L for MC-LR in daily drinking water (WHO, 1998). A maximum daily intake of 0.04 mg total MCs per kg body weight per day has also been recommended for safety (Chorus and Bartram, 1999). For these reasons, the treatment of MC-contaminated water has become an urgent issue and had received considerable attention in recent years (Chen et al., 2011, 2012).
MCs are cyclic peptides that share the structure of cyclo(D-Ala-L-X-D-erythro-ß-Methyl-D-isoAsp-LY-Adda-isoGlu-N-methyldehydro-Ala), where X and Y represent variable amino acids and Adda is shorthand for 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyl-deca-4,6-dienoic-acid. The cyclic structures of MCs consist of seven carbonyl groups,two or more free carboxyl groups and some amino groups. Due to these functional groups, MCs would form a complex with metal ions and exhibit affinity toward them (Humble et al., 1997; Yan et al., 2000;Dai et al., 2017). In our previous work, we achieved fast and effective removal of MCs with a microgel-Fe(III) complex by the immobilization of free Fe(III)onto the nanosized microgel (Dai et al., 2012). The Fe(III) in microgel-Fe(III) bound with carbonyl groups, carboxyl groups or amino groups in the microcystin molecules through a complexation reaction with a short equilibrium time. However, for large scale applications, less expensive Fe(III)-containing materials must be developed for the fast and efficient removal of aqueous MCs.
To date, many studies have focused on new technologies and methods for MC removal. TiO2photocatalysis has been widely studied for its destructive effects on different organic pollutants, and the use of TiO2for MC elimination has been shown to be a feasible and effective technology (Pelaez et al.,2011; Vilela et al., 2012). The oxidative elimination of MCs with ozone, chlorine, chlorine dioxide and permanganate has also been widely reported (Miao et al., 2010; Zhou et al., 2014), and some studies have demonstrated that the oxidation reaction product of MCs after water decontamination did not show any toxicity (Rodríguez et al., 2008). Additionally, the Fenton reagent (Fe(II)/H2O2) and photo-Fenton technology have been used in the degradation of MCs. With the help of H2O2and ultraviolet or visible radiation, Fe(II) generates hydroxyl radicals and completely degrades MCs (Zhong et al., 2009).Although much progress has been made in the development of these technologies, more work is required before they can be successfully applied to MC elimination in waterworks; signi ficant challenges are still presented by the speed or reaction equilibrium time of the methods, safety risks due to excess decontamination reagents and the high cost and complexity of related equipment and facilities(Pietsch et al., 2002; Zhang et al., 2011; Fan et al.,2013). The development of new technologies may help to overcome these obstacles.
Activated carbon is a relatively low cost adsorbent that has already been widely used in the removal of a wide range of pollutants (El-Sadaawy and Abdelwahab, 2014; Mercier et al., 2014). It has a high sorption capacity due to its porous structure that yields a high surface area per unit mass. Also,activated carbon has the potential to be used in largescale MC removal in waterworks (Lambert et al.,1996; Ho et al., 2011). Furthermore, it has also been widely used for the adsorption of metal ions in previous studies (Lo et al., 2012; De Lima et al.,2014); the adsorption capacity for the metal ions is determined by the number of hydrophilic groups on the surface of the activated carbon (Uchida et al.,2000). These hydrophilic groups, such as protonated C-OH2+, neutral C-OH and ionized CO-groups, have mutual chemical interactions with metal ions,resulting in the greater stability of the reaction product. In the present study, we use PAC modi fied with Fe(III) for MC removal. We aim to develop a simple and low cost technology for the fast and effective treatment of MC-contaminated water.
2 MATERIAL AND METHOD
2.1 Material
The MC-LR was provided by Express Technology Company (Beijing, China). HPLC grade methanol used as the HPLC mobile phase was purchased from Fisher (Loughborough, UK). KCl, Na2CO3, humic acid (HA), salicylic acid (SA) and powered activated carbon were obtained from Shanghai Chemical Reagent Company. All other chemicals were analytical grade.
