Astragaloside IV protects RGC-5 cells against oxidative stress
Ming Hao, Yu Liu, Ping Chen, Hong Jiang, Hong-Yu Kuang,
1 Department of Endocrinology, The First Clinical Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China
2 Department of Endocrinology, Heilongjiang Provincial Hospital, Harbin, Heilongjiang Province, China
3 Department of Endocrinology, The First Hospital of Harbin, Harbin, Heilongjiang Province, China
Introduction
Astragalus membranaceusis a traditional herb and food used in China and India that is also used as a food additive.Astragalus membranaceuscan treat the common cold, fatigue, diarrhea, and cardiac disease (Sun et al., 2008; Wang et al., 2008).Astragalus membranaceusis an immunomodulating agent that alleviates immunodeficiency disease (Ma et al., 2002). Recently, an increasing number of reports have shownAstragalus membranaceus-induced protection of the nervous system.Astragalus membranaceuscan enhance recovery of stroke patients by reducing the cerebral infarction area, and also has anti-oxidative qualities (Xu et al., 2008; Chen et al., 2012). Studies have shown thatAstrag-alus membranaceusmarkedly inhibits oxidative stress and improves oxygen-free radical-scavenging abilities (Zhang et al., 2009). Astragaloside IV (As-IV), or 3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosylcycloastragenol (chemical structure shown in Figure 1), is the main active compound ofAstragalus membranaceus. Previous studies have shown that As-IV has strong anti-inflammatory, anti-cerebral edema, anti-cardiac hypertrophy, anti-diabetic, and anti-oxidative activities, with protective effects against progression of peripheral neuropathy (Li et al., 2014; Lu et al., 2014; Sun et al., 2016; Qiao et al., 2017).
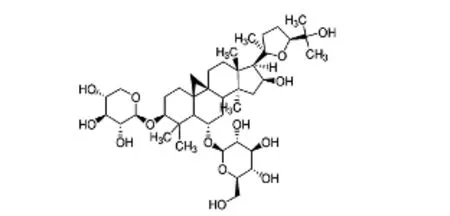
Figure 1 Chemical structure of astragaloside IV.
Oxidative stress is involved in vision-threatening diseases including diabetic retinopathy, age-related macular degeneration, glaucoma, uveoretinitis, and many other retinal diseases (Bearse et al., 2004; Klein et al., 2007; Meyer-Rüsenberg et al., 2007; Yau et al., 2012). Because of higher oxygen consumption in the retina, it is more easily damaged by oxidative damage than most other body tissues (Zhang et al.,2008b; Han et al., 2016). Increased reactive oxygen species(ROS) affect neuronal cells, causing neuronal cell apoptosis and visual impairment (Ozawa et al., 2011; Masuda et al.,2017). Although anti-oxidative stress is beneficial for the treatment of several ocular diseases, an effective treatment has not yet been developed.
In the present study, we used an oxidative stress model for retinal diseases. Specifically, we used hydrogen peroxide(H2O2) to induce cellular damage in the retinal ganglion cell(RGC)-5 line. The RGC-5 cell line has the advantages of generational stability and straightforward survival. Indeed, the molecular mechanism and maturation of cells are basically the same as for primary RGCs. Accordingly, this cell line has been widely used in ophthalmology research in recent years.In this study, we determined whether As-IV attenuates impairments in damaged RGC-5 cells and investigated the mitochondrial mechanism of As-IV-mediated protection.
