Morphological characters and DNA barcoding of Syngnathus schlegeli in the coastal waters of China*
CHEN Zhi (陈治) ZHANG Yan (张岩) HAN Zhiqiang (韩志强) SONG Na (宋娜) GAO Tianxiang (高天翔)
1 Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China
2 Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
3 Fisheries College, Zhejiang Ocean University, Zhoushan 316022, China
1 INTRODUCTION
Syngnathus, a species-rich genus with 56 recognized species in family Syngnathidae (Dawson,1985; Kuiter, 2000; Koldewey and Martin-Smith,2010; Lourie et al., 2016; Shao, 2016), was particularly common and widely distributed in the Atlantic Ocean(Dawson, 1985; Riley et al., 1986; Rogers and Millner, 1996; Rogers et al., 1998; Kuiter, 2001; de Lussanet and Muller, 2007). As for Chinese coastal waters, abundantSyngnathusindividuals also existed in this coastline, and these common individuals were identified asS.acusby native ichthyologists (Zhang et al., 1955, 1962; Meng et al., 1962; Feng and Cao,1979; Shen, 1984; Zhu et al., 1984; Cheng and Zhou,1997; Li, 2011, 2015). Apart fromS.acus, another rare species identified asS.schlegeliwas also once collected in this area. According to the original descriptions of this species, it’s SL/HL (SL: standard length; HL: head length) was clearly distinguished from that ofS.acus. Only when the SL/HL ranged from 4.8 to 7.2, could aSyngnathusindividual be counted asS.schlegeli. Because except these two original descriptions themselves, other known records didn’t satisfy this unique range (Zhu et al., 1963; Sun and Chen, 2013),S.schlegeliwas long-termly thought an uncommon occurrence with quite difficult finds and the dominatingSyngnathusspecies seen every day in the coastal waters of China wasS.acus(Zhang et al., 1955, 1962; Meng et al., 1962; Feng and Cao,1979; Shen, 1984; Zhu et al., 1984; Cheng and Zhou,1997; Li, 2011, 2015).
Although onlyS.acusandS.schlegelihave been reported in the coastal waters of China (Shao, 2016;Zhang et al., 2017), in our opinion, taxonomic problems were not fully resolved within these two mentioned species (Zhang et al., 1955, 1962; Zhu et al., 1963; Shen, 1984, 1993; Kuiter, 2000; Shao,2015). In such a context, the first confusing phenomenon was the fact that the type species and central distribution area ofS.acuswere located in Europe (Linnaeus, 1758; Dawson, 1985; Riley et al.,1986; Rogers and Millner, 1996; Rogers et al., 1998;Kuiter, 2001; Mwale, 2005; de Lussanet and Muller,2007). It was also reported thatSyngnathushad a pretty week diffusion ability to spread worldwide(Mwale, 2005). Furthermore, in the view of Japanese ichthyological systematics, only oneSyngnathusspeciesS.schlegelirather thanS.acusexisted in Japanese coastal waters (see Tokiharu, 1986; Nakabo,2000, but also Dawson, 1985; Tokiharu, 1986; Nakabo,2000; Mwale et al., 2013; Shao, 2015). Spreading from the North-eastern Atlantic Ocean to million miles-away Chinese coastal waters but not to the approximately same far-away Japanese waters, the identification ofS.acusin China was really doubtful.
Besides, European taxonomic researchers have also largely studied localS.acus(Linnaeus, 1758;Dawson, 1985; Riley et al., 1986; Rogers and Millner,1996; Rogers et al., 1998; Kuiter, 2001; Mwale, 2005;de Lussanet and Muller, 2007). Even if the taxonomic status of ChineseS.acusproved to be true, compared to European abundant introductions, the morphological description about Chinese nativeS.acuswas rough and deficient (Zhang et al., 1955,1962, 1997; Zhu et al., 1963). Neither traditional medical books nor contemporary taxonomic publications of fishes gave a sufficiently and authoritatively morphological description about this pipefish species (Zhang et al., 1955, 1962, 2017;Meng et al., 1962; Feng and Cao, 1979; Shen, 1984;Zhu et al., 1984; Cheng and Zhou, 1997; Li, 2011; Li et al., 2015). They just underlined the preponderant distribution of a conspecificSyngnathusspecies and took it asS.acushabitually. Such deficient introduction made the identification ofS.acusin the coastal waters of China lack conclusively morphological evidence despite lots of literature records (Zhang et al., 1997;Sadovy and Cornish, 2000; Clarke, 2002; Wu et al.,2009; Cheng and Ken, 2012). According to our present information, localS.acusdistributed in China was also conflict with some newly increased descriptions about EuropeanS.acus(Zhang et al.,1955, 1962; Nijssen and Buizer, 1983; Tokiharu,1986; Nakabo, 2000; Wu, 2002; Mwale, 2005; Hu,2005; Sun and Chen, 2013).
