Hepatoprotective effect of Opuntia dillenii seed oil on CCl4 induced acute liver damage in rat
Mohamed Bouhrim, Hayat Ouassou, Mohamed Choukri, Hassane Mekhfi, Abderrahim Ziyyat, Abdelkhaleq Legssyer, Mohammed Aziz, Mohamed Bnouham✉
1Laboratory of Physiology, Genetics and Ethnopharmacology, Department of Biology, Faculty of Sciences, University Mohamed Ist, Oujda, Morocco
2Faculty of Medicine and Pharmacy, Oujda, Morocco
3Biochemistry Laboratory, Central Laboratory Service - CHU, Mohammed VI, Oujda, Morocco
1. Introduction
In the human body, the liver is considered important among the largest organs, it takes care of several functions, like the process of metabolism that is essential for life[1]. It is always vulnerable to different toxic molecules of foreign origin due to its location in the human body. These xenobiotic absorbed by the intestine pass first through the liver, which makes it threatened by diseases[2].Currently, many people suffer from liver diseases induced by several hepatotoxic agents; among these agents are alcohol, infections and chemicals like carbon tetrachloride (CCl4) and paracetamol[3,4].Liver affections have become a global problem, and caused every year up to 20000 deaths arise due to liver affections[5]. Treatment of liver affections with conventional medications may be inadequate or have adverse effects[6]. That is why it is very important to replace chemical drugs with herbal products.
CCl4is a toxin among the oldest toxins, and is most used for experimentally induced liver injury in laboratory animals[7]. A hepatotoxic agent that causes liver cell damage is comparable to that of the viral infection. When it enters the liver, CCl4is encountered as a xenobiotic, and is converted into two free radicals, which are trichloromethyl and trichloromethyl-peroxyl by the microsomal system dependent on monooxygenase P-450. These two radicals cause lipid peroxidation, which provokes serious liver injury[8].Antioxidants have been reported to prevent oxidative injury to the liver, and they can prevent the risk of liver disease[9]. This is why we notice that there is a rising interest in natural products that have an antioxidant property, in order to use them to prevent liver pathologies related to oxidative stress[10].
Opuntia dillenii seed oil (ODSO) is an oil that is marked by a rise degree of unsaturated fatty acids, wherein linoleic acid is the prominent fatty acid, β- sitosterol is the sterol marker while γ-tocopherol is the only vitamin E in oil[11]. Antioxidant[12] and anti-inflammatory[13]activities are the only studies that have been done on this oil. The aim of this work is to evaluate the hepatoprotective effect of ODSO on Wistar rat with hepatotoxicity provoked by CCl4.
2. Materials and methods
2.1. Chemicals
CCl4was purchased from Sigma chemicals, USA. Alanine amino transferase (ALT), aspartate amino transferase (AST), alkaline phosphatase (ALP), total cholesterol, triglycerides, high density lipoprotein (HDL-c), bilirubin direct and total, glucose, total protein,urea, creatinine and acid uric kits were purchased from Biosystems,Spain. All other reagents used in this study were of high quality and analytical grade.
2.2. Collection of plant material
The fresh fruits of Opuntia dillenii used in this study were collected in February 2016 from regions in Essaouira, Morocco. The specimen was deposited at Mohammed First University, Oujda, Morocco,under the reference number HUMPOM 351 after its identification by the expert botanist Mohammed Fennan, from the scientific institute of the University Mohammed Ⅴ.
2.3. Preparation of Opuntia dillenii seeds powder
Fruits of Opuntia dillenii were peeled then the seeds were separated from the fruit, cleaned with the distilled water, dried in the oven at 37 ℃ for three days and then ground with a blender, until a fine and homogeneous powder was obtained and conserved at -20 ℃ until use.
2.4. Oil extraction
An amount of 100 g of seed powder was added in 500 mL of petroleum ether, and then the totality was shaken for 24 h under an ambient temperature. After filtration, the organic solvent was removed on a rotary evaporator under temperature at 40 ℃. Moreover, the oil was dried and stored at 4 ℃.
2.5. Animals
Twenty-four healthy adult Wistar rats [(200 ± 50) g, 10 weeks old]were employed in this research. Animals were taken from the animal house of the Faculty of Sciences, Mohammed First University,Oujda, Morocco. The rats were grouped in plastic cages in a wellventilated room with soft bedding and accessibility to water and food ad libitum in an environmentally controlled room (22-26 ℃, with a 12/12 h light/dark cycle). The rats were adapted one week preceding treatment.