Materials and Methods
RGC-5 cell culture and cell survival assay
Rat RGC-5 cells (PTA6600) were purchased from the cell collection of the American Type Culture Collection, which have been verified to be of the correct lineage. Cells cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone,Beijing, China), containing 10% fetal bovine serum with 100 U/mL of penicillin and 100 µg/mL of streptomycin,were incubated in 5% CO2at 37°C. Cells were passaged at a ratio of approximately 1:8 every 2 to 3 days. To passage,5000 cells/well were dispensed into 96-well plates. To build an oxidative stress cell model, the plate was pre-incubated for 24 hours in a humidified incubator at 37°C, 5% CO2(HF90; Heal Force, Hong Kong, China). Cells were switched to DMEM containing 0 µM H2O2group (control group) or 100, 200, 400, 600, or 800 µM H2O2to determine the optimal concentration of H2O2for establishing RGC-5 oxidative stress. Cells were incubated for 24 hours. Next, 10 µL of Cell Counting Kit-8 (CCK-8) solution (C0038; Beyotime,Hunan, China) was added to each well and incubated for 2 hours. Absorbance at 450 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA). The appropriate concentration of H2O2(600 µM) for the RGC-5 cell line was initially measured.
RGC-5 cells were plated into 96-well plates at a density of 5 × 103or 105cells per well of a 6-well plate. Cells were incubated for 24 hours in a humidified incubator at 37°C,5% CO2. Cells were then divided into six groups. The control group was incubated with normal culture medium without As-IV or H2O2. The intervention group included five subgroups, which were pre-treated with various concentrations(5, 10, 50, 100, or 200 mg/L) of As-IV for 1 hour. After removing As-IV, cells were incubated with medium containing H2O2(600 µM) for an additional 24 hours. RGC-5 cells were incubated in 96-well plates. After H2O2exposure, 10 µL of CCK-8 solution was added to each well and incubated for 2 hours. Absorbance at 450 nm was measured using a microplate reader (Bio-Rad), followed by initial measurement of the appropriate concentration of As-IV (100 mg/L) for the RGC-5 cell line.
Nuclear staining for assessment of apoptosis
RGC-5 cells were plated in 6-well plates, pre-treated with As-IV (100 mg/L), and then incubated with H2O2for 24 hours. Cells were washed with phosphate-buffered saline(PBS) and stained with 4′,6-diamidino-2-phenylindole(DAPI) (1 µg/mL) at 37°C for 10 minutes. Cells were washed with PBS. A fluorescence microscope (TE2000-U; Nikon,Tokyo, Japan) was used to capture fluorescence images.
Intracellular ROS production
Intracellular ROS was examined using the dye, 2′,7′-dichlorofluorescein diacetate (DCFH-DA). Intracellular radical species (H2O2, OH-, and O2-) can oxidize nonfluorescent dichloro fluorescein (DCFH) to fluorescent dichloro fluorescein (DCF). RGC-5 cells were pre-treated with As-IV (100 mg/L) and then incubated with H2O2for 24 hours. DCFHDA dye (10 µM) was added and incubated for 30 minutes at 37°C. Cells were washed twice with PBS and fluorescence analyzed by flow cytometry (FACS Aria™Cell Sorter; BD,Franklin Lakes, NJ, USA) through the FL1 channel.
Mitochondrial membrane potential
Mitochondrial membrane potential (Δψm) was measured using the fluorescent probe, JC-1 (Beyotime). JC-1 is capable of selectively entering mitochondria where it forms monomers and emits green fluorescence when Δψm is relatively low. At high Δψm, JC-1 aggregates and emits a red fluorescence. After drug treatment, cells were incubated in 6-well plates and 0.5 mL JC-1 working solution added for 20 minutes at 37°C. JC-1 staining buffer was used to wash cells twice. Cells were scanned and imaged on a confocal microscope (Zeiss 510; Zeiss, Oberkochen, Germany).