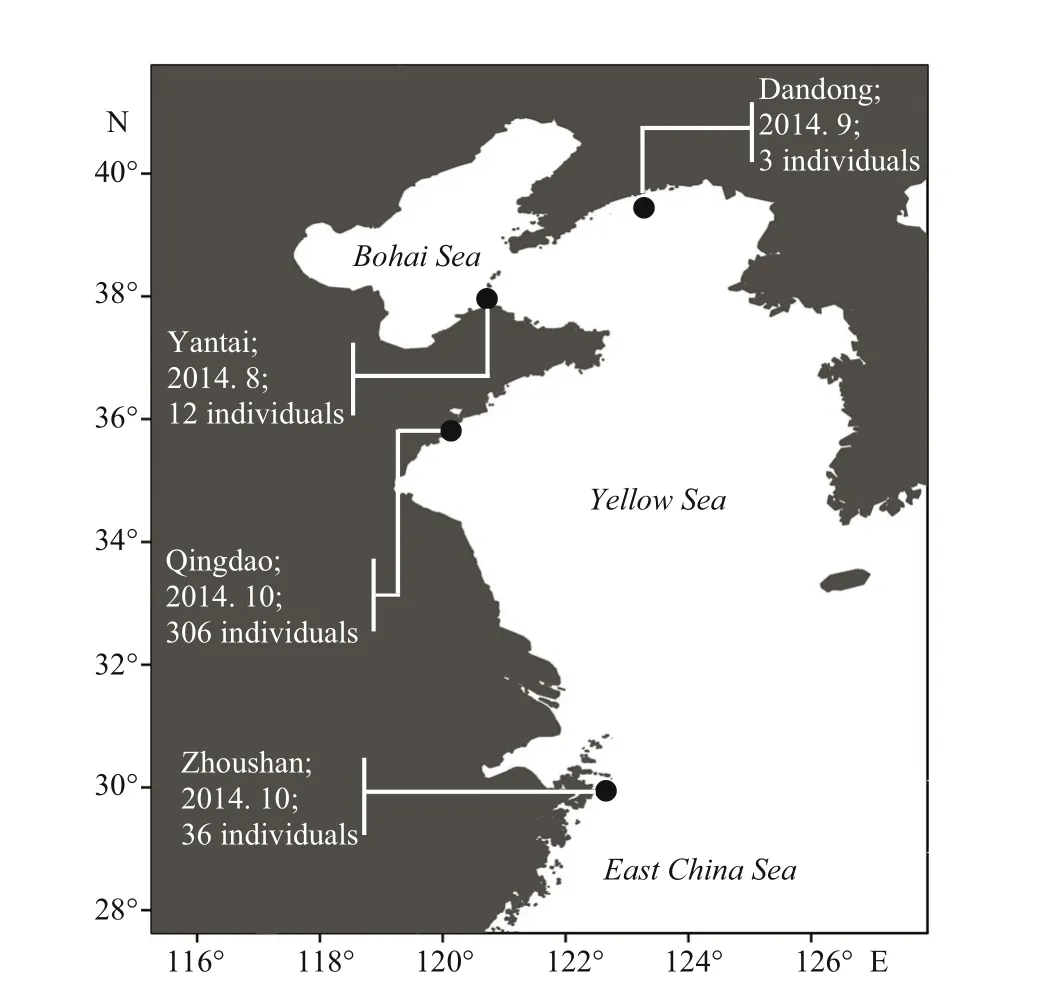
Fig.1 Sampling location, date, and number of Syngnathus individuals collected in this study
Meanwhile, it was reported thatS.schlegelispread from eastern Russia southward to Taiwan Island(Masuda et al., 1984), including the Japanese coast,Korean Peninsula, Ryuku Islands and Bonin Islands(Masuda et al., 1984; Dawson, 1985; Kim et al.,2005). It was noteworthy that the unique SL/HL criterion ofS.schlegeliproposed by Chinese gaugers was not adopted in any Japanese ichthyological systematics; thus, if we do not take SL/HL into consideration, an obviously morphological overlaps between ChineseS.acusandS.schlegeliwill be found (Zhang et al., 1955, 1962; Meng et al., 1962;Feng and Cao, 1979; Shen, 1984; Zhu et al., 1984;Cheng and Zhou, 1997; Li, 2011, 2015). The descriptions of ChineseS.acuswere synchronously consistent with the characteristics of JapaneseS.schlegeli.

