Extraction and structural investigation of jute cellulose nano fibers
Zhong-Xuan Bian•Xia-Ran Miao•Jin-You Lin•Feng Tian•Feng-Gang Bian•Hui Li
1 Introduction
Cellulose nano fibrils(CNFs)are a type of natural biopolymers in the nanometer range(several hundred nanometers in length and ~ 5–50 nm in width)that are mainly extracted from natural plant and animal tissues.In recent years,CNF has drawn increasing attention because of its nontoxicity,renewability,biodegradability,stability,superior mechanical properties,and low cost[1]and has shown potential applications in many fields such as nanocomposites,pharmaceuticals,and filtration.
The cellulose molecular chains consist of repeating units of b-1,four linked anhydro-D-glucose units assembled in parallel by covalent and hydrogen bonds.There are four types of polymorphs for cellulose,cellulose I,II,III,and IV,of which cellulose I and II have been studied the most for their structure and properties[1,2].In nature,cellulose I,which has higher Young’s modulus and lower thermal stability,is the most common polymorph found in plants.
CNF can be extracted from various natural resources such as wood[3],bacteria[4],tunicate[5],alga[6],and fiber plants such as cotton[7],hemp[8],sisal[9], flax[10],and jute[11].A kind of fiber plant,jute is widely grown in Bangladesh,China,Thailand,Nepal,and Indonesia.Jute fibers mainly consist of cellulose(72%),hemicellulose(13%),lignin(13%),and vegetable wax(<1%).The cellulose content of jute is higher than that of other high- fiber plants[12],and the mechanical properties of the jute itself and the internal cellulose fibrils are better[13].The cellulose molecules in the jute fiber are highly oriented and arranged in a spiral parallel to the axial direction[14].At the same time,its micro fibril angle is large(7°–12°),which is conducive to the separation of cellulose nano fibrils[15].The preparation of CNFs from jute is of great significance for the application of jute and can effectively reduce energy consumption and cost of production.
Up to now,CNFs have been successfully extracted by several methods such as mechanical defibrillation,acid hydrolysis[16],and 2,2,6,6-tetramethylpiperidine-1-oxyl(TEMPO)-mediated oxidation [11].The method of TEMPO-mediated oxidized cellulosefollowed by a mechanical defibrillation to generate CNF has attracted much attention in recent years[17,18].The reaction system is a TEMPO/NaBr/NaClO ternary aqueous system in which TEMPO is an oxidation catalyst,and NaClO is used as the main oxidant.The system selectively oxidizes the hydroxyl groups of C6 in the cellulose chain as negatively charged carboxyl groups[18],and the repulsive forces between the fibrils induce mechanical fibrillation that can yield CNFs of similar dimensions.
In this work,we report the fabrication of CNF from jute without alkali treatment or degumming by TEMPO-mediated oxidation with a TEMPO/NaBr/NaClO ternary system following mechanical disintegration.The structure and morphology were characterized by synchrotron radiation wide-angle X-ray scattering(SR-WAXS),Fourier transform infrared spectra (FTIR),transmission electron microscopy(TEM),and field-emission scanning electron microscopy(FE-SEM).At the same time,the results were compared with those of cellulose microcrystalline(MCC)fabricated from cotton to study the structural change during the preparation of the CNFs;this is favorable to further study the relationship between structure and physicochemical properties.
2 Materials and methods
2.1 Materials
Pristine jute fibers were provided by Redbud Textile Tech.Inc.,China,and were sufficiently dried in a vacuum drying oven at 70°C for over 24 h.Analytically pure sodium hydroxide(NaOH,97%),TEMPO(98%),NaBr,NaClO solution(12 wt%),and ethanol absolute were purchased from Shanghai Aladdin Chemical Regent Inc.,China.
2.2 Preparation of jute fibers
The dried jute fibers were ground into powder(less than 1-mm particle size)and dispersed in deionized water at room temperature for 1 h.Then,the suspension was allowed to stand for 10 min to remove the impurities in the upper layer of the suspension.After washing several times with deionized water,the mass was dried in a vacuum oven at 70°C for 2 h.
