Macrophage depletion and Schwann cell transplantation reduce cyst size after rat contusive spinal cord injury
Yee-Shuan Lee , Lucy H. Funk , Jae K. Lee , Mary Bartlett Bunge ,
1 The Miami Project to Cure Paralysis, University of Miami Miller School of Medicine, Miami, FL, USA
2 Department of Neurological Surgery, University of Miami Miller School of Medicine, Miami, FL, USA
3 Department of Cell Biology, University of Miami Miller School of Medicine, Miami, FL, USA
Introduction
Extensive work on rat Schwann cell transplantation has led to phase I clinical trials to evaluate the safety of autologous human Schwann cell transplants after subacute and chronic spinal cord injury (SCI) (Guest et al., 2013; Bunge et al.,2016). Human Schwann cells are capable of promoting axon regeneration as well when transplanted into nude athymic rats with completely transected spinal cords (Guest et al.,1997). The Phase I clinical trial for Schwann cell transplantation into subacute spinal cord injured subjects, completed in 2015 in the Miami Project, was found to be safe (Bunge et al., 2016). Clinical trials in Iran and China also have found Schwann cell transplantation to be safe (Saberi et al., 2008,2011; Zhou et al., 2012).
Whereas Schwann cell transplantation is a promising SCI therapy, there are concerns about Schwann cell survival and integration with the host tissue. Schwann cell transplantation alone leads to a reduction in cyst size and provides a scaffold to improve axon regeneration across the injury site (Takami et al., 2002). Past studies have shown that only a small percent of descending axons that enter the transplant exit into the caudal cord (Bamber et al., 2001; Plant et al., 2001; Williams et al., 2015). This could be due to axons preferring the more favorable environment of the transplant than the neighboring spinal cord and/or the presence of chondroitin sulfate proteoglycans, molecules that inhibit axon growth, at the caudal host-graft scar interface (Plant et al., 2001).
Combination strategies are likely required to enhance the repair of SCI because of the manifold tissue changes that occur after SCI. Various therapeutic combinations with Schwann cells, such as the addition of methylprednisolone(Chen et al., 1996), growth factors such as neurotrophins(Xu et al., 1995; Menei et al., 1998; Blits et al., 2003; Golden et al., 2007; Enomoto et al., 2013; Kanno et al., 2014),chondroitinase (Fouad et al., 2005; Kanno et al., 2014) or increased cyclic AMP (Pearse et al., 2004) have been shown to result in greater axon regeneration into the Schwann cell graft than when the Schwann cells are transplanted alone.Work in rats has shown that early after transplantation,70–80% of transplanted cells undergo cell death (Hill et al.,2006; Pearse et al., 2007).
An acute immune response after SCI including in filtration of hematogenous macrophages, activation of microglia, and release of inflammatory factors that attract other inflam-matory cells contribute to the poor survival of transplanted cells and lead to cavity formation (Okano et al., 2003; Coyne et al., 2006; Assinck et al., 2017). In rats, treatment to deplete hematogenous macrophages subsequent to SCI with clodronate liposomes without cell transplants improves axon sprouting/regeneration and reduces cavitation (Popovich et al., 1999). When Schwann cell transplants were combined with other treatments to provide anti-inflammatory effects,the combinatory treatments improved axon regeneration(Pearse et al., 2004; Hill et al., 2006) and functional recovery (Pearse et al., 2004) compared to just Schwann cell transplants alone. As improvements have been seen with Schwann cell transplantation or hematogenous macrophage depletion alone, we investigated here whether the combination of the two would lead to an enhanced repair effect.
Materials and Methods
Animals
A total of 22 adult female Fischer 344 rats (160–180 g, Envigo Inc., Frederick, MD, USA) were housed according to the National Institutes of Health (NIH) and United States Department of Agriculture (USDA) guidelines. After receiving contusion injury, rats were randomly assigned to receive macrophage depletionviainjection of liposomes filled with clodronate (SC/CLO group) or no depletionviainjection of liposomes filled with phosphate buffered saline (PBS) (SC alone group). The institutional Animal Care and Use Committee (IACUC) of the University of Miami approved all animal procedures (IACUC protocol #15-079). A detailed timeline of the experimental procedures is shown in Figure 1.