Fig.2 An illustration of some morphometric and meristic characters described in Table 1 (Dawson, 1986)
In present study, we first performed a survey on morphological characters ofSyngnathuscollected from four locations, with the aim of providing plentifully morphological characters of this widelydistributed species and determining whether it was misidentified in China. A comparison among morphological data belonging to typicalS.acus,S.schlegeliand specimens we collected was conducted consequently. At the same time, a mitochondrial DNA barcoding approach was also employed by means of cytochrome oxidase subunit 1 (COI) gene sequencing,in order to solve the chaotically taxonomic problems of these specimens at genetic level.COIgene variability highly diverges between species but little within a conspecifics (Hajibabaei et al., 2007; Gao et al., 2011; Gross, 2012). It is suitable for identifying animal species (Bian et al., 2008; Puckridge et al.,2013). When an appropriate fragment of theCOIgene is used to identify controversial fish species, whether formerly recorded or uncovering cryptic ones, it has widely been proved effective and credible (Zemlak et al., 2009; He et al., 2011).
2 MATERIAL AND METHOD
2.1 Sampling
Specimens were collected from the coastal waters of Dandong, Yantai, Qingdao, and Zhoushan from August 2014 to October 2014 (Fig.1). All 357 individuals were identified based on morphological characteristics commonly-used by Herald (1941),Dawson (1985, 1986) and Mwale (Mwale, 2005;Mwale et al., 2013). A piece of freezed body tissue from each individual was then obtained and preserved in 95% ethanol. All examined specimens were preserved at the Fishery Ecology Laboratory, Fisheries College, Ocean University of China in Qingdao.
2.2 Morphological study
Counts and measurements followed the standard methods given by Dawson (1985) and Herald (1941).Measurements of body lengths were done on a measuring board graduated in 1.0 mm intervals. All other measurements were taken using dial calipers and recorded to the nearest 0.1 mm. Fin rays were counted using a magnifier and the rays of both pectoral fins were counted. Some fin-ray counts could not be determined because of the poor condition of this specimen. In such instances the reported values were taken from the original description (for holotypes) or the specimen was discarded from analysis. The number of subdorsal rings was the sum of subdorsal trunk and tail rings (Dawson, 1986; Mwale, 2005;Mwale et al., 2013). Table 1 and Fig.2 show the meristic and morphometric characters examined for specimens.
2.3 DNA extraction and sequencing
After morphometric measurements of all 357 specimens, 4 individuals from Yantai, 5 from Qingdao,4 from Zhoushan and all from Dandong were randomly selected for genetic studies. The classical phenol-chloroform technique was used for DNA extraction. Polymerase chain reaction (PCR) was subsequently conducted. The F and R sequences of the primers used forCOIamplification were 5′-TCGACTAATCATAAAGATATCGGCAC-3′ and 5′-ACTTCAGGGTGACCGAAGAATCAGAA-3′(Ivanova et al., 2007) respectively. PCR was carried out in a 25 μL reaction mix containing DNA template(1 μL, 50 ng/uL), forward primer (F, 1 μL, 10 μmol/L),reverse primer (R, 1 μL, 10 μmol/L), dNTPs (2 μL,2.5 mmol/L each), EasyTaq DNA Polymerase(0.15 μL, 5 U/μL) and 10× PCR buff er (2.5 μL,25 μmol/L). A Biometra thermal cycler (Göttingen,Germany) with the following given procedure: one initial denaturation (95°C, 5 min), thirty-five cycles consisting of denaturation (94°C, 50 s), annealing(54°C, 50 s) and extension (72°C, 48 s), and one final extension (72°C, 10 min), was employed to put PCR amplification into effect. PCR products were sent to Shanghai Majorbio Bio-Pharm Technology Co., Ltd.to get originalCOIsequences.

Table 1 morphological characters measured in our present study
2.4 COI analysis
All 16 individuals’ original sequences were successfully obtained and revised by DNASTAR software (DNASTAR Inc., Madison, WI, USA). OneCOIsequence ofHippocampustrimaculatusand 15 sequences ofSyngnathuswere also downloaded from NCBI for phylogenetic tree study (Table 2). These 32COIsequences were then aligned using the above DNASTAR software. MEGA 5.0 (Tamura et al., 2011)was used to construct maximum likelihood tree.
3 RESULT
3.1 Morphological characters
No individual with SL/HL ranging from 4.8 to 7.2 was found. The generally morphological features were shown in Fig.3. Body and head were moderately compressed. The specimens were mainly brownish,usually mottled with pale. Encased in a series of bony rings without any scales, these pipefish were typically slender and elongate. Their superior ridges of trunk and tail were not conjoined, but the inferior were. As for lateral trunk ridge, it extended alone the trunk andeventually disappeared in the vicinity of anal ring.Sometimes the confluent ends were not straight but upward-sloping, even connected to the extension line of superior ridges of tail. Besides, the ends of lateral trunk ridge were located in the last trunk ring or the first tail ring. No pipefish in this study had any lateral tail ridges. Median dorsal ridge of snout was entire and low, although there was no lateral spine on snout. In contrast to the result that no median dorsal body ridge had been found in any samples, a median ridge could be seen in ventral trunk. Opercle ridges were usually prominent and complete in early juveniles but typically incomplete or vestigial in sub-adult individuals. All ridges present in specimens were smooth.