2.2 Modi fication of PAC by Fe(III)
For the modi fication of PAC, PAC was added into Fe(NO3)3·9H2O solution (pH=6.4, T=25°C) in a 25-mL dark glass reaction bottle with magnetic stirring.The final concentrations of PAC and Fe3+were 1.6 g/L and 0.56 g/L, respectively (For the ratio of PAC: Fe3+,please see Fig.S1 in the supporting information).After reacting for 12 h in a dark constant temperature incubator, the Fe(III)-modi fied PAC (PAC-Fe(III))was obtained by centrifugation at 7 400× g for 3 min and washed twice by 10 mL Milli-Q water to remove the residual free iron. The surface zeta potentials of PAC-Fe(III) at different pH values were measured on a Zen 3600 Malvern Zetasizer Nano system (Malvern Instruments, U.K.) (Fig.S2). PAC-microgel-Fe(III)was also prepared for comparison. The microgel was synthesized as our previous report (Dai et al., 2012);PAC (2 g/L) was added into a microgel solution(0.2 mg/L) to adsorb nano-microgel particles for 12 h.The microgel-immobilized PAC was obtained by centrifugation at 7 400× g for 5 min. It was then modi fied by Fe(III) as described above to obtain the product PAC-microgel-Fe(III).
2.3 Removal of MC-LR by PAC-Fe(III)
Initial concentrations of PAC-Fe(III) and MC-LR were 0.15 mg/mL and 10 μg/mL, respectively. PACFe(III) and MC-LR interacted in 1 mL water in a 1.5-mL dark glass bottle with constant shaking. Each experiment was conducted in duplicate. Samples were collected at 5 min, 15 min, 30 min, 60 min, 2 h, 4 h and 6 h, and different pH values (4.3, 5.7, 7.1, 8.4, and 9.6)were tested. The effect of ionic strength on MC-LR removal was studied by adding different concentrations of KCl (0.01, 0.1, and 1 mol/L) into the MC-LR solution at pH 7.4. Alkalinity was also studied as the carbonate ion has the potential to bind with Fe and interfere with MC-LR removal; Na2CO3was added to the MC-LR solution with final concentrations of 0.5 mmol/L, 1.0 mmol/L and 1.5 mmol/L to study the effect of alkalinity. The pH of the Na2CO3solutions along with the Mili-Q water control group were all adjusted to 7.1–7.3 with 0.1 mol/L HCl and 0.1 mol/L NaOH solution. Humic acid (HA) was chosen to represent a high molar mass, hydrophobic natural organic matter (NOM), while salicylic acid (SA) was chosen to represent a low molar mass, hydrophilic NOM. The final experimental concentrations of HA and SA were 5 and 20 mg/L at pH 7.3. To compare the efficiency of different MC-LR removal strategies in natural water, four different materials PAC, PAC-Fe(III),PAC-microgel-Fe(III) and TiO2(irradiation with a 365-nm UV light source, F4T5-BLB, 4W× 2) were applied in eutrophic natural water from Lake Donghu in Wuhan, China at the same concentration (0.1 mg/mL). MC-LR was added to the natural water (pH=7.82,T=17.4°C, TP=0.117 mg/L, TN=2.029 mg/L, COD=17.5 mg/L) to produce a final concentration of 8 μg/mL.
2.4 MC-LR recovery from PAC-Fe(III)
A MC-LR regeneration test was carried out to clarify the fate of MC-LR removed by PAC-Fe(III).PAC-Fe(III) was used to remove MC-LR at various pH levels (4.3, 5.7, 7.1, 8.4 and 9.6). MC-LR was removed to regenerate PAC-Fe(III) through centrifugation and washing in 2 mL methanol for 5 min. The MC-LR concentration of the methanolwashed solution was then analyzed by HPLC. The MC-LR recovery rate was calculated as:
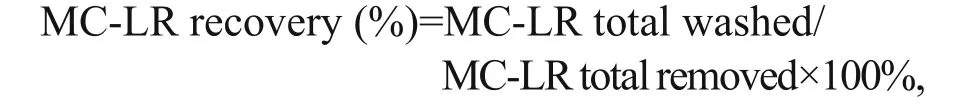
where MC-LR total removed was the amount of MCLR eliminated by PAC-Fe(III) from the solution at different pH levels and MC-LR total washed was the amount of MC-LR removed by methanol from PAC-Fe(III).
2.5 MC analysis
MCs were sampled for HPLC analysis on a Shimadzu LC-10A system with two LC-10A pumps and a UV detector. The column used was a Shimadzu shim-pack (CLO-ODS 6.0×150) with a mobile phase of 60% methanol (HPLC grade) mixed with 40%KH2PO4solution (0.05 mol/L, pH=3); the flow rate was 1 mL/min. The separation was performed at a constant temperature of 40°C with an injection volume of 10 μL. The indirect competitive ELISA procedure described by Hu was also employed when MCs of the sample was below HPLC detection limit(Hu et al., 2008).