All rats were cared for in compliance with the internationally accepted Guide for the care and use of laboratory animals, published by the US National Institutes of Health (NIH Publication No. 85-23,Revised in 1985).
2.6. Experimental procedure
One week after the adaptation, the rats were arbitrarily grouped into four groups, each including six rats, and treated as follows:The normal control group and CCl4control group received distilled water (10 mL/kg). Moreover, the ODSO control group and ODSO + CCl4group received ODSO (2 mL/kg). The animals of groups CCl4control group, and ODSO+ CCl4group received CCl4intraperitoneally at a dose 1 mL/kg body weight (25% CCl4,solubilized in olive oil; v/v) once a week for two weeks of treatment in order to induce liver injury. Body weights of the rats were measured before and after the treatment. All animals were treated and observed daily for two weeks.
2.7. Blood sampling
Twelve hours after the last intraperitoneal injection of CCl4, blood samples from these treated rats were drawn from their carotid arteries after being anesthetized under a light ethyl ether. Then the blood was centrifuged at 3000 rpm for 10 min and at 4 ℃ to obtain the plasma, thereafter the plasma was conserved at -20 ℃ until analysis. In addition, liver weights were measured and then used for making liver homogenate (10% w / v) in normal saline (pH 7.0) and conserved at -20 ℃ for biochemical analyzes.
2.8. Serum biochemical parameters determination
ALT and AST were estimated by IFCC method[14], ALP by paranitrophenyl phosphate method[15], total cholesterol by enzymatic method[16], triglycerides by glycerol phosphate oxidase method[17],HDL-c by the ultra HDL assay, total bilirubin by diazonium saltmethod[18], direct bilirubin by diazoreaction method[19], plasma glucose by the oxidase-peroxidase method[20], urea by Urease-GLDH method[21], creatinine by kinetic alkaline picrate method[22],uric acid by uricase-peroxidase method[23] were analyzed following to the reported methods. All analyses were performed in triplicate for every sample on the ARCHITECT c-Systems autoanalyzer(Hamburg, Germany) by using commercial reagent kits.
For low-density lipoprotein (LDL-c), its rate was estimated according to Friedewald et al.[24]. using the following formula:LDL-c = total cholesterol – [HDL-c + very low-density lipoprotein(VLDL-c)]
In addition, VLDL-c was calculated according to the formula of [24],as follows:
VLDL-c = triglycerides/5
2.9. Determination of malondialdehyde (MDA)
In this study, hepatic lipid peroxidation was determined by the Buege& Aust method[25], and this method measures the level of TBARS production as described by Iqbal et al.[26]. After the preparation of the homogenate, 0. 5 mL of the homogenate was added in 0. 5 mL of trichloroacetic acid (30% w/v) and the whole was subject to a centrifugation during 10 min at 3500 rpm and 4 ℃. Then 1 mL of thiobarbituric acid (0.67% w/v) was added to 1 mL of the supernatant obtained and the whole was placed in a boiling water bath during 10 min. Then to stop the reaction, the mixture was placed in an ice bath. At the end of this reaction, the spectrophotometer was used to measure the mixture of assays at 535 nm, and the calculation was made using the next molar extinction coefficient: 1.56×105M/cm-1.
The results were expressed in nanomoles of MDA produced per gram of tissue.
2.10. Statistical analyses
The results were expressed as the mean ± SEM and were subjected to statistical analyses using Graph Pad Prism 5 Software, San Diego,CA, USA. Multiple-group comparisons were analyzed by one-way analysis of variance (ANOVA). P < 0.05 was considered as statistical significant difference.
3. Results
3.1. Effect of ODSO on variation in relative liver weight and body weight gain
The variation in body weight of rats at the end of the experiment was used to assess the growth performance of all the groups studied.The relative weight of the liver and the body weight gain for rats of the groups studied were shown in Table 1. CCl4administration at the end of the first and second week significantly attenuated weight gain and increased the relative weight of the liver in untreated rats (CCl4control group) compared to the healthy rats. Moreover, the quotidian treating of hepatotoxic rats by ODSO (ODSO + CCl4group) during the two weeks markedly improved growth performance. Indeed, the treatment of hepatotoxic rats (ODSO + CCl4group) with ODSO showed a significant reduction in the relative liver weights and an increase in body weight gain compared with the CCl4control group rats. Furthermore, the feeding of ODSO alone did not affect the growth performance in healthy rats.