2.3 Preparation of CNFs
The pretreated jute fibers(1.0 g)were dispersed in 100 mL deionized water.Then,the NaBr(0.33 g)and TEMPO(0.033 g)were added to the suspension and stirred for 1 h.After they were completely dissolved,the reaction was initiated by adding the 12 wt%NaClO solution(20 g)and stirring for 3 h.At the same time,the 2 wt%NaOH solution was continually added to the solution to maintain the otherwise decreasing pH between 10.5 and 10.7.The reaction was stopped by adding ethanol absolute(7.0 mL)followed by stirring for 15 min.The final product was washed several times with deionized water by successive centrifugations(10,000 rpm for 10 min).The CNF was obtained by dispersing the cellulose jelly in deionized water with high-speed homogenization.
The 1-g CNF hydrogel was dried in a vacuum oven at 80°C for 24 h,and the weight of the residual solid was used to calculate the CNF solid content,which was 2 wt%.Then,25 g of the CNF hydrogel was dispersed in 75 mL deionized water followed by high-speed homogenization(12,000 rpm,10 min)and sonication(power 500 W,10 min)and then cooled to room temperature to obtain a 0.5 wt%CNF aqueous suspension.The prepared CNF aqueous suspension was poured into a cylindrical container and quickly frozen in liquid nitrogen.After several minutes,it was transferred to a freeze dryer(FD-80,Beijing BoYi Kang Experimental Equipment Ltd.Co.,China)for 48 h to obtain solidified jute CNF aerogels;the vacuum pressure was 4.5 Pa and the cold trap temperature-80°C.
2.4 Characterization
SR-WAXS experiments were performed at the smallangle X-ray scattering station(BL16B)in the Shanghai Synchrotron Radiation Facility(SSRF).The wavelength was 0.124 nm and the sample-to-detector distance 90.5 mm(corrected using the LaB6monocrystalline powder).A 2D MAR165 charge-coupled device(CCD;MAR USA,Inc.)was used for data collection.A schematic of the main experimental device is shown in Fig.1a,including the incident synchrotron X-rays,sample stage(including the experimental sample),beam-stop(diameter 20 mm),CCD,and other components.The beam-stop near the CCD was used to block the central transmission of X-rays to avoid CCD signal supersaturation.The jute fiber sample was placed on the sample holder perpendicular to the incident X-rays.The other powder samples were made into a solid wafer with a diameter of 10 mm and thickness 1 mm by pressing(pressure approximately 50 N)and packed between two Kapton films tightly to form the samples for the SR-WAXS experiments.All the samples were exposed for 60 s and the data of the blank Kapton films without any sample powders were collected for Compton films and air scattering correction.
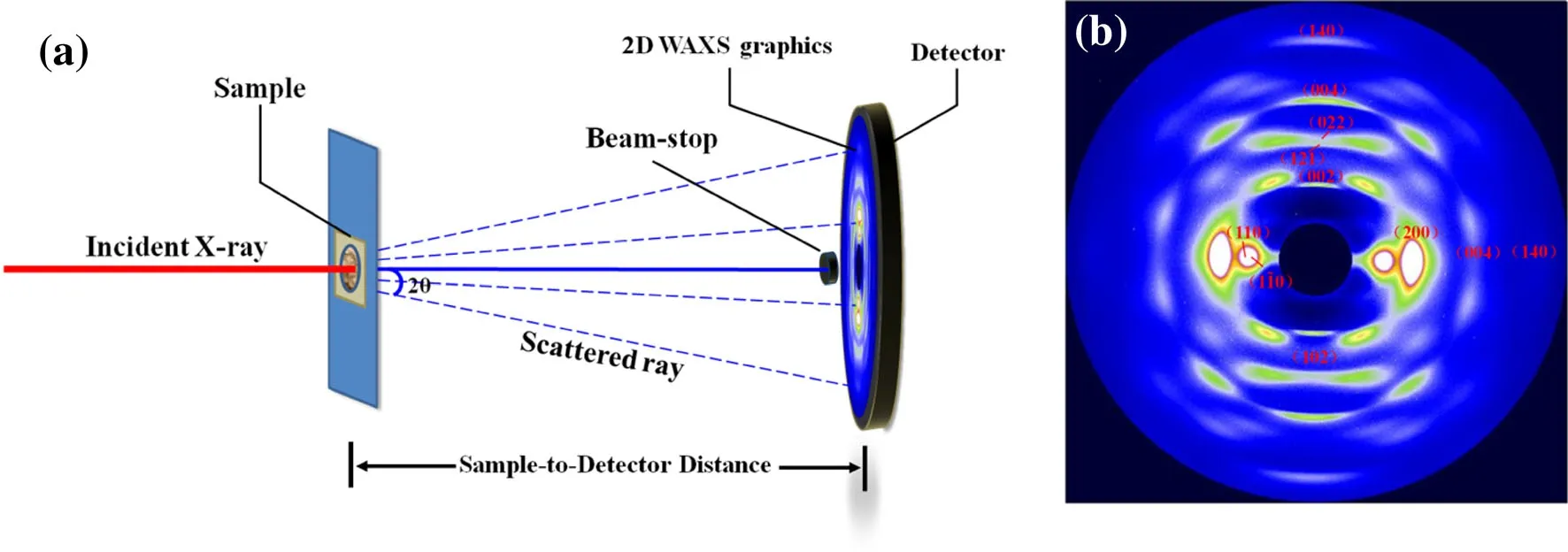
Fig.1(Color online)a Schematic of the SR-WAXS experiment and b 2D SR-WAXS data of elongated jute fibers