Western blot assay
After the designated treatment, cells were collected and washed with PBS. Cellular proteins were extracted with icecold radioimmunoprecipitation assay (RIPA) lysis buffer.Protein concentration was determined using the BCA Pro-tein Assay Kit (P0011; Beyotime). Protein samples (40 µg)were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5%nonfat dried milk at room temperature for 1 hour, and then incubated with primary antibodies overnight at 4°C. Membranes were washed with Tris-buffered saline–Tween 20 and incubated with the appropriate secondary antibodies at room temperature for 1 hour. The primary antibodies used were rabbit anti-cytochrome c polyclonal antibody (1:1000;sc-7159; Santa Cruz Biotechnology, Santa Cruz, CA, USA),rabbit anti-Bax polyclonal antibody (1:1000; sc-526; Santa Cruz), rabbit anti-Bcl-2 polyclonal antibody (1:1000; sc-492;Santa Cruz Biotechnology), rabbit anti-caspase-3 polyclonal antibody (1:1000; 9662; Cell Signaling Technology, Beverly, MA, USA) and rabbit anti-β-actin polyclonal antibody(1:1000; TA-09; ZSGB-BIO, Beijing, China). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit IgG (1:50,000; ZSGB-BIO). Afterwards, membranes were washed again with Tris-buffered saline–Tween 20.Membranes were incubated with enhanced chemiluminescence (P0018A; Beyotime) and detected using the Chemi-Doc XRS gel documentation system (Bio-Rad). Protein bands were quantified by Image Lab (Bio-Rad), with β-actin used as an internal control.
Statistical analysis
Values are presented as the mean ± SD, and were processed using SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). Oneway analysis of variance followed by Student-Newman-Keuls test was used to determine the significance of differences between means. All experiments were performed at least three times.P< 0.05 was considered statistically significant.
Results
As-IV protected RGC-5 cells against H2O2-induced toxicity
To investigate H2O2-induced cytotoxicity, RGC-5 cells were incubated with increasing concentrations of H2O2, and cell survival rate determined using CCK-8 at 24 hours after incubation. As shown in Figure 2A, H2O2at 100 to 800 µM significantly induced cytotoxicity (P< 0.05). Compared with the control group, cell survival rate in the 600 µM H2O2group was approximately 50%. Therefore, 600 µM H2O2was used in the following experiments.
To confirm the protective effect of As-IV on RGC-5 cells exposed to H2O2, serial concentrations of As-IV (200, 100,50, 10, and 5 mg/L) were incubated with RGC-5 cells prior to induction of H2O2. Cell survival rates of H2O2-induced RGC-5 cells incubated with As-IV improved (Figure 2B).Moreover, as the dose of As-IV increased, cell survival rate visibly increased. The optimum concentration of As-IV was 100 mg/L, which was used in the following study.
As-IV reduced H2O2-induced apoptosis of RGC-5 cells
Apoptotic morphology is typified by physiological cell death and includes chromatin condensation and DNA fragmentation. DAPI was used to measure apoptosis. The control group (Figure 3A) showed homogeneous and round nuclei,while nuclei in the H2O2group showed chromatin condensation and DNA fragmentation (Figure 3B). In Figure 3C,pre-treatment with As-IV at 100 mg/L induced a significant reduction of apoptosis in RGC-5 cells.
As-IV prevented intracellular ROS accumulation in RGC-5 cells in H2O2 medium
Accumulation of ROS is a critical indicator of H2O2-induced oxidative stress. As illustrated in Figure 4, average fluorescence intensity of DCFH increased after hydrogen peroxide was added (peak value to right deviation). Furthermore,when AS-IV was added, average fluorescence intensity decreased (peak value to left deviation). These results indicate that H2O2visibly increases intracellular ROS levels, whereas pre-treatment with As-IV induced a marked decrease in ROS production.
As-IV inhibited loss of mitochondrial membrane potential in H2O2 medium
Loss of mitochondrial membrane potential is a marker of primary cell apoptosis. After 24 hours of H2O2exposure,mitochondrial membrane potential in the H2O2group was reduced (Figure 5B), reflected by increased green fluorescence. However, As-IV-treated cells mitigated this loss of mitochondrial membrane potential, indicating a protective effect of As-IV (Figure 5C).
As-IV supported cytochrome c release, suppressed intracellular Bax and caspase-3 expression levels, and increased Bcl-2 expression
Expression of apoptosis-related proteins was examined by western blot assay. Figure 6 shows higher Bax expression in the H2O2group compared with the control group (P< 0.05).However, pre-treatment of RGC-5 cells with 100 mg/L As-IV, followed by 600 µM H2O2for 24 hours, significantly suppressed H2O2-induced expression of Bax. Compared with the H2O2group, As-IV prevented the decrease in Bcl-2 expression (P< 0.05). Release of cytochrome c from mitochondria to the cytoplasm is an indicator of mitochondrial dysfunction, which ultimately triggers apoptosisviaa caspase-3 pathway.