Table 2 GenBank accession numbers of related COI sequences downloaded from NCBI for phylogenetic tree study

Fig.3 Lateral view of a specimen, 174.2 mm SL

Table 3 Comparative counts of S. schlegeli and S. acus from different records
In addition to 18–20 trunk rings and 39–43 tail rings,the specimens had 10 caudal-fin rays, 36–46 dorsal-fin rays, 12–14 pectoral-fin rays, and 2–4 anal–fin rays.Dorsal fin base usually started from the last trunk ring,rarely from the first tail ring or the penultimate trunk ring. The number of trunk rings covered by the DFB was 1 mainly, 0 or 2 rarely, and that of tail rings covered by the DFB was 9 mostly, 8 or 10 scarcely. Each snout was slender and longer, its length was 1.61–2.08 in head length and 0.43–0.67 in post-orbital length.Besides, snout depth was 5.23–9.33 in snout length.Head length was 2.05–2.68 in trunk length, 7.25–8.93 in standard length, 0.25–0.31 in post–orbital length,and 3.09–3.59 in pre–dorsal fin distance. Standard length ranged from 117 mm to 213 mm with an average length of 180.3 mm and was 0.11–0.14 dorsal-fin length. Snout depth was 5.28–9.33 in snout length and 1.67–3.33 in trunk depth. Trunk depth was 0.37–0.67 in pectoral–fin length and 0.26–0.47 in caudal-fin length.

Fig.4 Phylogenetic tree based on Maximum Likelihood analysis of COI sequence
Table 3 shows summaries of selected morphological characters of specimens in present and previous studies.
3.2 Sequence analysis of the COI gene
Sixteen 651-bp-long sequences ofCOIgene fragments were obtained. After combined the downloadedCOIsequences ofSyngnathus, a total of 32 sequences were used for analysis. Table 4 reported the genetic distances between specimens. The mean distance within individuals and among species was 0.52% and 13.88% respectively. Genetic distance between our 16 specimens andS.schlegelidownloaded from NCBI was only 0.49%. Among specimens in present study and otherSyngnathusspecies, genetic distances ranged from 12.98% to 20.79%, which vastly exceeded the threshold of species delimitation (~2%).
A maximum likelihood (ML) phylogenetic tree was constructed using MEGA 5.0 (Fig.4).Hippocampustrimaculatuswas chosen as the outgroup to root the tree. AllCOIsequences of specimens in present study clustered in the same group, and 11 haplotypes were defined. All haplotype sequences were submitted to GenBank with the following accession numbers: KR152210–KR152220. The haplotype 4 (Hap-4) was shared by 1 specimen from Yantai and 3 from Qingdao. The haplotype 8 (Hap-8)were shared by 2 specimens from Dandong and the haplotype 2 (Hap-2) by 2 individuals from Zhoushan.The remaining haplotypes were unique and each of them was shared by one specimen. At the same time,a large genetic distance (13.17%) between specimens andS.acusindicated that they couldn’t be the same species.

4 DISCUSSION
The morphological characters of specimens used in this study were photographed, counted and compared with previously representative records on Table 3. These individuals were characterized by 18–20 trunk rings, 39–43 tail rings, 10 caudal-fin rays,36–46 dorsal-fin rays, 12–14 pectoral-fin rays and 2–4 anal-fin rays. These phenotypic traits were consistent with the descriptions ofS.schlegelifrom Japan (Kaup, 1856; Dawson, 1986; Tokiharu, 1986;Nakabo, 2000; Mwale et al., 2013) andS.acusfrom Europe (Linnaeus, 1758; Mwale, 2005). Besides, by reviewing all known references including our present results, it could be obviously found thatS.schlegeliandS.acuswere very similar in meristic values.Almost all morphological characteristics such as SL/HL were overlapped (Linnaeus, 1758; Kaup, 1856;Dawson, 1985, 1986; Tokiharu, 1986; Nakabo, 2000;Mwale et al., 2013). Such overlaps appeared to be very common amongSyngnathusthat were either closely related or lived in similar habitats (Herald,1965; Fritzsche, 1980; Kuiter, 2000).