The software Fit 2D(v12.077)was used to obtain the 2D WAXS patterns by one-dimensional integration of the 2D SR-WAXS data.The software PeakFit(v4.12,Sea Solve Software Inc.)was used for peak separation and analysis of the one-dimensional WAXS integral curve.The crystal index(Miller index)of each scattering peak was calculated according to Bragg’s formula,which can be written as

The result was compared with the standard result of cellulose Iβ.The cellulose crystallite scale Bhklwas determined by the sizes of the(004)crystal plane parallel to the cellulose chain and the(110),(110)and(200)planes perpendicular to the chain[19,20].Based on the Scherrer formula

the size of each facet(hkl)can be obtained;here,K=0.9[21]for cellulose,λ is the incident X-ray wavelength,and FWHM is the half-peak height of the fitting peak.The crystallinity of the samples was obtained by superimposing the peak area ratio of all the crystal peaks in the fitted onedimensional curve and was expressed by the crystallinity index(CI).
The FTIR data of the samples were obtained using an analyzer(Nicolet 6700,Thermo Fisher)in the attenuated total reflection(ATR)mode in the wavelength range 4000–600 cm-1.All the samples were vacuum-dried(70°C)for 24 h before testing.The data processing of each sample included the deduction of the absorption of H2O and air from the infrared spectrum and the corresponding baseline correction.
The morphology of CNF aerogels was investigated by FE-SEM (S4800,Japan).ATEM (Tecnai G2F20 S-TWIN)equipped with a Gatan 1 k*1 k CCD camera was used to characterize the morphology and scale of the CNF at an accelerating voltage of 100 kV.The 0.50-g TEMPO-oxidized jute cellulose jelly with a solid content of 2 wt%was dispersed in 49.50 g ethanol absolute followed by high-speed homogenization at 10,000 rpm for 10 min to obtain a 0.02 wt%suspension.Approximately 10 μL of the CNF ethanol dispersion was dropped on a glow-discharged carbon-coated Cu grid and dried by an infrared lamp for 30 min in ambient conditions.
3 Results and discussion
3.1 SR-WAXS analysis of the jute CNF
Figure 1b shows the 2D SR-WAXS pattern of the elongated jute fibers,where the scattering signal arcs in different orientations can be visually observed.The arcs with a highly anisotropic distribution indicate that the cellulose fibrils of jute have a good orientation structure along the fiber direction.The(110),(110),(200)planes in the equatorial axis and the(002),(004)planes in the meridian direction coincide with the spatial distributions of the different orientation cross sections in the reciprocal space.The one-dimensional integral curve for two directions of 2D SR-WAXS of the elongated jute fibers with the corresponding fit curves(component)is shown in Fig.2.The cellulose in the jute fiber is mainly cellulose Iβ,which belongs to the monoclinic system.The space group is P21,and the unit cell parameters are a=7.784,b=8.201,c=10.38,α = β =90°,γ=96.5°.Therefore,the crystal face index can be obtained according to the 2θ value of each scattering peak in the one-dimensional integral curve of SR-WAXS.