2.6 Statistical analysis
Data are presented as mean and standard error of mean unless otherwise stated. The results were analyzed using the Levene test on equality of variances, and One-Way ANOVA analysis by Tukey test for performing statistical comparisons. All statistical analyses were conducted using the Origin software version 7.5. All figures were also drawn using Origin 7.5.
3 RESULT AND DISCUSSION
3.1 PAC modi fication by Fe(III) and its MC-LR removal ability
PAC-Fe(III) (500 mg/L) was placed in distilled water for 24 h at a wide range of pH values, and the concentration of total Fe in the water phase after its adsorption to PAC was measured by atomic absorption spectroscopy, demonstrating less than 0.01 mg/L at all pH values, indicating a low risk of Fe(III) leakage from PAC-Fe(III). However, for large scale use of PAC-Fe(III) in natural waters , the dosage should still be limited. As previously reported, Fe pollution in Taihu Lake increased the biomass of cyanobacteria,which may result in more MC production in the natural waters (Xu et al., 2013)
Without modi fication by Fe(III), PAC required 16 to remove approximately 75% of the total MC-LR(Fig.1). After modi fication by Fe(III), however, 80%of the total MC-LR was removed in 5 min. We hypothesized that greater amounts of Fe(III)immobilized on PAC would signi ficantly increase the removal efficiency of MC-LR; to test this, we performed MC-LR removal experiments using PAC-microgel-Fe(III) because the PAC-microgel contains more carbonyl groups than PAC and therefore can accommodate more immobilized Fe(III). Compared with PAC-Fe(III), the MC-LR removal rate and efficiency of PAC-microgel-Fe(III) is dramatically increased (Fig.1); however, the cost of PAC-microgel-Fe(III) is much greater. To balance removal efficiency and material costs, we have chosen to study PACFe(III) instead of the higher-cost PAC-microgel-Fe(III) in the present work.
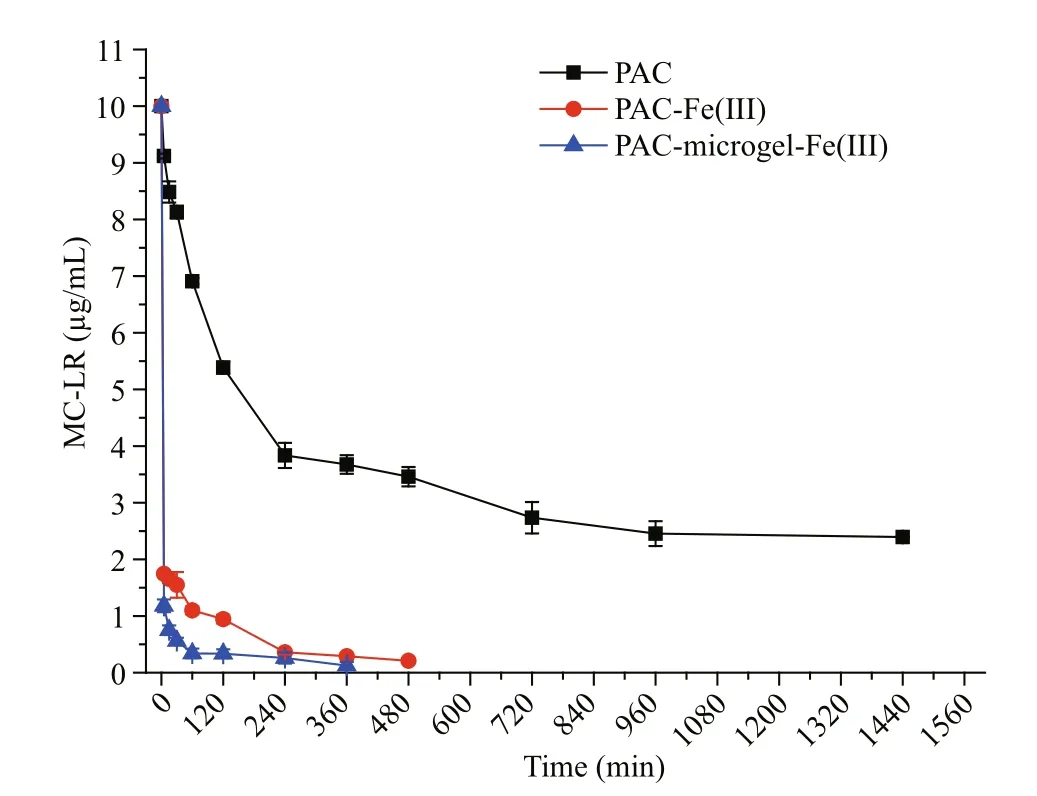
Fig.1 MC-LR removal by PAC, PAC-Fe(III) and PAC-microgel-Fe(III)
The dramatic increase in the MC-LR removal rate generated by Fe(III) may be indicative of a different removal mechanism. Before modi fication, the adsorption of MC-LR by PAC relied on its organic porous structure. After modi fication, Fe(III) is immobilized on PAC by functional groups including C-OH2+, C-OH and CO-. As MCs contain many carbonyl, carboxyl and amino groups, MC-LR is removed through its easy complexation with Fe(III)on PAC. A comparison of the different rates of adsorption of PAC and PAC-Fe(III) indicates that the fast initial removal of MC-LR by PAC-Fe(III) in the first 15 min of interaction was a result of Fe(III)complexation with MC-LR functional groups.Concurrently, the porous structure of PAC in PACFe(III) also likely functioned to generate additional MC-LR removal, though its reaction rate was much slower than the Fe(III)-MC-LR complexation. These results are in agreement with our previous work which indicated a faster MC-LR removal rate for microgel-Fe(III) compared to the porous-structured microgel alone.
