两个包含联苯三羧酸配体的铜ギ和锰ギ配合物的合成、晶体结构及磁性质
黎 彧 邹训重 顾金忠 成晓玲
(1广东轻工职业技术学院生态环境技术学院,佛山市特种功能性建筑材料及其绿色制备技术工程中心,广州 510300)(2兰州大学化学化工学院,兰州 730000)(3广东工业大学轻工化工学院,广州 510006)
0 Introduction
In recent years, the rational design and construction of coordination polymers have received remarkable attention due to their potential applications,architectures,and topologies[1-5].There are many factors,such as the coordination geometry of the metal centers,type and connectivity of organic ligands,stoichiometry,reaction conditions,template effect,presence of auxiliary ligands,and pH values influencing the structures of target coordination polymers during self-assembly[6-10].Among these factors,organic ligands play a noteworthy role in constructing coordination compounds.
Multi-carboxylate biphenyl ligands have been certified to be of great significance as constructors due to their strong coordination abilities in various modes,which could satisfy different geometric requirements of metal centers[8-9,11-14].In order to extend our research in this field,we chose one biphenyl tricarboxylic acid ligand,5-(3,4-dicarboxylphenyl)picolinic acid (H3dppa),to construct novel coordination compounds.The ligand possesses the following features:(1)it contains a pyridyl and a phenyl ring with structural flexibility and conformation.Rotation of the C-C single bond between pyridyl and phenyl rings could form numbers of coordination geometries of metal ions.(2)It has seven potential coordination sites,one N atom from pyridyl ring and six O atoms of three carboxylate groups,which is benifical to contruct coordination polymerw with interesting structures by its rich coordination modes. (3)It can act as hydrogen-bond acceptor as well as donor,depending upon the degree of deprotonation.
Taking into account these factors,we herein report the syntheses,crystal structures and magnetic properties of two Cuギ and Mnギ coordination compounds constructed from biphenyl tricarboxylic acid ligands.
1 Experimental
1.1 Reagents and physical measurement
All chemicals and solvents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded usingKBrpelletsandaBrukerEQUINOX 55 spectrometer.Thermogravimetric analysis(TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10℃·min-1.Magnetic susceptibility data were collected in the 2~300 K temperature range with a Quantum Design SQUID Magnetometer MPMS XL-7 with a field of 0.1 T.A correction was made for the diamagnetic contribution prior to data analysis.
1.2 Synthesis of[Cu2(Hdppa)2(4,4′-bipy)(H2O)4]·4,4′-bipy·6H2O (1)
A mixture of CuH2O (0.051 g,0.30 mmol),H3dppa(0.086 g,0.30 mmol),4,4′-bipy(0.047 g,0.3 mmol),NaOH(0.024 g,0.60 mmol),and H2O(10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃·h-1.Blue block-shaped crystals of 1 were isolated manually,and washed with distilled water.Yield:55%(based on H3dppa).Anal.Calcd.for C48H50Cu2N6O22(%):C 48.44,H 4.23,N 7.06;Found(%):C 48.59,H 4.27,N 7.02.IR(KBr,cm-1):3 667w,3 317w,2 979w,1 726w,1 603s,1 557w,1 493w,1 423w,1 382s,1 347s,1 307w,1 254 m,1 225w,1 143w,1 073w,1 044w,892w,852w,828w,805m,700w,664w,642w,583w.
1.3 Synthesisof{[Mn3(μ5-dppa)2(4,4′-bipy)(H2O)2]·4H2O}n(2)
The synthesis of 2 was similar as compound 1 using MnCl2·4H2O (0.059 g,0.30 mmol)instead of CuCl2·2H2O.Yellow block-shaped crystals of 2 were gained.Yield:60%(based on H3dppa).Anal.Calcd.for C38H32Mn3N4O18(%):C 45.76,H 3.23,N 5.62;Found(%):C 45.61,H 3.21,N 5.65.IR (KBr,cm-1):3 504w,3 312w,2 921w,1 592s,1 562s,1 487w,1 428w,1 399 m,1 307w,1 248w,1 213w,1 160w,1 090w,1 062w,1026w,1 003w,921w,903w,852m,811m,706w,658w,630w,589w.The compounds are insoluble in water and common organic solvents,such as methanol,ethanol,acetone and DMF.