Fig.2(Color online)1D integral curves for the a equatorial and b meridional direction of 2D SR-WAXS of elongated jute fibers with corresponding fit curves(component)
Figure 3 shows the one-dimensional SR-WAXS integral curves of jute fiber cellulose(JFC),jute CNF,and cotton linterMCC (length20 μm,purchasedfrom Sigma-Aldrich),in which the MCC was used as a reference for comparison.It can be observed that the scattering peaks corresponding to the(021)planes of MCC are more obvious than those of JFC and CNF,and the scattering peaks of the(110)and(110)planes of JF and CNF are stronger than those of MCC.In addition,the crystallinity can directly reflect the degree of ordered polymerization and folding arrangement of the CNF single-molecule chains[22].Table 1 shows that the crystallinity of the three samples increases in the order of JFC,CNF,and MCC.The reason may be that the hydroxyl groups on the cellulose chain are selectively oxidized to carboxyl groups and negatively charged in the solution environment during TEMPO-mediated oxidation,which can result in an increase in the electrostatic repulsions between the chains of cellulose and further promote the dissociation of the cellulose chains.In this process,most of the lignin,hemicellulose,and other amorphous phases are oxidized and dissolved,so the crystallinity of the obtained CNF is higher than that of JFC.The different relative intensities of the(021)scattering peaks also show that a small amount of the amorphous phase remains in the CNF,which causes some scattering peaks of cellulose I to be overlapped.

Fig.3(Color online)1D SR-WAXS integral curves of JF powder,CNF,and MCC

Table 1 Crystallinity indexes and crystallite widths of JFC,CNF,and MCC
Table 1 provides the crystallite dimensions of JFC,CNF,and MCC,calculated by the one-dimensional scattering results of SR-WAXS,and a schematic of the dimensions is shown in Fig.4.On one hand,it can be observed that the CNF microcrystals are larger than JFC,but the size ratios(1:1.12:1.28 and 1:1.08:1.26,respectively)are similar.On the other hand,the CNF radial length[(004)plane direction]is less than that of JFC.In addition,the calculated cross-sectional dimensions of the MCC microcrystals are(110)=4.4 nm,(110)=6.2 nm,and(200)=6.8 nm,which are similar to the previously reported dimensions for the CNFs prepared from different raw materials[19,23].The explanation for this result is that the combination of JFC single chains relies on the hydrogen bond interactions between the C6 hydroxyl groups(C6–OH)in the intramolecular and intermolecular directions,and the single-chain repulsion increases as thehydroxyl group is selectively oxidized to the carboxyl group after TEMPO-mediated oxidation.After washing with deionized water,the carboxyl group is present in the form of carboxylic acid or carboxylate,and the hydrogen bond interaction between the CNF chains is further weakened.In addition,with the dissolution of lignin and hemicellulose,the dissociated CNF is more likely to aggregate and crystallize[23].Thus,for jute CNF crystallites,the dimension is stretched in the cross-sectional direction and shrinks from 27.7 nm to 18.3 nm in the fiber direction.
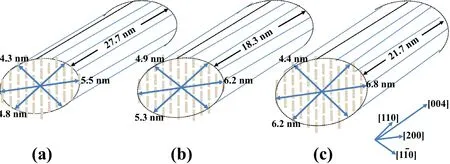
Fig.4(Color online)Schematic of the crystallite dimensions of a JFC,b CNF,and c MCC
Figure 5 shows the infrared spectra of the jute fiber powder,CNF,and MMC,which contains the main infrared spectral differences that enable us to determine the structural changes in these samples.For an intuitive and convenient comparison,Table 2 provides the different wavenumbers of the absorbance peaks in the FTIR spectra of the three samples.The features of the characteristic region(1700–850 cm-1)are due to the constituents of cellulose I,lignin,and hemicellulose[24].Compared to the JF,the absorption bands at 1735,1504,1456,and 1244 cm-1disappeared or were drastically reduced in CNF.At the same time,the characteristic bands,including 3351 and 1425 cm-1,in the JF powder were shifted to lower wavenumbers(3342 and 1421 cm-1)in CNF,and the absorption bands at 2899,1595,and 899 cm-1were shifted to higher wavenumbers (2901,1605,and 900 cm-1).As a reference sample,the MCC also revealed small differences in wavenumbers relative to JF and CNF.The explanation for this phenomenon is that the hydroxyl groups of C6 in the cellulose single chain of JF were oxidized to carboxyl groups after TEMPO-mediatedoxidation.The carboxyl group with the higher polarity and stronger intramolecular hydrogen bonds contributed to the transformation of the vibrational modes of the functional groups of cellulose Iβ.It is further demonstrated by the shift in the absorption bands of cellulose Iβ-glucoside chains at 900 cm-1and the C6-CH2bending vibrations in cellulose I at 1425 cm-1.