AlthoughS.schlegeliwas amazingly similar withS.acusand long-termly misidentified (Linnaeus,1758; Kaup, 1856; Zhang et al., 1955, 1962; Meng et al., 1962; Zhu et al., 1963, 1984; Herald, 1965; Feng and Cao, 1979; Fritzsche, 1980; Cheng and Zheng,1987; Cheng and Zhou, 1997; Kuiter, 2000; Li, 2011,2015), there were still some difference between these two species. The first distinguishable character was body color:S.acushad distinctive body rings, colored sandy brown with darker bars all along its body(Picton and Morrow, 2015). However,S.schlegeliwas mainly brownish, usually mottled with pale(Shao, 2015). The second distinction was body length:total length of specimens measured in this study ranged from 117 mm to 213 mm with an with an average 180.3 mm but that of adultS.acuswas generally 300 mm to 400 mm with a reported maximum length of 470 mm (Nakabo, 2000; Mwale,2005; Picton and Morrow, 2015; Shao, 2015). The maximum total length ofS.schlegeliup to now was only 300 mm (Shao, 2015). In fact, the English name ofS.acuswas “greater pipefish” (Linnaeus, 1758;Herald, 1941; Dawson, 1985). As the comparative word “greater” indicated,S.acuswas not only longer but also thicker much than most other species amongSyngnathus. The third difference wasS.schlegeliwith the name “Pacific seaweed pipefish” had a single northwest-Pacific distribution (Tokiharu, 1986;Nakabo, 2000).S.acus, however, was distributed in waters along the Eastern Atlantic Ocean including the Mediterranean and Black seas (Dawson, 1986;Mwale, 2005; Shao, 2015). These three difference could separateS.schlegelifromS.acuseasily when identifying.
COIsequence was recognized as an effective and reliable method for species identification (Hebert et al., 2003; Domingues et al., 2013; Qin et al., 2013).Our analysis indicated thatS.schlegelispecimens in this study could be morphologically different from otherSyngnathusspecies. Moreover, the genetic distance between specimens and twoSyngnathusspecies,S.acusandS.schlegeli, was 13.17% and 0.49% respectively. Taxonomic identifications as well asCOI-sequence accuracy of EuropeanS.acusand JapaneseS.schlegelihave already been recognized for many years. This reality provided our DNA barcoding studies a strongly genetic level support that the speciesS.schlegeliin the coastal waters of China was misidentified.
It should be noted that aS.acusCOIsequence(JF494653) from GenBank did not cluster with other twoS.acussequences. JF494653 was most likely wrong. As we have mentioned above,S.acuswas mainly existent along the Eastern Atlantic Ocean(Dawson, 1986; Mwale, 2005; Shao, 2015).Individuals of GQ502180 and KJ128631 were consistent with this distribution. Kuiter (2000) has also pointed out that the pipefish long-termly regarded asS.acusin southern Africa differed considerably from the trueS.acusin Mediterranean and Northeastern Atlantic. Local long-snout pipefish of southern Africa was later re-identified asS.temminckii(Dawson, 1985, 1986; Kuiter, 2000; Mwale et al.,2013). Thus, the individual of sequence JF494653 collected from Agulhas should beS.temminckiias well. The misidentification ofS.schlegeliin China andS.temminckiiin southern Africa once again manifested thatSyngnathusappeared to be a genus complex and easily confusable that required carefully taxonomic revision (Fritzsche, 1980; Kuiter, 2000).
Attention should be paid to the standard lengths of Zhoushan specimens. Because from Table 3, we can obviously see that these specimens were much smaller than other three populations. The standard lengths of specimens recorded inFishesofEastChinaSearanged from 102 mm to 138 mm (Zhu et al., 1963),which were consistent with our Zhoushan population and also shorter than those of Dandong, Yantai,Qingdao specimens. Based on the observation that Zhoushan specimens lack brood pouch trace and were colored little flaxen meaning immaturation inS.schlegeli(Shao, 2015), these specimens were thought juvenile individuals. However, it was pretty strange that not only us but also others didn’t once collect adult specimens (Zhu et al., 1963; Wu, 2002, 2005;Sun and Chen, 2013). Furthermore, populations of larger size were found in colder environments, and specimens of smaller size were found in warmer regions. Bergmann’s rule has been proved by a mass of studies of terrestrial vertebrates (Rypel, 2014).According to a latest study, this science does also apply to some fishes (Rypel, 2014). Body size in our present study correlated positively with latitude. This may be another reason why Zhoushan specimens seemed so short.