Representative immunoblots (Figure 7A) show cytochrome c and caspase-3 expression in the control, H2O2, and As-IV groups. Compared with the H2O2group (P< 0.05),substantial decrease of cytosolic cytochrome c was found in cells pre-treated with As-IV (Figure 7B), while caspase-3 was partially suppressed (Figure 7C). Thus, our results indicate that As-IV has an anti-apoptotic effect.
Discussion
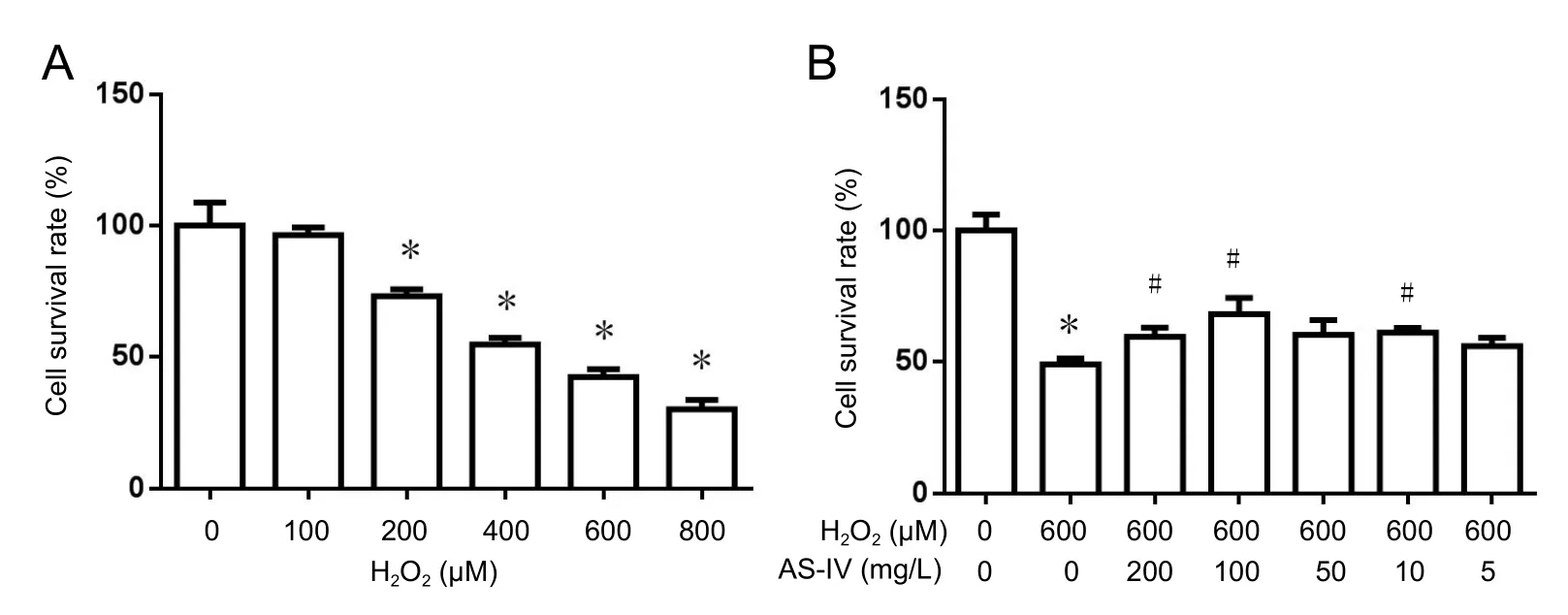
Figure 2 Astragaloside IV improved survival rate of RGC-5 cells following exposure to hydrogen peroxide (CCK-8 assay).