1.4 Structure determinations
The diffraction data of two single crystals with dimensions of 0.25 mm×0.23 mm×0.21 mm(1)and 0.28 mm×0.23 mm×0.21 mm(2)was collected at 293(2)K on a Bruker SMART APEXⅡCCD diffractometer with Mo Kα radiation (λ=0.071 073 nm).The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-2014 program[15].All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.A summary of the crystallography data and structure refinements for 1 and 2 is given in Table 1.The selected bond lengths and angles for compounds 1 and 2 are listed in Table 2.Hydrogen bond parameters of compounds 1 and 2 are given in Table 3.
CCDC:1814154,1;1814155,2.

Table 1 Crystal data for compounds 1 and 2
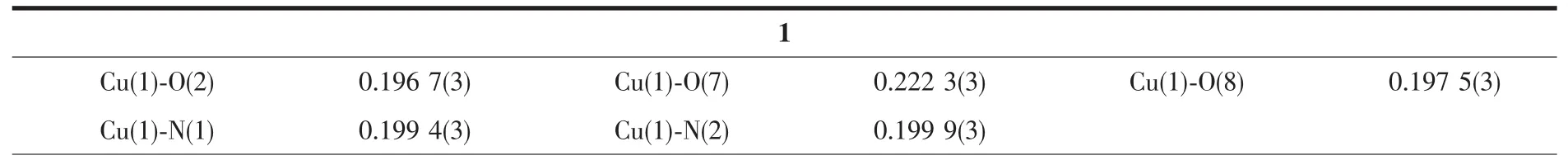
Table 2 Selected bond distances(nm)and bond angles (°)for compounds 1 and 2
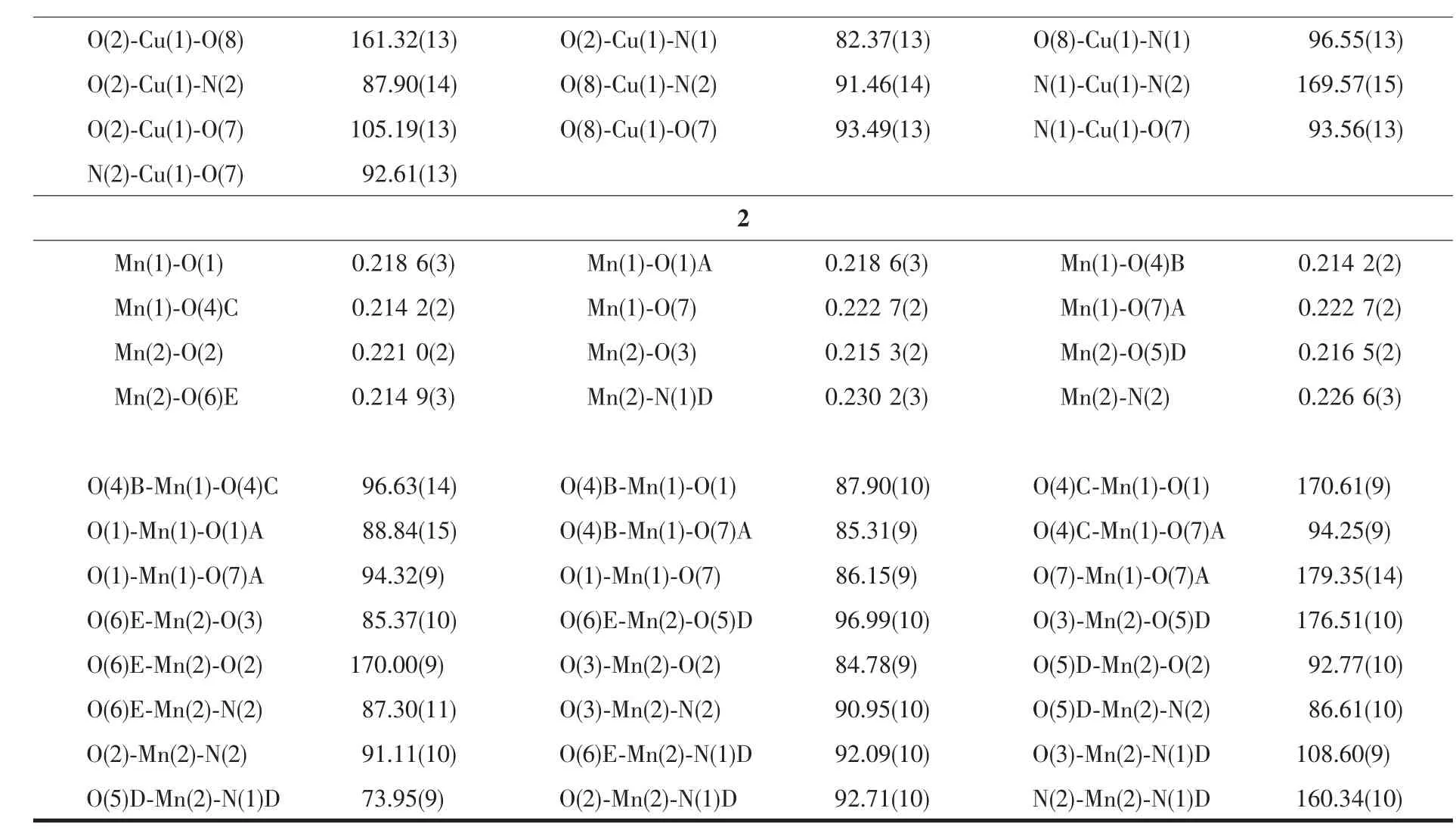