GFP-Schwann cell preparation
Purified Schwann cell cultures were obtained from sciatic nerves of 2 adult female Fischer 344 rats (Envigo Inc., Frederick, MD, USA) following previously published protocols(Meijs et al., 2004). The resulting cultures were purified to greater than 95% (Takami et al., 2002). At passage 2 and at approximately 50% confluence, Schwann cells were transduced in D10/mitogen medium overnight with a lentiviral vector encoding enhanced green fluorescent protein (GFP),genes from Aequorea victoria, at a multiplicity of infection of 30. D10 medium consisted of Dulbecco’s modified Eagle’s medium (DMEM, ThermoFisher Scientific, Carlsbad CA, USA) and 10% fetal bovine serum (FBS, GE Healthcare Life Sciences, Logan UT, USA); the added mitogens were pituitary extract (20 μg/mL, Biomedical Technologies S.L.,Madrid, Spain), forskolin (2 μM, Sigma-Aldrich, St. Louis MO, USA), and heregulin (2.5 nM, Genentech, San Francisco CA, USA). The production of the lentiviral vectors has been detailed elsewhere (Follenzi and Naldini, 2002; Blits et al., 2005). The transduction efficiency in the Schwann cell cultures was greater than 90%.

Figure 1 Experimental timeline for this study.
Contusion injury
At day 0, adult female Fischer 344 rats (n= 22) were anesthetized with 3–5% isoflurane (AttaneTM isoflurane, Minrad International Inc., Orchard Park, NY, USA) in oxygen using a tight-fitting facemask. The back was shaved and a 3 to 4 cm longitudinal incision was made. A laminectomy was performed at thoracic level 8 (T8) to expose the dorsal surface of the spinal cord. Moderate contusion was induced using the Infinite Horizons Impactor at T8 (175 kDynes;Precision Systems and Instrumentation, LLC, Fairfax Station VA, USA). The animals were then sutured to close the muscles and stapled to close the skin. Postoperative treatment for the first week included subcutaneous injections: twice daily of Lactated Ringer’s solution (5 mL) and once daily of an antibiotic (Gentamicin, 10 mg/kg; APP Pharmaceuticals,LLC, Schaumburg, IL, USA) for 7 days, and twice daily for 3 days of an analgesic (buprenorphine, 0.05 mg/kg; Buprenex,Reckitt Benckiser Healthcare Ltd, Berkshire, UK). Twice daily bladder expressions continued until rats regained bladder control.
Clodronate administration
Liposomes filled with clodronate (50 mg/k g body weight;Encapsome, Brentwood TN, USA) were injected intraperitoneally at 1, 3, 6, 11, and 18 days following contusion injury(timeline in Figure 1) into the animals in the SC/CLO group.For animals in the SC alone group, the same volume of liposomes filled with PBS, based on the animal’s body weight, was injected via the same route. The rats were anesthetized with 5% isoflurane and their abdomens were cleaned with 70% ethanol prior to each injection. The liposome vials were brought to room temperature and the contents mixed by gently inverting the containers before loading into the syringe. Macrophage depletionviathis administration route was tested by giving the same dose of clodronate or PBS to 3 naïve animals (adult female Fischer 344 rats, 160–180 g, Envigo Inc.) in each group at days 1, 3, and 6, and then perfused at day 7.
GFP-Schwann cell transplantation
Rats were anesthetized 1 week post injury using 3–5% isoflurane (Minrad International Inc.) in oxygen using a tight- fitting facemask. The staples were removed from the incision created for contusion injury. The wound was re-opened by gently pulling the skin, removing the sutures in the fascia and muscles, separating the muscles and removing the scar tissue that had formed over the contusion. The T7 vertebral process was then stabilized using a clamp in the stereotaxic frame. Two million GFP-Schwann cells were re-suspended in DMEM/F12 to a final volume of 6 μL. The cells were then loaded into a 10 μL Hamilton syringe (701RN 10 μL SYR syringe, barrel ID 0.485 mm/0.019 in, Hamilton Company,Reno, NV, USA) attached to a syringe pump (sp310i, World Precision Instruments, Sarasota, FL, USA). A pulled glass pipette was placed on the end of the needle for injecting the GFP-Schwann cells. The syringe was then positioned over the center of the injury site avoiding any intact vessels. To ensure there was no dead space between the injector and the head of the plunger in the syringe, the volume setting on the pump was advanced until a drop of cells was seen at the glass tip. The glass tip was inserted about 1.4 mm into the spinal cord and cells were injected at 2 μL/min for a total of 6 μL. To reduce leakage after injection, the glass tip was leftin the animal for 3 minutes and the animal was not moved for an additional 3 minutes following removal of the glass tip. The animals were then sutured to close the muscles and stapled to close the skin. Animals received postoperative treatment for the first week as described above.