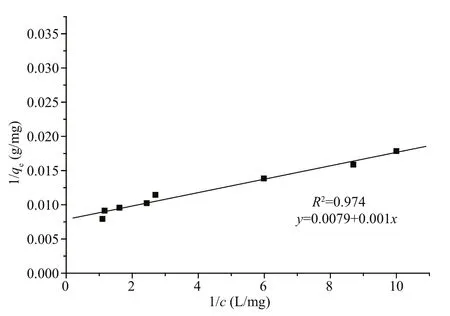
Fig.2 MC-LR Langmuir adsorption isotherms for PACFe(III)
To clarify the possible mechanisms of removal of MCs by PAC-Fe(III), we used methanol to elute adsorbed MC-LR from PAC-Fe(III) and determined the MC-LR recovery. At a wide range of pH, the MCLR recovery ranged from 83% to 98%, demonstrating that adsorption is the major mechanism of MC-LR removal by PAC-Fe(III) and that Fe(III) does not trigger the degradation of MC-LR. Many studies have reported the Fe(II)/Fe(III)-initiated degradation of MCs by a Fenton or Fenton-like process (Zhong et al.,2009; Fang et al., 2011); however, no evidence was found for these degradation processes, and they could be ignored or excluded in our study. Therefore, the MC-LR removal by PAC-Fe(III) is considered to be a chemical/physical adsorption process. Also, the PACFe(III) could be reused 3 times by methanol elution,after which the MC-LR removal efficiency was still over 70% (Fig.S3). This would make some sense from an economic perspective when the material is used in large scale. As the immobilization of Fe3+onto PAC was very simple, no other chemicals were needed. The cost of PAC-Fe(III) preparation would be low. Also, PAC-Fe(III) could be used just as the common PAC in the waterworks, no additional facilities should be added. These all indicated a low cost of PAC-Fe(III) used in treating MC-contaminated waters in the waterworks.
3.2 Advantages of PAC-Fe(III) compared to traditional PAC
The adsorption isotherms of PAC-Fe(III) for MCLR are shown in Fig.2 and Table 1. The isotherms are in the form of the Langmuir isotherm model (Huang et al., 2007),

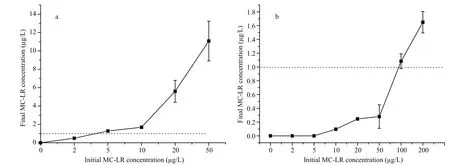
Fig.3 Final aqueous MC-LR concentrations as a function of the initial concentration with treatment by (a) PAC-Fe(III) and(b) PAC

Table 1 Isotherm parameters for Fe(III)-modi fied activated carbon
where qeis the MC-LR removal capacity by PAC or PAC-Fe(III), c is the equilibrium MC-LR concentration, qmrepresents the maximum adsorption or removal capacity and KLis a constant related to adsorption energy. PAC-Fe(III) adsorbed more MCLR than PAC at all equilibrium MC-LR concentrations,and the maximum adsorption capacity of PAC-Fe(III)was more than twice that of PAC (for PAC qm=58.82 mg/g, See Dai et al., 2012). MC-LR removal by unmodi fied PAC was slow because it relied on MC-LR adsorption in the PAC mesopore volume.After modi fication, the Fe(III) on the surface and in the mesopores of PAC had a strong affinity for the carbonyl, carboxyl groups or amino groups of the MC; therefore PAC-Fe(III) had an additional MC-LR removal pathway (complexation between Fe(III) and MC-LR). In addition to increasing the maximum adsorption capacity for MC-LR, this new pathway functions faster than the mesopore adsorption pathway, demonstrating the signi ficant value of Fe modi fication for MC-LR removal.