Continued Table 2
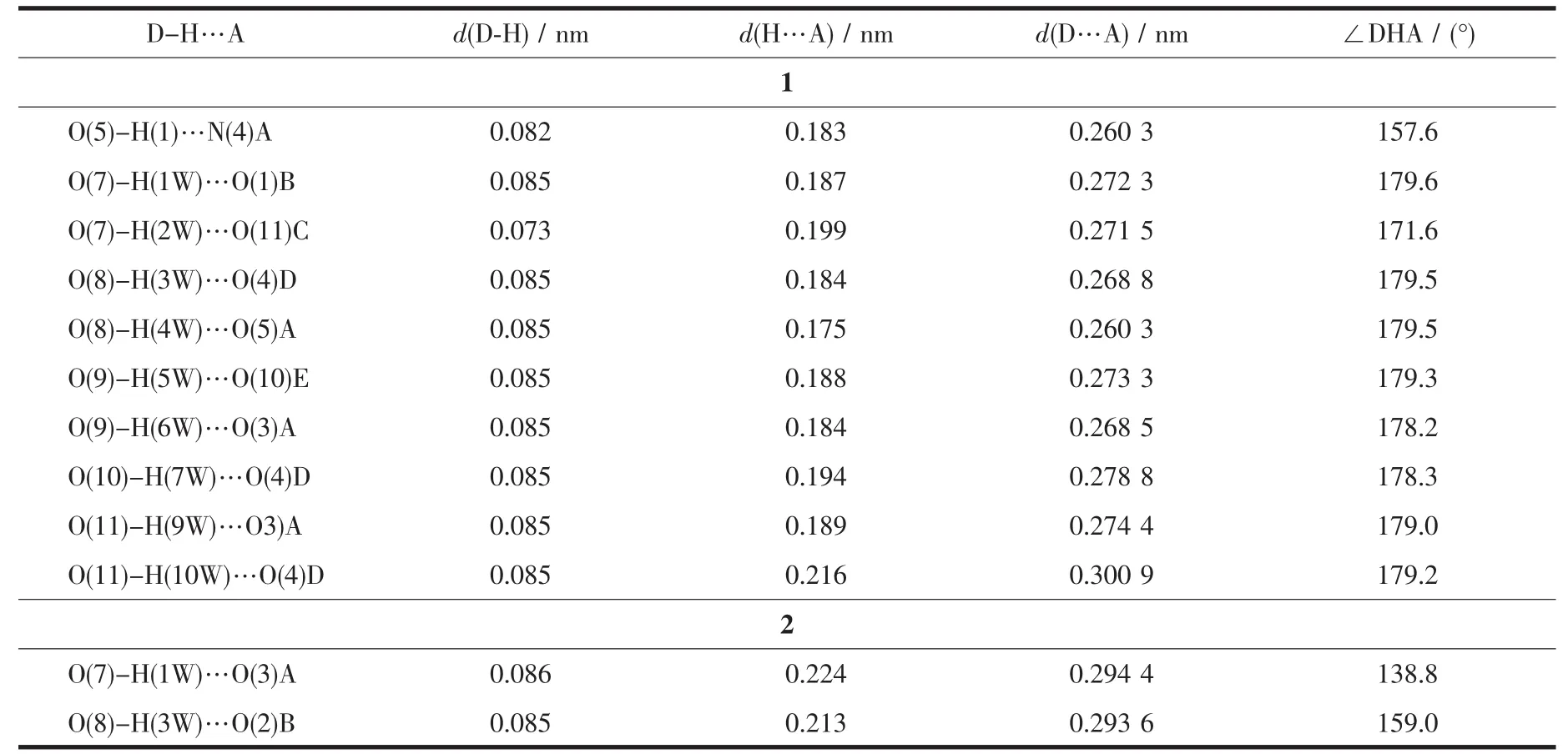
Table 3 Hydrogen bond parameters of compounds 1 and 2
2 Results and discussion
2.1 Description of the structure
2.1.1 [Cu2(Hdppa)2(4,4′-bipy)(H2O)4]·4,4′-bipy·6H2O(1)
Single-crystal X-ray diffraction analysis reveals that compound 1 crystallizes in the triclinic space group P1. Its asymmetric unit contains one crystallographically unique Cuギatom,one Hdppa2-block,a half of one 4,4′-bipy moiety,two H2O ligands,a half of one free 4,4′-bipy ligand,and three lattice water molecules.As depicted in Fig.1,Cu1 atom is surrounded by three O and two N atoms in a slightly distorted{CuO3N2}square-pyramidal geometry with the τ value of 0.138(τ=0 for a regular squarepyramidal geometry and τ=1 for a perfect trigonalbipyramidal geometry)[16].The two O(O2 and O8)and two N(N1 and N2)atoms occupy the basal plane,and one O(O7)atom resides at the apical position of the coordination polyhedron.The lengths of the Cu-O bonds range from 0.196 7(3)to 0.222 3(3)nm,whereas the Cu-N distances vary from 0.199 4(3)to 0.199 9(3)nm;these bonding parameters are comparable to those found in other reported Cuギcompounds[14,17].In 1,the Hdppa2-ligand adopts terminal coordination mode(modeⅠ,Scheme 1),in which the deprotonated carboxylate groups show the monodentate or uncoordinated modes.The dihedral angle between pyridyl and phenyl rings in the Hdppa2-is 17.51°.Two crystallographically equal Cuギ centers are bridged by the 4,4′-bipy ligand to form a discrete dinuclear copperギstructure with a Cu…Cu separation of 1.104(3)nm (Fig.2).These Cu2units are assembled to a 3D supramolecular framework through O-H…O/N hydrogen bond(Fig.3 and Table 3).