Fig.5(Color online)FTIR results of JF powder,CNF,and MCC

Table 2 Wavenumbers of the absorbance peaks in the FTIR spectra of JF powder,CNF,and MCC
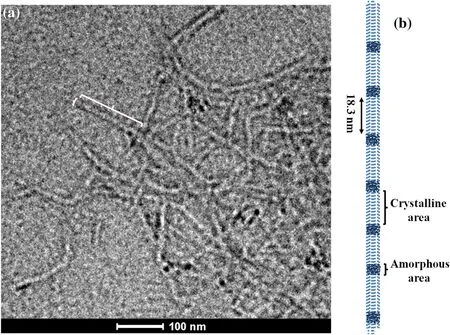
Fig.6(Color online)a TEM image of jute CNF and b the corresponding schematic diagram
As shown in Fig.5,all the samples have two absorption bands,ascribed to the functional group of cellulose I,between 3500 and 2750 cm-1,of which the relatively wider bands between 3360 and 3330 cm-1are assigned to–OH stretching,and the band at 2900 cm-1is ascribed to C–H stretching[25].For the infrared absorption spectrum of the jute fiber,the strong absorption band at 1735 cm-1is assigned to the C–O stretching in the carboxyl or ketone groups of hemicellulose,and the 1244 cm-1band is ascribed to the C–O stretching in the acetyl or xylan groups of the jute fiber,whereas the bands significantly decrease in CNF and MCC[24,26,27],which means that most of the hemicellulose and xylan in the JF powder is dissolved and removed during the preparation of the CNF.Similarly,the bands at 1504[25,28]and 1456 cm-1,respectively,corresponding to C=C stretching in aromatic hydrocarbon and CH deformation and CH2symmetric bending in lignin,appeared only in the JF infrared spectrum,which indicates the removal of lignin from JF after TEMPO-mediated oxidation.These results also explain the reason why the degree of crystallinity of CNF is greater than that of JF.The 1650 cm-1absorption band is ascribed to the functional group that appeared after cellulose I absorbed water[16,24,26],which is not detected in the case of CNF.The reason may be that the 1650 cm-1band in CNF is weak and covered by a stronger absorption band at 1605 cm-1,or the freeze-dried CNF has weaker water absorption compared to JF and MCC.

Fig.7 FE-SEM images of CNF aerogel
Figure 6 shows a TEM image of the CNFs prepared from jute fiber via TEMPO-mediated oxidation.As can be seen from the image,the CNF was defibrillated from the treated jute fiber successfully.A single CNF is approximately 150 nm long with a diameter of approximately 13 nm.All the CNFs observed in the figure had a uniform size distribution.Most of CNFs are formed due to an agglomeration phenomenon occurring at relatively low concentrations because ofthe strong intramolecular hydrogen bonds and the high specific surface area of CNF.Figure 6b presents the structure and arrangement of the CNFs,which were calculated on the basis of these results and the size of the CNF microcrystals revealed in the SRSAXS results.In the horizontal direction,each CNF single fiber consists of approximately four to six pieces of cellulose microcrystals with elliptical cross sections and relying on hydrogen bonds and covalent interactions to form a crystalline area.In the radial direction,there is an amorphous phase of cellulose between the crystalline areas,which is also connected by hydrogen bonding.It should be noted that the results shown above correspond to the single CNF microcrystalline structure,in which the size change(stretching or shrinkage)occurs after the TEMPO-mediated oxidation of the JFC.
Figure 7 shows the FE-SEM images of the CNF aerogel.The CNF aerogel exhibited complex cellular architectures and open porosity.The CNF aerogel consisted of irregular thin plates,on which were piled up nano fibrils.The diameter of the pores built up by the plates was in the 30-μm range.In the freeze-drying process,the CNFs were locked in ice crystals.With the sublimation of ice,the morphology of the CNF in the aqueous solution was fixed.In the inset of Fig.7,a nanoporous structure was observed on the thin plates.Individual nano fibrils could be observed clearly on the plates,which confirmed that the plates were composed of CNFs.