MC removal during water disposal was simulated by using a 16 mg/L application dose of PAC or PACFe(III) and a limited experimental time of 20 min to ascertain whether the concentration of MC could be maintained below the WHO safety guideline value(1 μg/L). PAC could keep the MC-LR concentration below 1 μg/L when the initial MC-LR concentration did not exceed 3.6 μg/L (Fig.3a); PAC-Fe(III)achieved this standard for MC-LR concentrations below approximately 95 μg/L (Fig.3b). Furthermore,in the experiments with unmodi fied PAC, the final MC-LR concentration rose sharply as the initial concentration increased; an increase in the initial concentration from 5 to 50 μg/L increased the final MC-LR concentration after disposal from 1.2 to 11 μg/L. In contrast, PAC-Fe(III) maintained the final MC-LR concentration in the narrow range of 0.1 to 1.6 μg/L, even when the initial concentration was increased from 5 to 200 μg/L. These results demonstrate that PAC-Fe(III) can ensure safety in MC-contaminated water disposal, even in the case of extreme situations that cause high MC concentrations such as cyanobacterial blooms.
3.3 Effect of pH on MC-LR removal by PAC-Fe(III)
To study the effect of pH on MC-LR removal by PAC-Fe(III), batch adsorption experiments were performed at different pH levels from 4.3 to 9.6. The maximum removal efficiency was observed at pH 4.3,and MC-LR removal efficiency increased as pH decreased from 9.6 to 4.3 (Fig.4). The initial adsorption rate in the first 5 min was signi ficantly in fluenced by pH; the rate at pH 4.3 was 12.37 mg/(g·min), while it was 8.80 mg/(g·min) at pH 9.6.
Due to the carboxyl, carbonyl and amino groups in the MC-LR structure, the overall charge of MC-LR is sensitive to pH. Below pH 2.09, the cationic species[(COOH)2(NH2+)] is produced, and the overall charge of MC-LR is positive. MC-LR exists in a neutral state in the narrow pH range of 2.09–2.19. Above pH 2.19,the anionic species [(COO−)2(NH2+)] and [(COO−)2(NH)] are produced, and MC-LR is negatively charged(De Maagd et al., 1999; Klein et al., 2013). In this study, MC-LR was negatively charged because the experimental pH ranged from 4.3 to 9.6. The effect of pH on the charge of PAC-Fe(III) was also studied; the isoelectric point of PAC-Fe(III) was approximately pH 6.8 (Fig.S2). When the pH was below 6.8, PACFe(III) was positively charged, and the positive charge increased with decreasing pH. Above pH 6.8, PACFe(III) was be negatively charged, and the negative charge increased with increasing pH. Since MC-LR was negatively charged in our study, the electrostatic attraction between MC-LR and PAC-Fe(III) was enhanced below pH 6.8 (when PAC-Fe(III) is positively charged), resulting in superior MC-LR removal efficiency. At pH values above 6.8, both PACFe(III) and MC-LR are negatively charged, resulting in electrostatic repulsion and reduced MC-LR adsorption efficiency. Additionally, the negativelycharged sites of MC-LR generate repulsion within the molecule, resulting in the stretching of MC-LR structure (Sathishkumar et al., 2010; Klein et al.,2013). Therefore, decreasing the pH would reduce MC-LR repulsion and stretching, leading to smaller MC-LR molecules that are more suitable for adsorption in the PAC pore structures of PAC-Fe(III).