Scheme 1 Coordination modes of Hdppa2-/dppa3-ligands in compounds 1 and 2
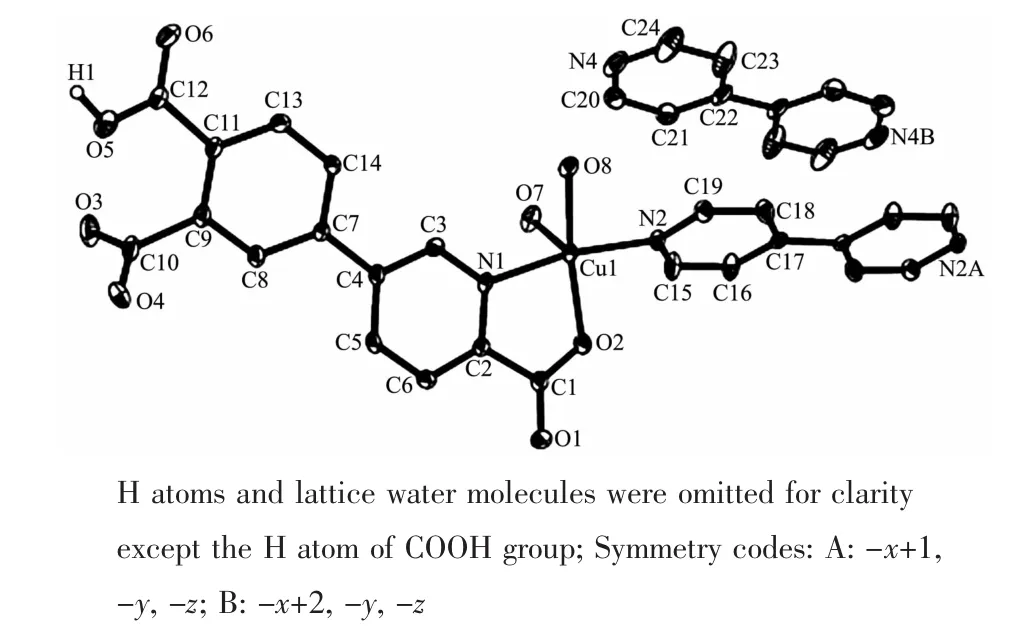
Fig.1 Drawing of the asymmetric unit of compound 1 with 30%probability thermal ellipsoids
2.1.2 {[Mn3(μ5-dppa)2(4,4′-bipy)(H2O)2]·4H2O}n(2)
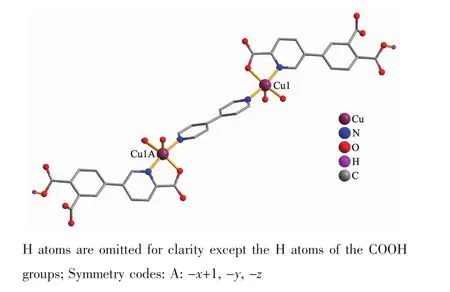
Fig.2 Dinuclear Cuギunit of 1

Fig.3 Perspective of 3D supramolecular framework parallel to the bc plane in 1

Fig.4 Drawing of the asymmetric unit of compound 2 with 30%probability thermal ellipsoids
The asymmetric unitof2 consistsoftwo crystallographically distinct Mn atoms(Mn1 with half occupancy;Mn2 with full occupancy),one μ5-appa3-block,a half of one 4,4′-bipy ligand,one coordinated and two lattice water molecules.As shown in Fig.4,six-coordinate Mn1 atom reveals a distorted octahedral{MnO6}environment,filled by four carboxylate O atoms from four individual μ5-dppa3-blocks and two O atoms from two H2O ligands.The Mn2 center is coordinated by four carboxylate O atoms from three distinct dppa3-moieties and two N atoms from two different 4,4′-bipy ligands,thus composing octahedral{MnO4N2}geometry.The Mn-O distances range from 0.214 2(2)to 0.222 7(2)nm, whereas the Mn-N distances vary from 0.226 6(3)to 0.230 2(3)nm;these bonding parameters are comparable to those observed in other Mnギcompounds[9,11,13].In 2,the dppa3-block acts as a μ5-N,O6-spacer and its COO-groups take a bidentate bridging mode (modeⅡ,Scheme 1).In dppa3-,a dihedral angle(between pyridyl and benzene rings)is 46.31°.The carboxylate groups of dppa3-blocks bridge alternately neighboring Mn atoms to form the infinite right-handed or left-handed helical Mn-O-C-O-Mn chains(Fig.5)with the Mn…Mn separation of 0.545 7(2)and 0.534 8(2)nm.Two types of these helical chains are interconnected to each other through the Mnギcenters to produce a double-helix chain (Fig.5).The adjacent double-helix subunits are further linked by the cptc3-blocks into a 2D sheet(Fig.6).These 2D sheets are arranged into a 3D framework by further coordination interactions of the dppa3-and 4,4′-bipy ligands to Mn atoms(Fig.7).