4 Conclusion
In this study,we successfully fabricated CNFs from raw jute fiber via TEMPO-mediated oxidation and mechanical disintegration.The SR-WAXS results showed the crystallinity and crystallite sizes of JFC and CNF.FTIR characterization showed changes in the composition of jute fiber and the microstructure of cellulose I during the preparation of CNF.TEM results showed that the length and diameter of the CNF microcrystals were approximately 150 nm and 13 nm,respectively,which demonstrates that the CNF has a high degree of uniformity,revealing the structure of the microcrystals in the CNF.
At present,before oxidation,to prepare the CNFs,the raw materials(protozoa and plants)need to be alkali or acid treated.In this study,we have successfully prepared homogeneous CNFs without any alkali treatment,but rather by changing the experimental conditions of TEMPO-mediated oxidation(such as concentration of oxidants,pH value of reaction,and reaction time).This process of preparing CNF is less polluting and consumes less energy;it is an effective attempt to achieve green and large-scale extraction of CNFs in the future.
1.R.J.Moon,A.Martini,J.Nairn et al.,Cellulose nanomaterials review:structure,properties and nanocomposites.Chem.Soc.Rev.40,3941–3994(2011).https://doi.org/10.1039/c0cs00108b
2.Y.Habibi,L.A.Lucia,O.J.Rojas,Cellulose nanocrystals:chemistry,self-assembly,and applications.Chem.Rev.110,3479–3500(2010).https://doi.org/10.1021/cr900339w
3.F.Jiang,A.R.Esker,M.Roman,Acid-catalyzed and solvolytic desulfation of H2SO4-hydrolyzed cellulose nanocrystals.Langmuir 26,17919–17925(2010).https://doi.org/10.1021/la1028405
4.M.Iguchi,S.Yamanaka,A.Budhiono,Bacterial cellulose—a masterpiece of nature’s arts.J.Mater.Sci.35,261–270(2000).https://doi.org/10.1023/A:1004775229149
5.W.Helbert,Y.Nishiyama,T.Okano et al.,Molecular imaging ofhalocynthia papillosacellulose.J.Struct.Biol.124,42–50(1998).https://doi.org/10.1006/jsbi.1998.4045
6.N.-H.Kim,W.Herth,R.Vuong et al.,The cellulose system in the cell wall ofMicrasterias.J.Struct.Biol.117,195–203(1996).https://doi.org/10.1006/jsbi.1996.0083
7.S.Elazzouzi-Hafraoui,Y.Nishiyama,J.-L.Putaux et al.,The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose.Biomacromol 9,57–65(2007).https://doi.org/10.1021/bm700769p
8.Y.Habibi,A.Dufresne,Highly filled bionanocomposites from functionalized polysaccharide nanocrystals.Biomacromol 9,1974–1980(2008).https://doi.org/10.1021/bm8001717
9.N.L.Garcia de Rodriguez,W.Thielemans,A.Dufresne,Sisal cellulose whiskers reinforced polyvinyl acetate nanocomposites.Cellulose 13,261–270(2006).https://doi.org/10.1007/s10570-005-9039-7
10.X.Cao,H.Dong,C.M.Li,New nanocomposite materials reinforced with flax cellulose nanocrystals in waterborne polyurethane.Biomacromol 8,899–904(2007).https://doi.org/10.1021/bm0610368
11.X.Cao et al.,Cellulose nanowhiskers extracted from TEMPO-oxidized jute fibers.Carbohydr.Polym.90,1075–1080(2012).https://doi.org/10.1016/j.carbpol.2012.06.046
12.L.Y.Mwaikambo,M.P.Ansell,Chemical modification of hemp,sisal,jute,and kapok fibers by alkalization.J.Appl.Polym.Sci.84,2222–2234(2002).https://doi.org/10.1002/app.10460
13.A.Bledzki,J.Gassan,Composites reinforced with cellulose based fibres.Prog.Polym.Sci.24,221–274(1999).https://doi.org/10.1016/S0079-6700(98)00018-5
14.J.W.Hearle,W.E.Morton,Physical Properties of Textile Fibres(Elsevier,Amsterdam,2008)