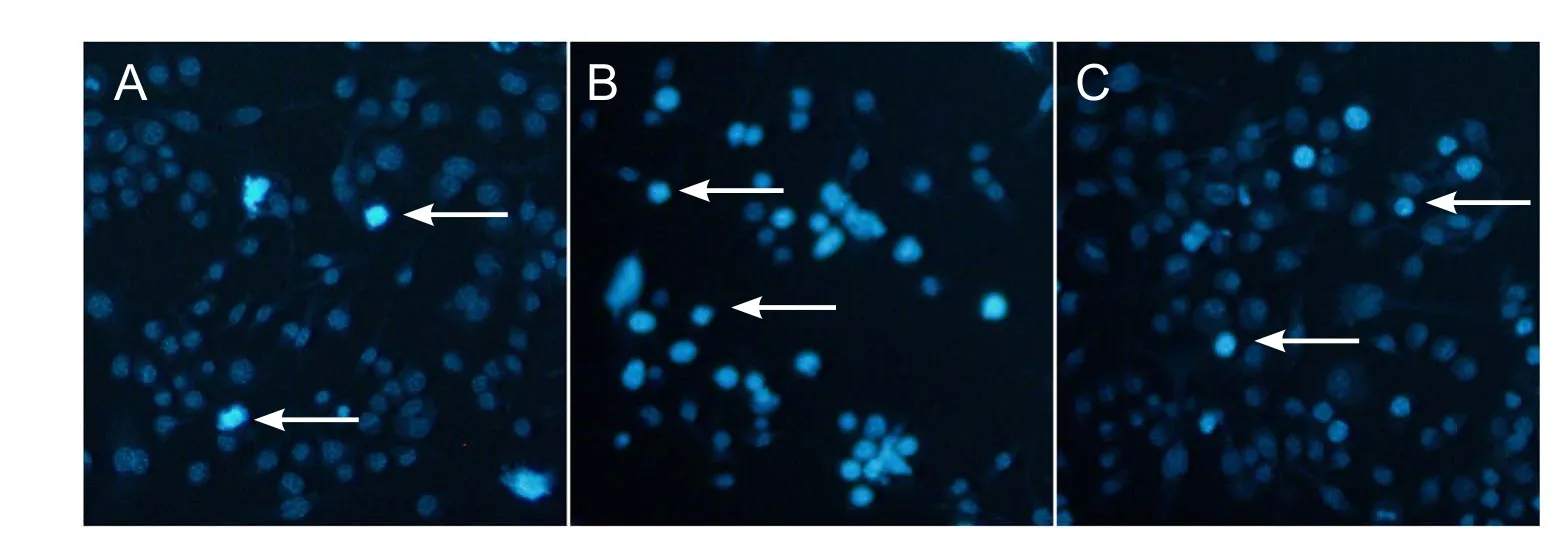
Figure 3 Astragaloside IV suppressed RGC-5 cell apoptosis induced by hydrogen peroxide DAPI staining, fluorescence microscopy).

Figure 4 Astragaloside IV prevented intracellular reactive oxygen species accumulation in RGC-5 cells.
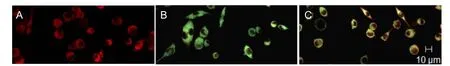
Figure 5 Astragaloside IV inhibited hydrogen peroxide-induced loss of mitochondrial membrane potential in RGC-5 cells.
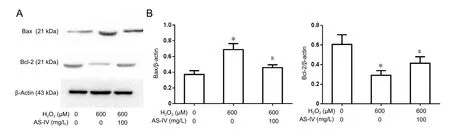
Figure 6 Effect of astragaloside IV on Bax and Bcl-2 expression.