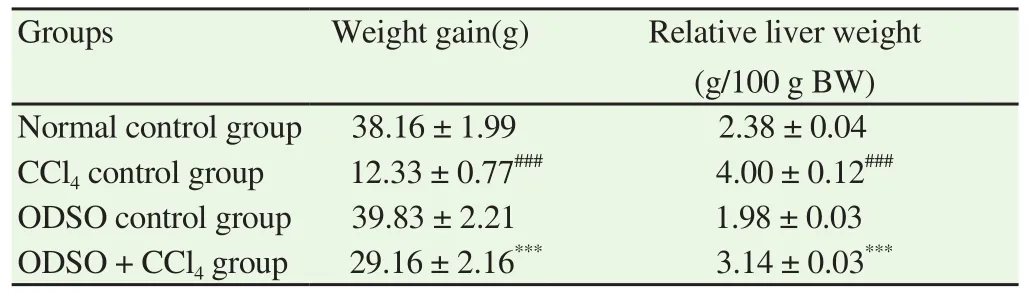
Table 1 Effect of ODSO on the growth parameters in CCl4-intoxicated rats.
3.2. Effect of ODSO on plasma hepatic markers (AST, ALT and ALP) in hepatotoxic rats.
The intoxication of the liver by CCl4was confirmed by measuring the activity of AST, ALT and ALP in the plasma (Figure 1). The CCl4injection of rats induced a significant rise in the activity of ALT, AST and ALP in comparison to the healthy rats. However, the feeding of rats with ODSO at a dose of 2 mL/kg significantly attenuated the elevation of these parameters compared to CCl4control group rats.Furthermore, the intake of ODSO alone did not affect the hepatic markers in healthy rats.
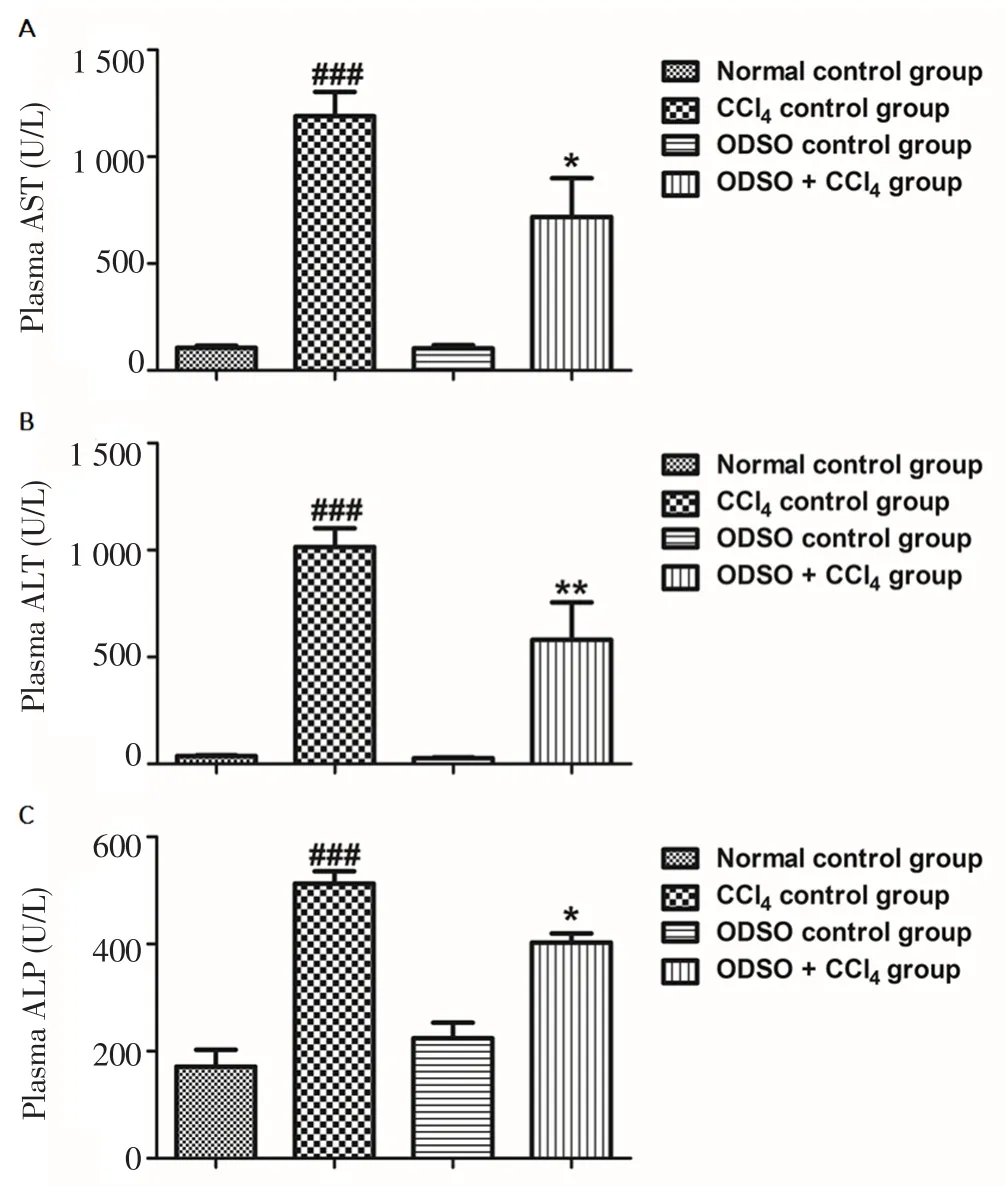
Figure 1. Effect of ODSO on CCl4-induced alterations in plasma hepatic markers.