15.R.M.Rowell,J.S.Han,J.S.Rowell,Characterization and factors effecting fiber properties.Natural Polymers and Agro fibers Bases Composites. Embrapa Instrumentacao Agropecuaria, P.O.Box 741,Sao Carlos,13560-970 SP,Brazil,2000.,2000:115–134
16.N.Kasyapi,V.Chaudhary,A.K.Bhowmick,Bionanowhiskers from jute:preparation and characterization.Carbohydr.Polym.92,1116–1123(2013).https://doi.org/10.1016/j.carbpol.2012.10.021
17.A.E.De Nooy,A.C.Besemer,H.van Bekkum,Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans.Carbohydr.Res.269,89–98(1995).https://doi.org/10.1016/0008-6215(94)00343-E
18.A.Isogai,T.Saito,H.Fukuzumi,TEMPO-oxidized cellulose nano fibers.Nanoscale 3,71–85(2011).https://doi.org/10.1039/c0nr00583e
19.T.Virtanen,K.Svedström,S.Andersson et al.,A physicochemical characterisation of new raw materials for microcrystalline cellulose manufacturing.Cellulose 19,219–235(2011).https://doi.org/10.1007/s10570-011-9636-6
20.P.Rämänen,P.A.Penttilä,K.Svedström et al.,The effect of drying method on the properties and nanoscale structure of cellulose whiskers.Cellulose 19,901–912(2012).https://doi.org/10.1007/s10570-012-9695-3
21.F.Xu,Y.-C.Shi,D.Wang,Structural features and changes of lignocellulosic biomass during thermochemical pretreatments:a synchrotron X-ray scattering study on photoperiod-sensitive sorghum.Carbohydr.Polym.88,1149–1156(2012).https://doi.org/10.1016/j.carbpol.2012.01.041
22.R.Zhou,Q.Xiang,J.Song,A study on displacement of crystalline diffraction peaks in electron-beam irradiated filter paper cellulose.Nuclear Tech.20,631–635(1997).(in Chinese)
23.K.Leppänen,S.Andersson,M.Torkkeli et al.,Structure of cellulose and microcrystalline cellulose from various wood species,cotton and flax studied by X-ray scattering.Cellulose 16,999(2009).https://doi.org/10.1007/s10570-009-9298-9
24.F.Carrillo,X.Colom,J.J.Sun˜ol et al.,Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres.Eur.Polym.J.40,2229–2234(2004).https://doi.org/10.1016/j.eurpolymj.2004.05.003
25.S.M.L.Rosa,N.Rehman,M.I.G.Miranda et al.,Chlorine-free extraction of cellulose from rice husk and whisker isolation.Carbohydr.Polym.87,1131–1138(2012).https://doi.org/10.1016/j.carbpol.2011.08.084
26.J.I.Morán,V.A.Alvarez,V.P.Cyras et al.,Extraction of cellulose and preparation of nanocellulose from sisal fibers.Cellulose 15,149–159(2007).https://doi.org/10.1007/s10570-007-9145-9
27.E.Sinha,S.K.Rout,Effect of neutron irradiation on the structural,mechanical,and thermal properties of jute fiber.J.Appl.Polym.Sci.110,413–423(2008).https://doi.org/10.1002/app.28504
28.E.Sinha,S.Rout,In fluence of fibre-surface treatment on structural,thermal and mechanical properties of jute fibre and its composite.Bull.Mater.Sci.32,65–76(2009).https://doi.org/10.1007/s12034-009-0010-3
 Nuclear Science and Techniques2018年7期
Nuclear Science and Techniques2018年7期
- Nuclear Science and Techniques的其它文章
- Monte Carlo simulation of incident electrons passing through thin metal layer
- Annual effective dose values from137Cs activity concentrations in soils of Manisa,Turkey
- Preliminary analysis of tritium fuel cycle in Z-pinch-driven fusion– fission hybrid reactor
- Investigation of high-temperature-resistant rhenium–boron neutron shields by experimental studies and Monte Carlo simulations
- Preparation and characterization of Bi2O3/XNBR flexible films for attenuating gamma rays
- Gamma irradiation-induced effects on the properties of TiO2 on fluorine-doped tin oxide prepared by atomic layer deposition