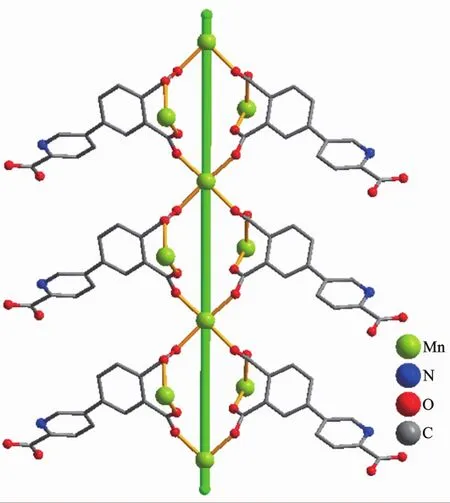
Fig.5 Double-helix chain unit in compound 2
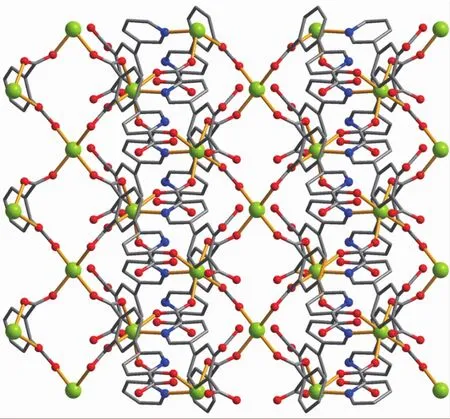
Fig.6 Two dimensional sheet along the c axis in compound 2
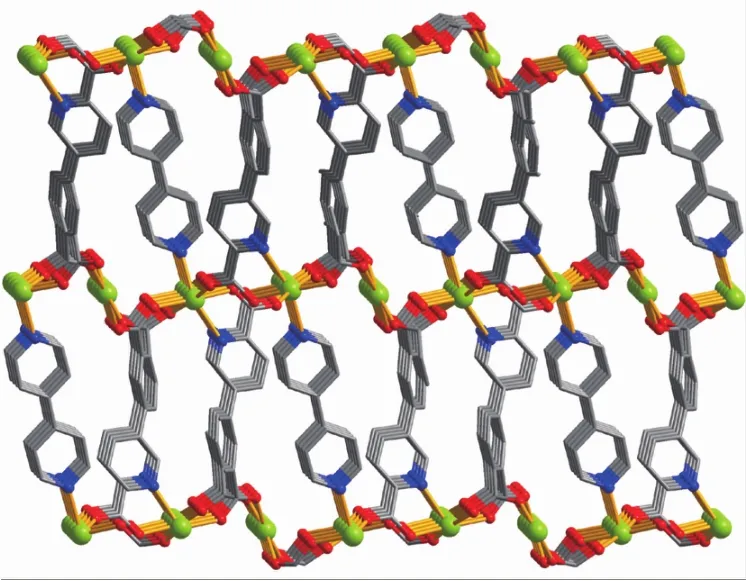
Fig.7 Three dimensional framework along the b axis in compound 2
2.2 TGA analysis
To determine the thermal stability of compounds 1 and 2,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis(TGA).As shown in Fig.8,compound 1 loses its six lattice water molecules in the range of 41~162℃(Obsd.8.8%,Calcd.9.1%),followed by the decomposition at 218℃.The TGA curve of 2 reveals that four lattice and two coordinated water molecules are released between 78 and 230℃(Obsd.10.5%,Calcd.10.8%),and the dehydrated solid begins to decompose at 334℃.