3.3. Effect of ODSO on plasma direct and total bilirubin in hepatotoxic rats
The activity of ODSO administration on direct and total bilirubin level in all rats of the groups studied was shown in the Figure 2. The treatment of rats with CCl4induced a significant elevation in plasma bilirubin (direct and total). Moreover, the elevation in bilirubin concentration showed the dysfunction of the excretory function of hepatic cells. This abnormality was significantly corrected after the daily intake of ODSO at a concentration of 2 mL/kg. In addition, the administration of ODSO alone without induced CCl4injury did not influence the excretory function of the hepatic cells in the healthy rats.
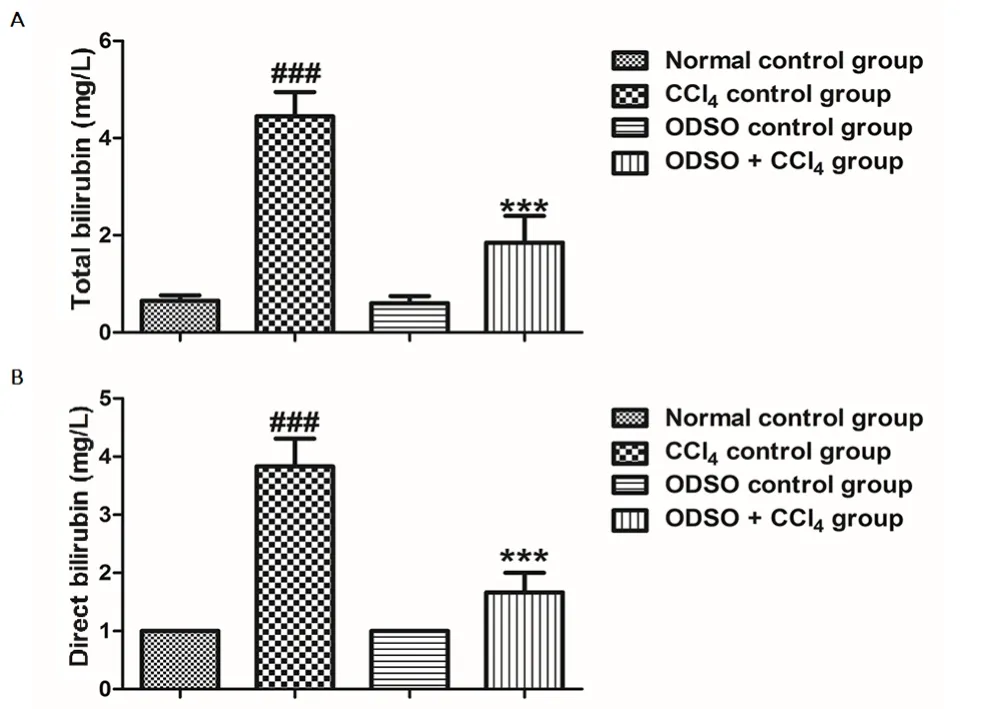
Figure 2. Effect of ODSO on plasma total bilirubin (A) and direct bilirubin(B) in CCl4-intoxicated rats.