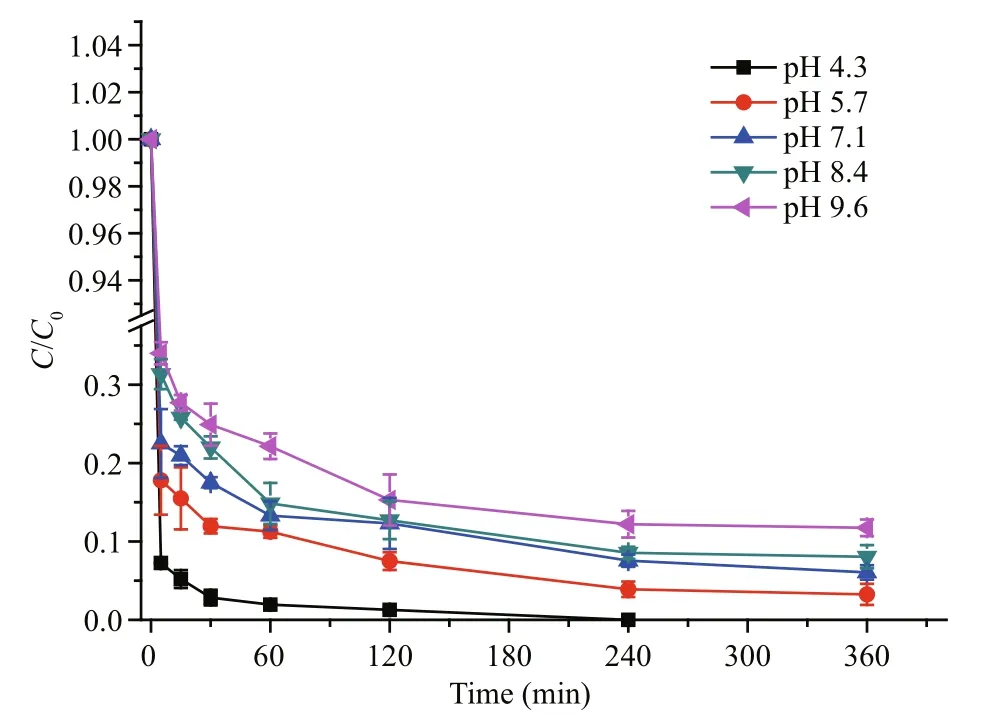
Fig.4 MC-LR removal dynamic by PAC-Fe(III) at different pH levels
3.4 Effect of ionic strength and alkalinity
Various concentrations of KCl (0.01, 0.1 and 1 mol/L) at pH 7.4 were used to study the effect of ionic strength on MC-LR removal by PAC-Fe(III). The MC-LR removal efficiency was enhanced with increasing ionic strength (Fig.5a). At pH 7.4, both PAC-Fe(III) and MC-LR were negatively charged,creating electrostatic repulsion between them. With the addition of KCl, the K+surrounding PAC-Fe(III)and MC-LR would shield their negative charges,diminishing some of the intermolecular repulsion between them and improving MC-LR adsorption. On the other hand, the shielding effect would also interfere with the intramolecular repulsion generated by the functional groups of MC-LR; through coiling,folding, or compression, the K+ions would enable MC-LR molecules to access a larger surface area fraction of PAC-Fe(III), facilitating its transport through the narrow PAC pores (Campinas and Rosa,2006). Although the addition of KCl has a positive impact on MC-LR adsorption, a 100-fold increase in KCl concentration from 0.01 to 1 mol/L only increased the MC-LR adsorption rate by 6.26% in the initial 5 min (from 10.22 to 10.86 mg/(g·min)).
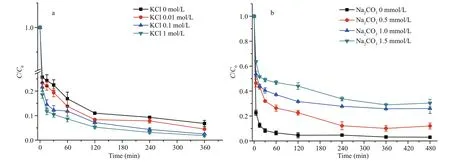
Fig.5 MC-LR removal dynamic in the presence of various concentrations of (a) KCl and (b) Na 2 CO 3
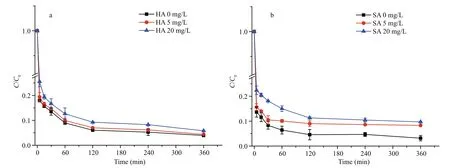
Fig.6 MC-LR removal dynamic by PAC-Fe(III) in the presence of (a) humic acid and (b) salicylic acid
To understand the effect of alkalinity on MC-LR removal by PAC-Fe(III), experiments were conducted with added Na2CO3while keeping pH constant. As shown in Fig.5b, the addition of 0.5 mmol/L Na2CO3decreased the MC-LR removal rate by 30.65% (from 10.31 to 7.15 mg/(g·min)), while a 1.5 mmol/L addition further decreased the rate to 4.85 mg/(g·min).Water alkalinity is mainly in fluenced by the concentrations of bicarbonate (HCO ˉ3), carbonate(CO23ˉ) and hydroxide (OH–) ions; these ions would affect MC removal technology such as TiO2photocatalysis by diminishing radical species (Pelaez et al., 2011). In our alkalinity experiments at pH 7.2,the predominant species was bicarbonate ion, while carbonate ion would predominate at high pH levels above 10. Both bicarbonate and carbonate have a strong affinity for Fe, and would compete with MCLR for Fe complexation. Therefore, the addition of Na2CO3signi ficantly decreases the initial MC-LR removal rate from water because the added bicarbonate and carbonate ions occupy many of the MC-LR adsorption sites on PAC-Fe(III). In addition, the adsorption of bicarbonate and carbonate ions increase the negative charge on PAC-Fe(III), resulting in enhanced electrostatic repulsion between MC-LR and PAC-Fe(III) and a subsequent reduction in the MCLR removal rate. In natural waters, the release of bicarbonate, carbonate and hydroxide ions would also cause increase solution pH, further decreasing the efficiency of MC-LR removal by PAC-Fe(III).