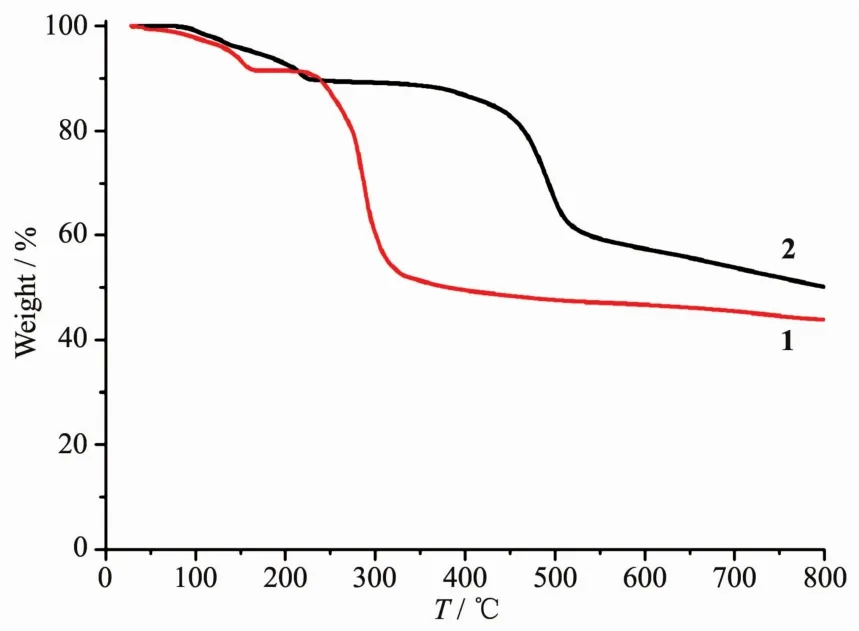
Fig.8 TGA curves of compounds 1 and 2
2.3 Magnetic properties
Variable-temperature magnetic susceptibility studies were carried out on powder sample of 2 in the 2~300 K temperature range.The χMT value at 300 K is 14.48 cm3·mol-1·K,which is larger than the value of 13.12 cm3·mol-1·K expected for three magnetically isolated high-spin Mnギcenters(SMn=5/2,g=2.0).Upon cooling,the χMT value drops down very slowly from 14.48 cm3·mol-1·K at 300 K to 13.97 cm3·mol-1·K at 100 K and then decreases steeply to 3.04 cm3·mol-1·K at 2 K(Fig.9).The χM-1vs T plot for 2 in the 2~300 K range obeys the Curie-Weiss law with a Weiss constant θ of-6.88 K and a Curie constant C of 14.78 cm3·mol-1·K.The negative value of θ and the decrease of the χMT should be attributed to the overall antiferromagnetic coupling between the Mnギcenters within double-helix chain unit.We attempted to fit the data for 2 by applying the following expression[18]for a 1D Mnギ chain:
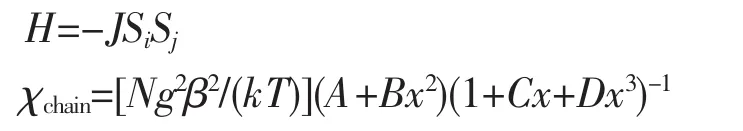
with A=2.916 7,B=208.04,C=15.543,D=2 707.2,and x=|J|/(kT).
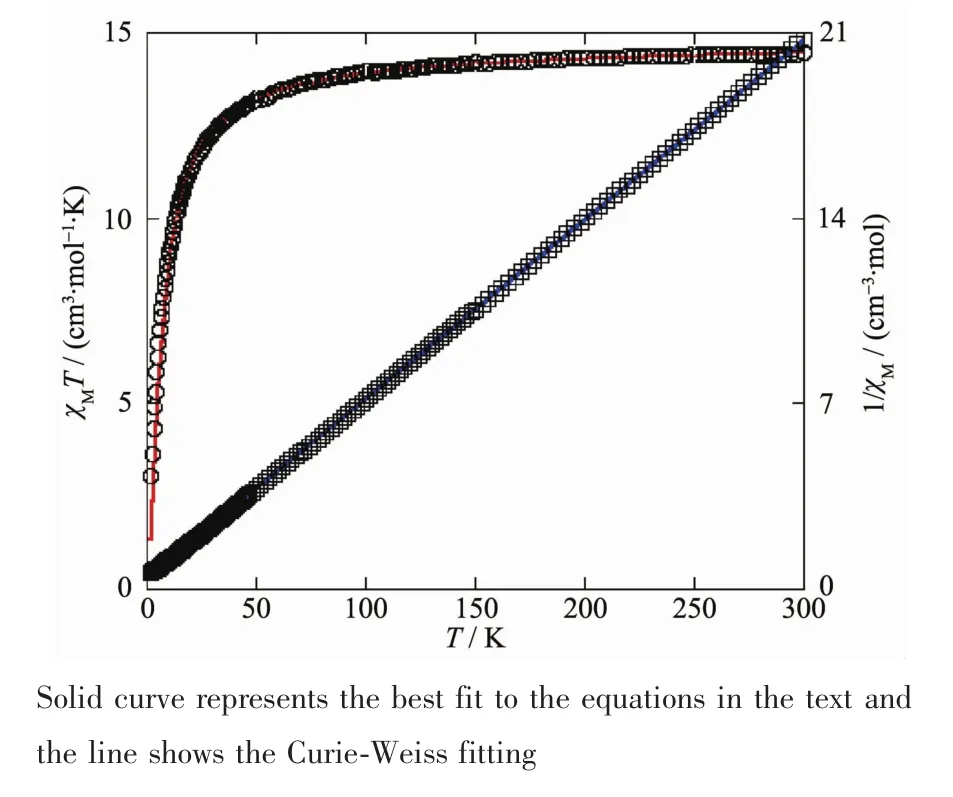
Fig.9 Temperature dependence of χMT(○)and 1/χM(□)vs T for compound 2
The susceptibility for 2 was simulated using this rough model,and resulting in J=-3.01 cm-1,g=2.07,and R=4.98×10-5.The negative J parameter indicates a weak antiferromagnetic exchange coupling between the adjacent Mnギcenters in 2,which is in agreement with a negative θ value.
3 Conclusions