3.4. Effect of ODSO on plasma total cholesterol, triglycerides and glucose in hepatotoxic rats
In this study, the levels of triglycerides, total cholesterol and glucose were investigated in order to evaluate the effect of ODSO on metabolic function of the liver (Figure 3). In our study, the intraperitoneal injection of CCl4to the rats induced a significant rise in the triglyceride amount, a significant decrease in glucose while the total cholesterol level was not impaired, in comparison with the normal control group rats. However, this alteration in triglyceride and glycemia level was recovered after the intake of ODSO at a concentration of 2 mL/kg. The administration of ODSO alone without CCl4-induced injury did not influence the metabolic function of the cells hepatic in the healthy rats.
3.5. Effect of ODSO on plasma lipoproteins LDL-c and VLDL-c, HDL-c in hepatotoxic rats
The effect of ODSO on the plasma lipoprotein levels was shown in Figure 4. The administration of CCl4in rats induced a significant elevation in VLDL-c amount, a significant reduction in HDL content and did not affect the LDL-c content in comparison to the healthy rats. However, this change in VLDL-c and HDL-c level was slightly recovered after administration of ODSO at a concentration of 2 mL/kg.The administration of ODSO alone without CCl4- induced injury did not influence the lipoprotein levels of the hepatic cells in the healthy rats.

Figure 3. Effect of ODSO on plasma total cholesterol (A), triglycerides (B)and glucose (C) in CCl4-intoxicated rats.
The plasma concentration of uric acid, creatinine and urea was examined as biomarkers of renal function (Figure 5). The results of our study indicated that the intraperitoneal injection of CCl4in rats triggered a significant increase in uric acid, a slight increase of urea and does not affect creatinine level. In addition, the intake of ODSO at a concentration of 2 mL/kg slightly corrected the change of uric acid and urea that was induced by CCl4.
3.6. Effect of ODSO on lipid peroxidation in hepatotoxic rats
The impact of ODSO on lipid peroxidation in rats for all groups studied was illustrated in Figure 6. The intake of CCl4provoked a substantial increase in the lipid peroxidation, as indicated by an elevated MDA level, compared to the healthy rats. The daily intake of rats (injected by CCl4) with ODSO during two weeks induced a significant reduction in the content of MDA compared to CCl4control group. However, the MDA level in the rats treated only with ODSO was similar to that of the healthy rats.
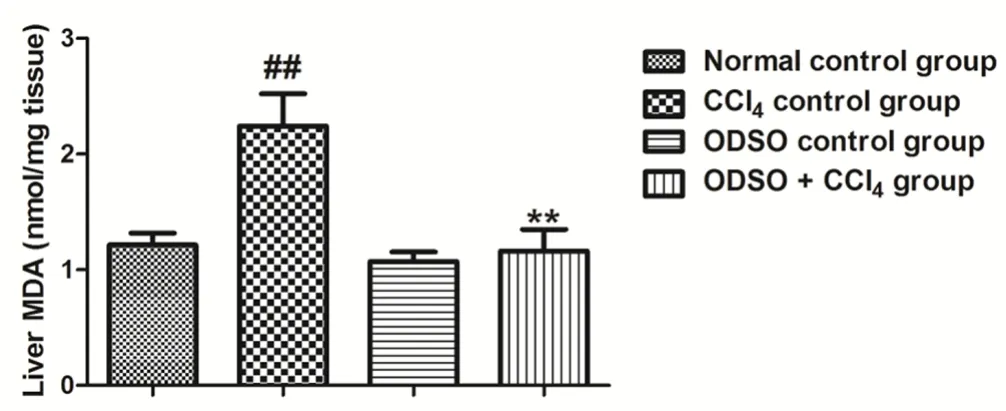
Figure 6. Effect of ODSO on lipid peroxidation in CCl4-intoxicated rats.