It was also reported thatSyngnathuswas widely distributed in the coastal waters of China including the South China Sea (Zhang et al., 1955, 1962, 2017;Meng et al., 1962; Feng and Cao, 1979; Shen, 1984;Zhu et al., 1984; Cheng and Zhou, 1997; Li, 2011,2015). We have also tried our best to collect the specimens from southern regions. But it seemed that fishermen and researchers could hardly see the occurrence ofSyngnathusspecies in the South China Sea. The inundant distribution in Shandong province and infrequent presence in the South China Sea made a sharp contrast.FishesofTaiwanhas also stated it clearly that people in Taiwan Island scarcely witnessedS.schlegeli(Shen, 1984, 1993). ThisSyngnathusspecies may prefer cold water and live in higher latitude areas. Further domestic and overseas specimen collection is also indispensable in order to define its clearly geographic limits.
5 CONCLUSION
The morphological characters andCOIsequence analysis demonstrated there were significant differences between specimens and other species of the same genus, which revealed that specimens collected from the coastal waters of China wereS.schlegeliin reality.S.schlegeliwas recorded asS.acusfor a long time. Our present study clarified this previous species misidentification. We hope this study will not only promote the sustainable exploitation,biodiversity conservation and fisheries management ofSyngnathusbut also contribute to species identification within this genus in the future.
6 ACKNOWLEDGEMENT
Mrs. FANG Y. L. and Mrs. LIU L. contributed much to this paper. Thanks were also given to Mr.CHENG P. and Mr. LI C. for sample collection.
Bian X D, Zhang X M, Gao T X, Xiao Y S. 2008. Morphological and genetic identification of Japanese halfbeak(Hyporhamphussajori) eggs.JournalofFisheriesof China,32(3): 342-352. (in Chinese with English abstract)
Cheng L, Ken C S H. 2012. A summary of pilot research searching for pipefish in Hong Kong.Eco-Education&ResourcesCentreandGreenPower,16: 1-13.
Cheng Q T, Zheng B S. 1987. Systematic Synopsis of Chinese Fishes. Science Press, Beijing, China. 1 458p. (in Chinese)
Cheng Q T, Zhou C W. 1997. The Fishes of Shandong Province.Shandong Science and Technology Press, Jinan, China.549p. (in Chinese)
Clarke S. 2002. Trade in Asian dried seafood: characterization,estimation and implications for conservation. Wildlife Conservation Society Working Paper 22. United States,New York, USA. 1-95.
Dawson C E. 1985. Indo-Pacific Pipefishes (Red Sea to the Americas). The Gulf Coast Research Laboratory,Pascagoula, Mississippi, America. 230p.
Dawson C E. 1986. Family No.145: syngnathidae.In: Smith M M, Heemstra P C eds. Smiths’ Sea Fishes. Macmillan,South Africa. p.445-458.
de Lussanet M H E, Muller M. 2007. The smaller your mouth,the longer your snout: predicting the snout length ofSyngnathusacus,Centriscusscutatusand other pipette feeders.JournaloftheRoyalSocietyInterface,4(14):561-573.
Domingues R R, de Amorim A F, Hilsdorf A W S. 2013.Genetic identification ofCarcharhinussharks from the southwest Atlantic Ocean (Chondrichthyes:Carcharhiniformes).JournalofAppliedIchthyology,29(4): 738-742.
Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap.Evolution,39(4): 783-791.
Feng Z X, Cao Q. 1979. Ichthyology. Agriculture Press,Beijing, China. 217p. (in Chinese)
Fritzsche R A. 1980. Revision of the eastern pacific syngnathidae (Pisces: Syngnathiformes), including both recent and fossil forms.ProceedingsoftheCalifornia AcademyofSciences,42(6): 181-227.
Gao T X, Ji D P, Xiao Y S, Xue T Q, Yanagimoto T, Setoguma T. 2011. Description and DNA barcoding of a newSillagospecies,Sillagosinica(Perciformes: Sillaginidae), from coastal waters of China.ZoologicalStudies,50(2): 254-263.
Gross M. 2012. Barcoding biodiversity.CurrentBiology,22(3): R73-R76.
Hajibabaei M, Singer G A C, Hebert P D N, Hickey D A. 2007.DNA barcoding: how it complements taxonomy,molecular phylogenetics and population genetics.Trends inGenetics,23(4): 167-172.
He W P, Cheng F, Li Y X, Liu M, Li Z J, Xie S G. 2011.Molecular identification ofCoiliaectenesandCoilia mystusand its application on larval species.Acta HydrobiologicaSinica,35(4): 565-571.
Hebert P D N, Ratnasingham S, de Waard J R. 2003. Barcoding animal life: cytochromecoxidase subunit 1 divergences among closely related species.ProceedingsoftheRoyal SocietyB:BiologicalSciences,270(S1): S96-S99.
Hebert P D, Stoeckle M Y, Zemlak T S, Francis C M. 2004.Identification of birds through DNA barcodes.PLoS Biology,2(10): e312.