In summary,two new coordination compounds,namely[Cu2(Hdppa)2(4,4′-bipy)(H2O)4]·4,4′-bipy·6H2O(1)and{[Mn3(μ5-dppa)2(4,4′-bipy)(H2O)2]·4H2O}n(2),have been synthesized under hydrothermal conditions.The compounds feature the 0D dinuclear and 3D framework structures,respectively.Magnetic studies show an antiferromagnetic coupling between the adjacent Mnギcenters in 2.
[1]Hu Z G,Zhao D.CrystEngComm,2017,19:4066-4081
[2]Lv R,Li H,Su J,et al.Inorg.Chem.,2017,56:12348-12356
[3]Li P Z,Wang X J,Liu J,et al.CrystEngComm,2017,19:4157-4161
[4]Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2012,112:1126-1162
[5]Kuppler R J,Timmons D J,Fang Q R,et al.Coord.Chem.Rev.,2009,253:3042-3066
[6]Gu J Z,Cui Y H,Liang X X,et al.Cryst.Growth Des.,2016,16:4658-4670
[7]Ji P F,Manna K,Lin Z,et al.J.Am.Chem.Soc.,2016,138:12234-12242
[8]Gu J Z,Gao Z Q,Tang Y.Cryst.Growth Des.,2012,12:3312-3323
[9]Gu J Z,Wu J,Lv D Y,et al.Dalton Trans.,2013,42:4822-4830
[10]Du M,Li C P,Liu C S,et al.Coord.Chem.Rev.,2013,257:1282-1305
[11]Gu J Z,Liang X X,Cui Y H,et al.CrystEngComm,2017,19:117-128
[12]LU Ya(鲁雅),LÜ Tian-Xi(吕天喜),HU Wei-Ji(胡未极),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(10):1869-1875
[13]ZHAO Su-Qin(赵素琴),GU Jin-Zhong(顾金忠).Chinese J.Inorg.Chem.(无机化学学报),2016,32(9):1611-1618
[14]CHEN Jin-Wei(陈金伟),WEN Bing-Song(温炳松),CAO Fang-Li(曹芳利),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(12):2322-2328
[15]Spek A L.Acta Crystallogr.Sect.C:Struct.Chem.,2015,C71:9-18
[16]Addison A W,Rao T N,Reedijk J,et al.J.Chem.Soc.Dalton Trans.,1984:1349-1356
[17]Gu J Z,Liang X X,Cai Y,et al.Dalton Trans.,2017,46:10908-10925
[18]Hiller W,Strhel J,Datz A,et al.J.Am.Chem.Soc.,1984,106:329-335