4. Discussion
The liver is considered important among the largest and most complex organs in the body. It is an organ that provides several physiological functions, such as protein synthesis, excretory and secretory function, storage of nutrients and maintenance of homeostasis. It also protects the body against the side effects induced by some drugs and xenobiotic[27]. The usual model used to study the hepatoprotective action of natural products is hepatotoxicity induced by CCl4solvent[28]. The latter is a chemical solvent that causes liver damage like to that produced by viral hepatitis[29]. The liver receives CCl4as a foreign toxin, and it turns it into two free radicals, which are trichloromethyl and trichloromethyl-peroxyl, by the microsomal system dependent on monooxygenase P-450. Moreover, these two free radicals cause the start of the oxidation of unsaturated lipids[8]and the oxidation of unsaturated lipids is recognized as the main causes of CCl4-provoked hepatic affections[30]. In addition, numerous compounds are recognized by their beneficial effects against the liver impairment caused by CCl4, by applying their protective action either by the attenuation of CCl4-derived free radical production process, or by their antioxidant activities[31]. In our study, the hepatoprotective effect of ODSO against CCl4provoked liver injury in healthy adult Wistar rats was evaluated. Indeed, the hepatoprotective effect of ODSO was evaluated by assessing AST, ALT and ALP activities,as enzyme markers of liver injury[32]. In this work, we also studied the activity of ODSO on the growth performance (body weight gain and relative liver weight), metabolic function (triglycerides, total cholesterol, plasmatic glucose, HDL-c, LDL-c and VLDL-c) and excretory function (total and direct bilirubin) of the liver. Moreover,we evaluated the effect of ODSO on renal excretory function (serum uric acid, urea and creatinine), besides MDA, which represents the final product of the oxidation of unsaturated lipids in liver oxidative stress. The MDA level represents an important indicator of CCl4-damaged liver[33]. In the current study, the intraperitoneal injection of CCl4to rats produced a significant rise in the plasma AST, ALT, ALP,direct bilirubin, total bilirubin, triglycerides, VLDL-c, uric acid, urea(slight increase), MDA and relative liver weight. In contrast, CCl4induced a significant reduction in plasma glucose, HDL-c and body weight gain compared to normal control group rats. In addition, CCl4did not affect the total cholesterol, LDL-c and creatinine. ODSO was found to exert a hepatoprotective effect by diminishing ALT, AST and ALP activities in plasma, in comparison with rats treated only by CCl4. In addition, ODSO ameliorated the excretory function of the liver, and this activity was shown by decreasing of the total and direct bilirubin plasma levels. Moreover, ODSO also ameliorated the metabolic function of liver by restoration of the triglycerides, glucose and VLDL-c to normal value in comparison with CCl4control group.On the contrary, the oil did not restore the HDL-c to its normal value. In addition, ODSO ameliorated the renal excretory function by a slight correction of uric acid and urea levels in comparison with rats treated only by CCl4. Finally, the oil was found also to improve the growth performance by diminishing relative liver index and raising the body weight gain. The effectiveness of a hepatoprotective drug depends principally on its capacity to maintain standard physiological function or to reduce the damaging impact caused by hepatotoxic agents[34]. Therefore, based on these results obtained from our study, ODSO showed very high hepatoprotective effect in animals with CCL4-provoked hepatotoxicity. Regarding the process by which CCL4acts in the liver, the generation of free radicals has a crucial role in the hepatotoxic effect[35]. Then, the hepatoprotective activity is related to the antioxidant activity, since it actions of damage are caused by the free radicals[36]. In a study regarding the chemical composition of ODSO, it has been reported that this oil was rich in phenolic compounds. In the same study, 11 phenolic compounds have been identified, namely, catechol, cinnamic acid,phenylpropionic acid, psoralen, syringic acid, sinapaldehyde, 3′-O-methylcatechin, (+)-gallocatechin, bisdemethoxycurcumin, 4′-O-methyl-(-)-epicatechin 3′-O-glucuronide, viscutin 1[37]. Other reports showed that this oil possesses a considerable antioxidant activity[12-37]. In that case, the protective effect of this oil against hepatotoxic CCl4can be due to its antioxidant effect, by the elimination of the free radicals resulting from the metabolism of CCl4in the liver,which are involved in the generation of liver damage. However, more studies on the active compounds of the ODSO and their biochemical mechanisms responsible for the hepatoprotective effect will be necessary.
In conclusion, based on results obtained, ODSO has a significant hepatoprotective effect on rats rendered hepatotoxic by CCl4.Moreover, this effect has been represented by the improvement of the state of the enzyme marks related to hepatocellular damage, the metabolic and excretory function of the liver and lipid peroxidation in the liver. Therefore, it may be possible to use this oil as a hepatoprotective agent.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgements
This study was financed by CNRST, Morocco (PPR2). The members in this work wish to express their appreciation to Badraoui Mustapha and Ramdaoui Karim for their technical assistance
[1] Kandimalla R, Kalita S, Saikia B, Choudhury B, Singh YP, Kalita K, et al.Antioxidant and hepatoprotective potentiality of Randia dumetorum lam.leaf and bark via inhibition of oxidative stress and inflammatory cytokines.Front Pharmacol 2016; 7: 205.