3.5 Effect of NOM
Hydrophobic humic acid (HA, ~3 000 Da) and hydrophilic salicylic acid (SA, 138 Da) were selected as model NOM to study the effects of NOM on MCLR removal efficiency. HA and SA contain functional groups including carbonyl and carboxyl groups that have the potential to complex with Fe and compete with MC-LR for adsorption on PAC-Fe(III). In addition, as natural organic molecules, HA and SA can be adsorbed by the organic carbon pore structure of PAC-Fe(III), possibly leading to reduced MC-LR removal.
Figure 6a shows the effect of HA on MC-LR removal; the average MC-LR removal in 360 min was reduced by 0.8% at a NOM concentration of 5 mg/L and by 3.1% at 20 mg/L. This small effect may be explained by competition between MC-LR and NOM for Fe complexation. A single ring of the MC-LR cyclic structure contains seven carbonyl groups, two or more free carboxyl groups and some amino groups;these functional groups may react together with Fe at the same time (e.g., one carbonyl group and its neighboring carbonyl or carboxyl group binding with a single Fe(III)). In this way, the MC-LR affinity for Fe(III) in PAC-Fe(III) would be stronger that of other NOM such as HA. Furthermore, the molecular weight of HA is much larger than that of MC-LR. This indicates a large size for HA, which, compared to MC-LR, makes HA less likely to be adsorbed in the small organic pores of PAC-Fe(III). For these reasons,HA did not strongly interfere or compete with MCLR during adsorption by PAC-Fe(III).
At the same concentrations (5 and 20 mg/L), the number of molecules of the low molecular weight(138 Da) SA was signi ficantly larger than HA or MCLR (994.5 Da). Due to the much larger number of molecules, SA more strongly interfered with MC-LR removal by PAC-Fe(III) compared to HA (Fig.6b).Additionally, the small size of SA molecules allow them to fit in the narrow organic pores of PAC-Fe(III),further interfering with MC-LR adsorption. However,as SA contains only one carboxyl and hydroxyl group,its affinity for Fe and subsequent competition ability would be much weaker than those of MC-LR. Even the SA (20 mg/L) molar concentration was 14.4 times that of MC (10 mg/L), it only showed a limited interfere with MC-LR removal by PAC-Fe(III), with a decrease of removal rate by 7%.
3.6 Comparison of MC-LR removal by PAC, PACFe(III), PAC-microgel-Fe(III), and TiO 2 in natural waters
As shown in Fig.7, in the first 5 min the removal efficiencies of the four materials, PAC, PAC-Fe(III),PAC-microgel-Fe(III) and TiO2(with UV light) were 19.7%, 62.5%, 94.4% and 18.3%, respectively. After 4 h, the removal efficiency was 62.9%, 76.0%, 99.2%and 62.4%, respectively. MC-LR removal by TiO2(with UV light) was similar to PAC in the first 5 min.However, TiO2completely destroyed MC-LR within 24 h. Both PAC-Fe(III) and PAC-microgel-Fe(III)exhibited fast and efficient MC-LR removal. The natural water contained many pollutants including organics and nutrients. These pollutants, along with bicarbonate and carbonate ions, can bind to the active Fe sites in PAC-Fe(III) to reduce MC-LR adsorption.Our results demonstrate that comparing to other materials, the Fe-modi fied materials showed a short equilibrium time and fast removal rate. And the interference of these pollutants with Fe(III)-modi fied materials such as PAC-Fe(III) and microgel-Fe(III)was slight. Therefore, Fe-modi fied materials including PAC-Fe(III) are promising for large-scale application in MC-contaminated water puri fication.