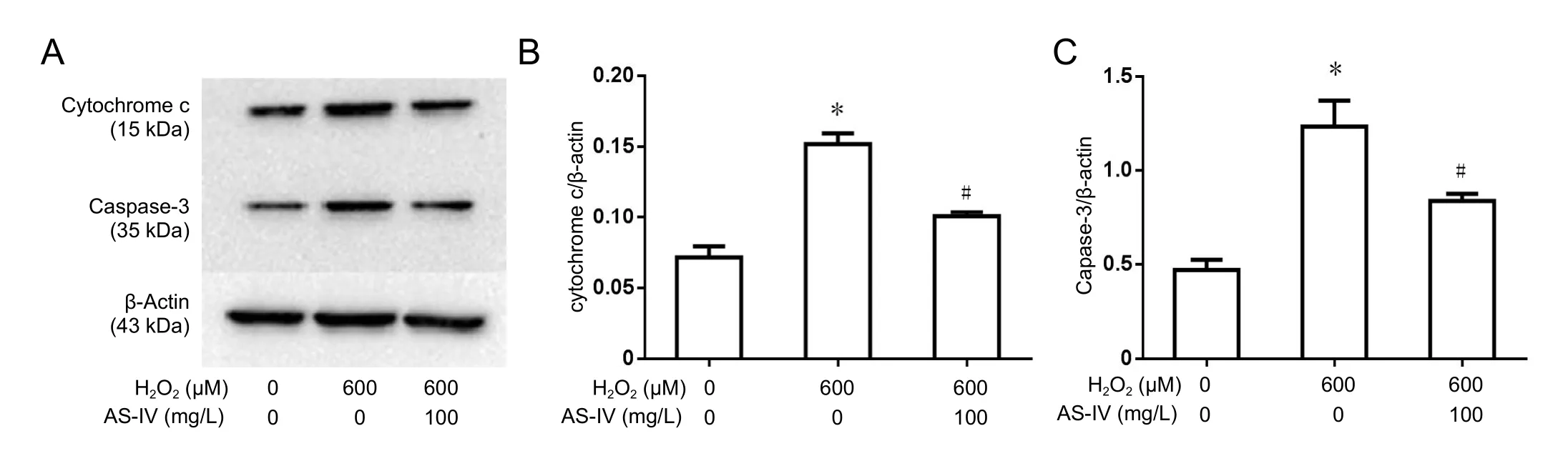
Figure 7 Effect of astragaloside IV on cytochrome c and caspase-3 expression in RGC-5 cells following exposure to H2O2 (western blot assay).
Oxidative stress participates in over 50 common diseases and is defined by an imbalance between the oxidant and antioxidant systems (Kowluru et al., 2007; Zhang et al., 2008a;Zhao et al., 2010; Zhu et al., 2012). However, intracellular signaling pathways of H2O2-induced cell death have not extensively investigated (Cai., 2013). Compared with other ROS, H2O2plays a key role because it is relatively stable,generated from nearly all sources of oxidative stress (Zhao et al., 2011), and is freely diffusible within and between cells.Exogenous H2O2can penetrate cells and induce high membrane permeability (Halliwell et al., 1992), which is predominantly generated during mitochondrial oxidative metabolism (Ray et al., 2012). Our study confirmed that RGC-5 cells incubated with H2O2led to a dose-dependent loss in viability.Astragalus membranaceuscan improve energy metabolism and inhibit apoptosis to alleviate nerve injury(Zhang et al., 2010; Huang et al., 2012). Many studies have shown that As-IV suppresses cell apoptosis (Chan et al.,2009, Sun et al., 2014). Furthermore, As-IV pre-treatment markedly and dose-dependently protects neurons (Chan et al., 2009), which is consistent with our study.
Pathogenesis of retinopathy not only relates to retinal vessels but is also strongly associated with neurons and glial cells of the retina. Appropriately, application of neuroprotective drugs can delay the occurrence of retinopathy (Zhang et al., 2011). In the retina, RGCs reflect the earliest differentiation of neurons and play a crucial role in visual signal processing of dark adaptation. Neurotrophic factors have been shown to protect neuronal and retinal cells, and delay the start of retinopathy (Hammes et al., 1995; Zhang et al.,2008b). Nevertheless, at present there is no study using As-IV for treatment for retinopathy. Here, using the RGC-5 cell line, we show a protective effect of As-IV that involves neuroprotective mechanisms through the anti-oxidative stress pathway. These results are coincident with previous studies showing that As-IV has diverse pharmacological properties such as anti-oxidative stress and neuroprotective effects(Chan et al., 2009). Thus, in RGCs, As-IV may have a neuroprotective effect that delays the start of retinopathy. This is a novel finding that has not been previously reported.