[2] Stickel F, Brinkhaus B, Krahmer N, Seitz HK, Hahn EG, Schuppan D.Antifibrotic properties of botanicals in chronic liver disease. Hepatogastroenterol 2002; 49: 1102-1108.
[3] Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al.Acetaminophen-induced acute liver failure: Results of a United States multicenter prospective study. Hepatology 2005; 42: 1364-1372.
[4] Domenicali M, Caraceni P, Giannone F, Baldassarre M, Lucchetti G,Quarta C, et al. A novel model of CCl4-induced cirrhosis with ascites in the mouse. J Hepatol 2009; 51(6): 991-999.
[5] Latha TB, Srikanth A, Kumar EK, Srinivasa MSK, Rao Y, Bhavani B.Comparative hepatoprotective efficacy of Kumaryasava and livfit against carbon tetrachloride induced hepatic damage in rats. Pharmacol Online 2009; 1: 1127-1134.
[6] Takate S, Pokharkar R, Chopad V. Hepatoprotective activity of the aqueous extract of Launaea intybacea Beauv against carbon tetrachloride induced hepatic injury in Albino rats. J Pharm Sci Tech 2010; 2(7): 247-251.
[7] Chiu HW, Hua KF. Hepatoprotective effect of wheat-based solid-state fermented antrodia cinnamomea in carbon tetrachloride-induced liver injury in rat. PLoS One 2016; 11(4): e0153087.
[8] Zhao Q, Peng Y, Huang K, Lei Y, Liu HL, Tao YY, et al. Salvianolate protects hepatocytes from oxidative stress by attenuating mitochondrial injury. Evid Based Complement Altern Med 2016; 2016: 5408705.
[9] Bertolami MC. Mechanisms of hepatotoxicity. Arq Bras Cardiol 2005; 85 Suppl 5(1): 25-27.
[10] Dhanasekaran M, Ignacimuthu S, Agastian P. Potential hepatoprotective activity of ononitol monohydrate isolated from Cassia tora L. on carbon tetrachloride induced hepatotoxicity in wistar rats. Phytomedicine 2009;16(9): 891-895.
[11] Ghazi Z, Ramdani M, Fauconnier ML, El Mahi B, Cheikh R. Fatty acids sterols and vitamin E composition of seed oil of Opuntia ficus indica and Opuntia dillenii from Morocco. J Mater Environ Sci 2013; 4(6): 967-972.
[12] Ghazi Z, Ramdani M, Tahri M, Rmili R, El msellem H, El Mahi B, et al. Chemical composition and antioxidant activity of seeds oils and fruit juice of Opuntia ficus Indica and Opuntia dillenii from Morocco. J Mater Environ Sci 2015; 6(8): 2338-2345.
[13] El Hachimi F, Hajjaj G, Bendriss A, Cherrah Y, Alaoui K. Antiin flammatory activity of seed oils of Opuntia ficus-indica L. and Punica granatum L. from Morocco. World J Pharm Res 2015; 4(1): 284-294.
[14] Karmen A, Wroblewski F, Ladue JS. Transaminase activity in human blood. J Clin Invest 1955; 34(1): 126-133.
[15] Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD. A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem 1983; 29(5): 751-756.
[16] Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974; 20(4): 470-475.
[17] Fossati P, Prencipe L, Berti G. Use of 3, 5-dichloro-2-hydroxybenzenesulfonic acid/4-amino-phenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 1980; 26: 227-231.
[18] Winsten S, Cehelyk B. A rapid micro diazo technique for measuring total bilirubin. Clin Chim Acta 1969; 25(3): 441-446.
[19] Burtis CA, Ashwood ER. Tietz textbook of clinical chemistry. Third edition. Philadelphia: WB Saunders Co; 1999.
[20] Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 1969; 6(1): 24-27.
[21] Talke H, Schubert GE. Enzymatic determination of urea using the coupled urease-GLDH enzyme system. Mediat Inflamm1965; 43: 174-176.
[22] Jaffe M. About the precipitate which picric acid produces in normal urine and about a new reaction of creatinine. J Physiol Chem 1886; 10(5): 391-400.