4 CONCLUSION
PAC-Fe(III) was developed for fast removal of microcystin-LR. In this study, we con firmed the stability of the synthesized PAC-Fe(III). Maximum MC-LR adsorption capacity of PAC-Fe was about twice that of PAC. To control MC-LR concentration below the WHO guideline, initial MC-LR concentration for PAC-Fe(III) should not be above 95 μg/L, while for PAC, not higher than 3.6 μg/L. MCLR removal efficiency by PAC-Fe(III) increased with pH decreasing. Surface charge of PAC-Fe(III) as a function of pH was an important factor for MC-LR removal. The high ionic strength of the water was found to have a positive effect on MC-LR adsorption onto PAC-Fe(III), while the alkalinity has a negative impact. The natural organic matter (NOM) such as humic acid (HA) and salicylic acid (SA) have little interference with MC-LR adsorption by PAC-Fe(III).Complexation reaction between Fe3+of PAC-Fe(III)and functional groups of MC-LR was suggested as the key mechanism of MC-LR removal by PAC-Fe(III).
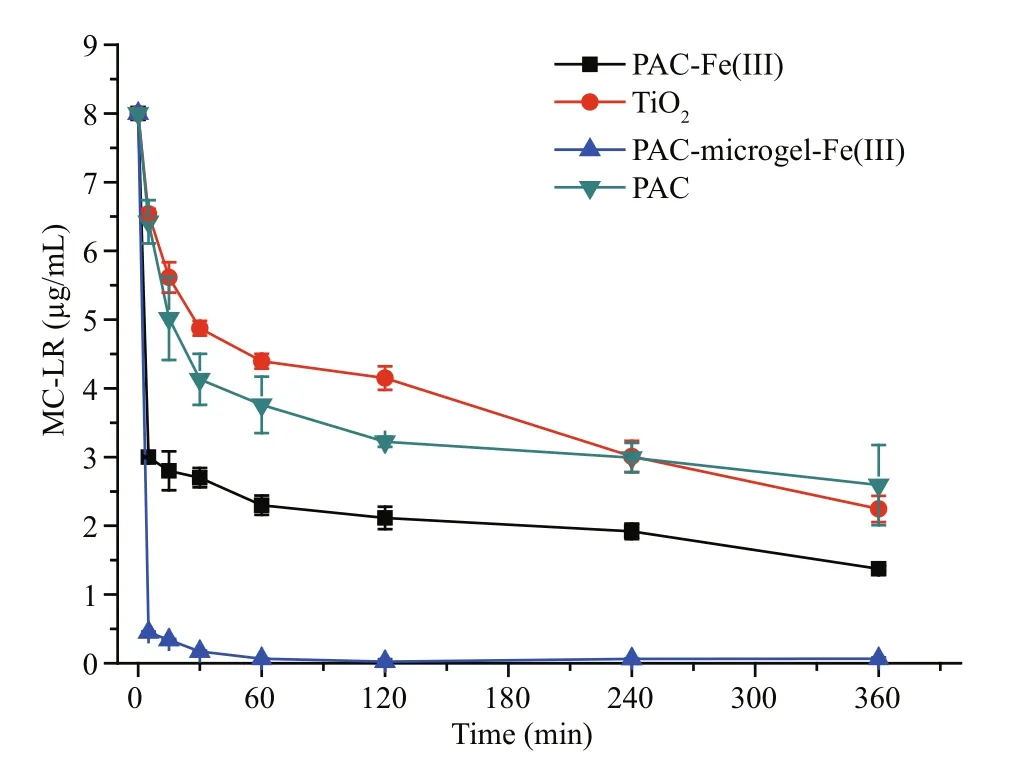
Fig.7 MC-LR removal by PAC, PAC-Fe(III), PAC-microgel-Fe(III) and TiO 2 in natural Donghu water
5 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2018年4期
Journal of Oceanology and Limnology2018年4期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Effects of seawater acidi fication on the early development of sea urchin Glyptocidaris crenularis*
- Dietary effects of A zolla pinnata combined with exogenous digestive enzyme (Digestin™) on growth and nutrients utilization of freshwater prawn, Macrobrachium rosenbergii(de Man 1879)
- Preliminarily study on the maximum handling size, prey size and species selectivity of growth hormone transgenic and non-transgenic common carp Cyprinus carpio when foraging on gastropods*
- Hydrodynamic characteristics of the double-winged otter board in the deep waters of the Mauritanian Sea*
- De novo transcriptome sequencing reveals candidate genes involved in orange shell coloration of bay scallop Argopecten irradians*