Herald E S. 1941. A systematic analysis of variation in the western American pipefish,Syngnathuscaliforniensis.StanfordIchthyologicalBulletin,2: 49-73.
Herald E S. 1965. Studies on the Atlantic American pipefishes with descriptions of new species.Proceedingsofthe CaliforniaAcademyofSciences,32: 363-375.
Ivanova N V, Zemlak T S, Hanner R H, Hebert P D N. 2007.Universal primer cocktails for fish DNA barcoding.MolecularEcologyNotes,7(4): 544-548.
Kaup L S. 1856. Catalog of Lophobranch Fishes. British Museum, London, Britain. 48p.
Koldewey H J, Martin-Smith K M. 2010. A global review of seahorse aquaculture.Aquaculture,302(3-4): 131-152.
Kuiter R H. 2000. Seahorses, Pipefishes and Their Relatives: A Comprehensive Guide to Syngnathiformes. TMC Publishing, Chorleywood, UK. 859p.
Kuiter R H. 2001. Revision of the Australian seahorses of the genusHippocampus(Syngnathiformes: Syngnathidae)with descriptions of nine new species.Recordsofthe AustralianMuseum,53(3): 293-340.
Li C X, Bian H R, Zou G L. 2001. Study on sea medical annimal Syngathidae-Chinese.AminoAcids&Biotic Resources,23(2): 6-9. (in Chinese with English abstract)
Li J D, Huang L Q, Li C Y. 2015. The Illustrated Book of Chinese Medicinal Animals. Fujian Scientific and Technical Publishers, Fuzhou, China. 1 340p. (in Chinese)
Li M D. 2011. Taxonomy of Fishes. China Ocean Press,Beijing, China. 294p. (in Chinese)
Linnaeus C. 1758. Systema Naturae Per Regna Tria Naturae:Secundum Classes, Ordines, Genera, Species, Cum Characteribus, differentiis, Synonymis, Locis. Decima Company, Stockholm, Sweden. 824p.
Lourie S A, Pollom R A, Foster S J. 2016. A global revision of the SeahorsesHippocampusRafinesque1810(Actinopterygii: Syngnathiformes): taxonomy and biogeography with recommendations for further research.Zootaxa,4146(1): 1-66.
Meng Q W, Miao X Z, Su J X, Yu T J, Liu M. 1962. Ichthyology(Part 2). Agriculture Press, Beijing, China. 261p. (in Chinese)
Mwale M, Kaiser H, Barker N P, Wilson A B, Teske P R. 2013.Identification of a uniquely southern African clade of coastal pipefishesSyngnathusspp.JournalofFish Biology,82(6): 2 045-2 062.
Mwale M. 2005. The biology and systematics of south African pipefishes of the genusSyngnathus. A thesis submitted in fulfilment of the requirements for the degree of doctor of philosophy of Rhodes University. Rhodes University Press, Grahamstown, Cape province, South Africa. 222p.
Nakabo T. 2000. Fishes of Japan with Pictorial Keys to the Species. Tokai University Press, Tokyo, Japan. 1 748p.
Nijssen H, Buizer D A G. 1983. First record of the worm pipefish, Nerophis lumbriciformis (Pennant, 1776) in coastal waters of the Netherlands, with notes on other animal species recently recorded from the Oosterschelde.BulletinZoologischMuseum,9(22): 209-214.
Picton B E, Morrow C C. 2015.Syngnathusacus(Linnaeus,1758).In: Encyclopedia of Marine Life of Britain and Ireland. National Museums of Northern Ireland. http://www.habitas.org.uk/marinelife/species.asp?item=ZG3760.Accessed on 2016-03-02.
Puckridge M, Andreakis N, Appleyard S A, Ward R D. 2013.Cryptic diversity in flathead fishes (Scorpaeniformes:Platycephalidae) across the Indo-West Pacific uncovered by DNA barcoding.MolecularEcologyResources,13(1):32-42.
Qin Y, Song N, Zou J W, Zhang Z H, Cheng G P, Gao T X,Zhang X M. 2013. A new record of a flathead fish(Teleostei: Platycephalidae) from China based on morphological characters and DNA barcoding.Chinese JournalofOceanologyandLimnology,31(3): 617-624.
Riley J D, Symonds D J, Woolner L E. 1986. Determination of the distribution of the planktonic and small demersal stages of fish in the coastal waters of England, Wales and adjacent areas between 1970 and 1984. Fisheries Research Technical Report Number 84. Ministry of Agriculture,Fisheries and Food, Directorate of Fisheries Research.p.1-23.
Rogers S I, Millner R S, Mead T A. 1998. The Distribution and Abundance of Young Fish on the East and South Coast of England (1981 to 1997). Centre for Environment Fisheries and Aquaculture Science, Lowestoft, UK. 130p.