[23] Remaley AT, Rifai N, Warnick GR. Lipids, lipoproteins, apolipoproteins,and other cardiovascular risk factor. In: Burtis CA, Ashwood ER, Bruns DE. (eds). Tietz textbook of clinical chemistry and molecular diagnostics. St.Louis, Missouri: Elsevier; 2006, p. 903-968.
[24] Friedewald WT, Levy RI, Fredrickson DS. Estimation of concentration of low-density lipoprotein cholesterol in plasma without use of preparative centrifuge. Clin Chem 1972; 187: 589.
[25] Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978; 52: 302-310.
[26] Iqbal M, Giri U, Giri DK, Alam MS, Athar M. Age-dependent renal accumulation of 4-Hydroxy-2 Nonenal (HNE)-modified proteins following parenteral administration of ferric nitrilotriacetate commensurate with its differential toxicity: Implications for the involvement of HNE-protein adducts in oxidative stress and carcinogenesis. Arch Biochem Biophys 1999;365: 101-112.
[27] Williamson EM, Okpako DT, Evans FJ. The liver and biliary system:Selection, preparation and pharmacological evaluation of plant material.London, UK: John Wiley and Sons; 1996.
[28] Lu Y, Hu D, Ma S, Zhao X, Wang S, Wei G, et al. Protective effect of wedelolactone against CCl4-induced acute liver injury in mice. Int Immunopharmacol 2016; 34: 44-52.
[29] Ponmari G, Annamalai A, Gopalakrishnan VK, Lakshmi PTV,Guruvayoorappan C. NF-kB activation and proinflammatory cytokines mediated protective effect of Indigofera caerulea Roxb. On CCl4induced liver damage in rats. Int Immunopharmacol 2014; 23: 672-680.
[30] John Buege A, Steven Aust D. Microsomal lipid peroxidation. Methods Enzymol 1978; 52: 302-310.
[31] Hewawasam RP, Jayatilaka KAPW, Pathirana C, Mudduwa LKB.Hepatoprotective effect of Epaltes divaricata extract on carbon tetrachloride induced hepatotoxicity in mice. Indian J Med Res 2004;120(1): 30-34.
[32] Anand KK, Singh B, Chand D, Chandan BK. An evaluation of Lawsonia alba extract as hepatoprotective agent. Planta Med 1992; 58: 22-25.
[33] Cheng N, Ren N, Gao H, Lei X, Zheng J, Cao W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem Toxicol 2013; 55(3):234-240.
[34] Kazeem MI, Bankole HA, Fatai AA. Protective effect of ginger in normal and carbon-tetrachloride induced hepatotoxic rats. Annals Biol Res 2011;2(1): 1-8.
[35] Zhang Y, He Y, Yu H, Ma F, Wu J, Zhang X. Liquiritigenin protects rats from carbon tetrachloride induced hepatic injury through PGC-1alpha pathway. Evid Based Complement Altern Med 2015; 649568(10): 25.
[36] Muthy KNC, Jayaprakash GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extracts using in vivo models. J Agric Food Chem 2002; 50(17): 4791-4795.
[37] Koubaa M, Mhemdi H, Barba FJ, Angelotti A, Bouaziz F, Chaabouni SE, et al. Seed oil extraction from red prickly pear using hexane and supercritical CO2: Assessment of phenolic compound composition,antioxidant and antibacterial activities. J Sci Food Agric 2017; 97(2): 613-620.
 Asian Pacific Journal of Tropical Biomedicine2018年5期
Asian Pacific Journal of Tropical Biomedicine2018年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Co-detection and isolation of Leishmania and Crithidia among naturally infected Tatera indica (Rodentia: Muridae) in Fars province, southern Iran
- Larvicidal efficacy of crude and fractionated extracts of Dracaena loureiri Gagnep against Aedes aegypti, Aedes albopictus, Culex quinquefasciatus, and Anopheles minimus mosquito vectors
- Antiplasmodial activity of silver nanoparticles: A novel green synthesis approach
- Combination treatment of bisphosphonate (pamidronate) and Quercus infectoria semipurified fraction promotes proliferation and differentiation of osteoblast cell via expression of Osterix and Runx2 marker
- Phytochemical bioprospecting, antioxidant, antimicrobial and cytotoxicity activities of saline extract from Tithonia diversifolia (Hemsl) A. Gray leaves
- Molecular methods for detection of pathogenic viruses of respiratory tract-A review