A major limitation of our current study is culture of the RGC-5 cell linein vitro. Consequently, we solely examined the effect of oxidative stress on cells and have not reproduced the in vivo environment. As-IV has been shown to have potential for prevention and treatment of retinal diseases including diabetic retinopathy, age-related macular degeneration, and glaucoma. In the future, we will further study the effect of As-IVin vivo.
In summary, our study shows for the first time that As-IV protects RGC-5 cells from H2O2-induced oxidative stress and apoptosis. Our findings will aid understanding of the underlying mechanisms of retinal injury.
Author contributions:HYK designed the study. MH performed experiments. YL and PC analyzed data. HJ and MH wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:The authors declare no competing financial interests.Financial support:This study was supported by a grant from the Education Department of Heilongjiang Province of China, No. 12541398. The funder did not participant in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Bearse MA Jr, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ(2004) Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest Ophthalmol Vis Sci 45:3259-3265.
Cai X, Chen X, Wang X, Xu C, Guo Q, Zhu L, Zhu S, Xu J (2013)Pre-protective effect of lipoic acid on injury induced by H2O2 in IPEC-J2 cells. Mol Cell Biochem 78:73-81.
Chan WS, Durairajan SS, Lu JH, Wang Y, Xie LX, Kum WF, Koo I,Yung KK, Li M (2009) Neuroprotective effects of Astragaloside IV in 6-hydroxydopamine-treated primary nigral cell culture. Neurochem Int 55:414-422.
Chen CC, Lee HC, Chang JH, Chen SS, Li TC, Tsai CH, Cho DY, Hsieh CL (2012) Chinese herb astragalus membranaceus enhances recovery of hemorrhagic stroke: double-blind, placebo-controlled, randomized study. Evid Based Complement Alternat Med 2012:708452.
Halliwell B, Gutteridge JM, Cross CE (1992) Free radicals, antioxidants,and human disease: where are we now? J Lab Clin Med 119:598-620.
Hammes HP, Federoff HJ, Brownlee M (1995) Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med 1:527-534.
Han ML, Liu GH, Guo J, Yu SJ, Huang J (2016) Imipramine protects retinal ganglion cells from oxidative stress through the tyrosine kinase receptor B signaling pathway. Neural Regen Res 11:476-479.
Huang XP, Tan H, Chen BY, Deng CQ (2012) Astragalus extract alleviates nerve injury after cerebral ischemia by improving energy metabolism and inhibiting apoptosis. Biol Pharm Bull 35:449-454.
Klein BE (2007) Overview of epidemiologic studies of diabetic retinopathy. Ophthal Epidemiol 14:179-183.
Kowluru RA, Kanwar M, Kennedy A (2007) Metabolic memory phenomenon and accumulation of peroxynitrite in retinal capillaries.Exp Diabesity Res 2007:21976.
Li M, Ma RN, Li LH, Qu YZ, Gao GD (2014) Astragaloside IV reduces cerebral edema post ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Cell Stress Chaperon 19:105-114.
Lu M, Wang H, Wang J, Zhang J, Yang J, Liang L, Maslov LN (2014) Astragaloside IV Protects against Cardiac Hypertrophy via Inhibiting the Ca2+/CaN Signaling Pathway. Planta Med 80:63-69.
Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW (2002) Chemical analysis of Radix Astragali (Huangqi ) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem 50:4861-4866.
Masuda T, Shimazawa M, Hara H (2017) Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone). Oxid Med Cell Longev 2017:9208489.
Meyer-Rüsenberg B, Pavlidis M, Stupp T, Thanos S (2007) Pathological changes in human retinal ganglion cells associated with diabetic and hypertensive retinopathy. Graefes Arch Clin Exp Ophthalmol 245:1009-1018.