Rogers S I, Millner R S. 1996. Factors affecting the annual abundance and regional distribution of English inshore demersal fish populations: 1973 to 1995.OxfordJournals Science&MathematicsICESJournalofMarineScience,53(6): 1 094-1 112.
Rypel A L. 2014. The cold-water connection: bergmann's rule in North American freshwater fishes.TheAmerican Naturalist,183(1): 147-156.
Sadovy Y, Cornish A S. 2000. Reef Fishes of Hong Kong.Hong Kong University Press, Hong Kong, China. 321p.
Shao K T. 2015. The fish database of Taiwan. WWW Web Electronic Publication. http://fishdb.sinica.edu.tw.Accessed on 2015-03-12.
Shao K T. 2016. The Fish Database of Taiwan. WWW Web electronic Publication. http://fishdb.sinica.edu.tw/mobi/home.php. Accessed on 2016-09-30.
Shen S C. 1984. Synopsis of Fishes of Taiwan. Nantian Press,Taipei, Taiwan, China. 533p. (in Chinese)
Shen S C. 1993. Fishes of Taiwan. National Taiwan University Press, Taipei, Taiwan, China. 960p. (in chinese)
Sun D R, Chen Z. 2013. Synopsis of South China Sea. China Ocean Press, Beijing, China. 606p. (in Chinese)
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods.Molecular BiologyandEvolution,28(10): 2 731-2 739.
Tokiharu A. 1986. Keys to the Japanese Fishes Fully Illustrated in Colors. Hokuryukan, Tokyo, Japan. 228p. (in Japanese)
Ward R D, Zemlak T S, Innes B H, Last P R, Hebert P D. 2005.DNA barcoding Australia’s fish species.Philosophical TransactionsoftheRoyalSocietyB:BiologicalSciences,360(1462): 1 847-1 857.
Wu H L. 2002. New Records of Chinese Toxic and Medicinal Fish. China Agriculture Press, Beijing, China. 679p. (in Chinese)
Wu H L. 2005. An Atlas of Ichthyotoxic, Medicinal and Dangerous Fishes. Shanghai Scientific and Technical Publishers, Shanghai, China. 482p. (in Chinese)
Wu Y, Liu J, Wang M Y, Ju P J, Li X B. 2009. Identification onSyngnathusand its adulterants with random amplified polymorphic DNA.ChinaJournalofChineseMateria Medica,34(14): 1 758-1 760. (in Chinese with English abstract)
Zemlak T S, Ward R D, Connell A D, Holmes B H, Hebert P DN. 2009. DNA barcoding reveals overlooked marine fishes.MolecularEcologyResources,9(S1): 237-242.
Zhang C L, Cheng Q T, Zheng B Z, Li S Z, Zheng W L, Wang W B. 1955. The Investigation Reports of Fishes in Yellow and Bohai Seas. Science Press, Beijing, China. 362p. (in Chinese)
Zhang C L, Cheng Q T, Zheng B Z, Wu X W, Meng Q W. 1962.Fishes of South China Sea. Science Press, Beijing, China.1 184p. (in Chinese)
Zhang Y H, Qin G, Zhang H X, Wang X, Lin Q. 2017. DNA barcoding reflects the diversity and variety of brooding traits of fish species in the family Syngnathidae along China’s coast.FisheriesResearch,185: 137-144.
Zhang Z H, Xu G J, Xu L H, Wang Q. 1997. Survey on medicinal resources of Syngnathidae.JournalofChinese MedicinalMaterials,20(5): 224-226. (in Chinese with English abstract)
Zhu Y D, Wu X W, Meng Q W, Su J X. 1984. The Fishes of Fujian Province (Part 1). Fujian Science and Technology Press, Fujian, China. 528p. (in Chinese)
Zhu Y D, Zhang C L, Cheng Q T. 1963. Fishes of East China Sea. Science Press, Beijing, China. 642p. (in Chinese)
 Journal of Oceanology and Limnology2018年2期
Journal of Oceanology and Limnology2018年2期
- Journal of Oceanology and Limnology的其它文章
- An aftereffect of global warming on tropical Pacific decadal variability*
- Analysis of monthly variability of thermocline in the South China Sea*
- A numerical study of the South China Sea Warm Current during winter monsoon relaxation*
- Predicting the sinkage of a moving tracked mining vehicle using a new rheological formulation for soft deep-sea sediment*
- Chemical characterization of fractions of dissolved humic substances from a marginal sea—a case from the Southern Yellow Sea*
- The morphological and molecular detection for the presence of toxic Cylindrospermopsis (Nostocales, Cyanobacteria) in Beijing city, China*