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS)homeostasis and redox regulation in cellular signaling. Cell Signal 4:981-990.
Sun H, Wang W, Han P, Shao M, Song G, Du H, Yi T, Li S (2016)Astragaloside IV ameliorates renal injury in db/db mice. Sci Rep 6:32545.
Sun L, Li W, Li W, Xiong L, Li G, Ma R (2014) Astragaloside IV prevents damage to human mesangial cells through the inhibition of the NADPH oxidase/ROS/Akt/NF κB pathway under high glucose conditions. Int J Mol Med 34:167-176.
Sun Y, Jin L, Wang T, Xue J, Liu G, Li X, You J, Li S, Xu Y (2008) Polysaccharides from Astragalus membranaceus promote phagocytosis and superoxide anion (O2-) production by coelomocytes from sea cucumber Apostichopus japonicus in vitro. Comp Biochem Physiol C Toxicol Pharmacol 147:293-298.
Wang PC, Zhang ZY, Zhang J, Tong TJ (2008) Two isomers of HDTIC isolated from Astragali Radix decrease the expression of p16 in 2BS cells. Chin Med J (Engl) 121:231-235.
Xu D, Hu ZP (2008) The effect of astragalus injection on the brain water content and neuron ultrastructure of the experimental intracerebral hemorrhage in rats. J Practical Med 24:3308-3311.
Yau JW, Rogers SL, Kawasaki R (2012) Meta-analysis for eye disease(META-EYE) study group: global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556-564.
Ozawa Y, Kurihara T, Sasaki M, Ban N, Yuki K, Kubota S, Tsubota K(2011) Neural Degeneration in the retina of the streptozotocin-induced type 1 diabetes model. Exp Diabetes Res 2011:108328.
Qiao Y, Fan CL, Tang MK (2017) Astragaloside IV protects rat retinal capillary endothelial cells against high glucose-induced oxidative injury. Drug Des Devel Ther 11:3567-3577.
Zhang DG, Zhao Y, Huang XQ, Liu ZM, Xia PJ (2008) Effect of melatonin on oxidative stress and peripheral nerve function in rats with diabetic peripheral neuropathy. Zhongguo Zuzhi Gongcheng Yanjiu 12:9917-9920.
Zhang J, Wu Y, Jin Y, Ji F, Sinclair SH, Luo Y, Xu G, Lu L, Dai W, YanoffM, Li W, Xu GT (2008b) Intravitreal injection of ethropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci 49:732-742.
Zhang RP, Zhang XP, Ruan YF, Ye SY, Zhao HC, Cheng QH, Wu DJ(2009) Protective effect of Radix Astragali injection on immune organs of rats with obstructive jaundice and its mechanism. World J Gastroenterol 15:2862-2869.
Zhang XP ,Weng K ,Yu YP, Zhao HC, Cheng QH (2010) Protective effect and mechanisms of radix astragali injection on the intestinal mucosa of rats with obstructive jaundice. Mediators Inflamm 2010:757191.
Zhang Y, Zhang J, Wang Q, Lei X, Chu Q, Xu GT, Ye W (2011) Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci 52:278-285.
Zhao XC, Zhang L, Yu HX, Sun Z, Lin XF, Tan C, Lu RR (2011) Curcumin protects mouse neuroblastoma Neuro-2A cells against hydrogen-peroxide-induced oxidative stress. Food Chem 129:387-394.
Zhu XF, Zou HD (2012) PEDF in diabetic retinopathy: a protective effect of oxidative stress. J Biomed Biotechnol 2012:580-687.
- 中国神经再生研究(英文版)的其它文章
- Role of brain-derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish
- Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases
- How random is the random forest ? Random forest algorithm on the service of structural imaging biomarkers for Alzheimer’s disease: from Alzheimer’s disease neuroimaging initiative (ADNI) database
- Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury
- INVITED REVIEW
- Role of presynaptic calcium stores for neural network dysfunction in Alzheimer’s disease