Biotinylated Dextran Amine (BDA) injections into the spinal cord at T5
At 8 weeks post-transplantation, a T5 laminectomy was performed and the animals were then clamped into the stereotaxic frame. A 10 μL Hamilton syringe and a glass tip were used to inject 0.3 μL of BDA (10,000 MW, 10% in phosphate buffered saline (PBS); ThermoFisher Scientific, San Jose, CA,USA) at the following coordinates: 7.0 and 5.5 mm rostral to the rostral edge of the injury, +/– 0.3 mm lateral to the midline, and 1.5 mm beneath the cord surface. The injection rate for the BDA was 0.3 μL/min and subsequent to each injection the glass tip was left for 1 minute before moving to the next coordinate. The animals were then sutured to close the muscles and stapled to close the skin. Animals received postoperative treatment for the 5 days as described aboveand then perfused. BDA injection was performed after function assessments; some animals were lost due to the additional surgery (n= 16 total;n= 8/group remaining).
Paraformaldehyde (PFA) perfusion
At Day 68 or an additional 5 days after BDA injection (Figure 1), rats were terminally anesthetized with ketamine (60 mg/kg; Vedco Inc., Saint Joseph, MO, USA) and xylazine(12 mg/kg; Lloyd Laboratories, Manila, Philippines) and transcardially perfused with chilled heparinized saline (0.9%NaCl, pH 7.4) followed by ice-cold 4% PFA in PBS (pH 7.4).Following perfusion, the spinal cord was removed and postfixed in 4% PFA for 2 hours. Cords were then placed in 30%sucrose for 1 to 2 days or until they no longer floated in the solution. The spleen was removed in animals perfused for macrophage depletion testing, fixed overnight in 4% PFA,and then placed in 30% sucrose.
Function assessments
The Gridwalk test was used to assess the rat’s motor control (Kunkel-Bagden et al., 1993). Rats were placed on a metal grid with 2 inch × 2 inch spacing. Prior to the injury,the animals underwent baseline training in which the animals walked continuously for at least 2 minutes on the grid.At 8 weeks post transplantation, the animals were filmed for 4 minutes; continuous walking for 30 seconds was used to score the number of foot falls or slips.
Assessment of sensory recovery was performed using specifically calibrated von Frey filaments (Chaplan et al., 1994).Rats were placed in a Plexiglas chamber on top of a wire mesh floor and their hind paws were stimulated with the von Frey filaments. The withdrawal response was assessed;if the paw was withdrawn, a lower force filament was used.The stimulation continued until the lowest force stimulated a response consecutively for 3 times or to a maximum of 20 stimulations. All animals responded before 20 stimulations.The animals were assessed at baseline and 2, 4, 6, and 8 weeks following Schwann cell transplantation.
Immunohistochemical staining
The spleen was embedded in O.C.T. (optimum cutting temperature) compound and 20 μm coronal sections were prepared. A 1.5 cm spinal segment centered at the injury site was embedded in 12% gelatin and sectioned sagittally on a cryostat into 20 μm serial sections (n= 16 total;n= 8/group). Slides with sections were stored at –20°C and thawed at room temperature for 1 hour before staining. Thawed sections were washed 3 times with 1 × PBS for 5 minutes each and incubated for 1 hour in 5% normal goat serum(NGS) in 1 × PBS with 0.3% TritonX-100. Sections were subsequently immunostained with primary antibodies for:rabbit anti-Iba1 (macrophage/microglia, Wako Chemicals,#019-19741, 1:500, only in naïve animals), mouse anti-glial fibrillary acidic protein (GFAP, MilliporeSigma, Burlington MA, USA; #NE1015, 1:500), rabbit anti-neurofilament(medium-chain, NF-M, Encor Biotechnologies, Gainesville FL, USA; #RPCA-NF-M, 1:500), and chicken anti-GFP(Abcam, Cambridge MA, USA; #ab13970; 1:1000) in 5%NGS, and 0.3% TritonX-100 overnight at 4°C with the exception of NF-M staining, performed at room temperature.The next day the sections were washed 3 times in PBS-0.3%TritonX-100 (NF-M staining, 5 times) and the sections were then incubated with the corresponding Alexa Fluor secondary antibodies (ThermoFisher Scientific, goat anti-rabbit,mouse, or chicken IgG, 1:500) for 1 hour at room temperature. Following a final washing stage (3 times in PBS-0.3%TritonX-100; 5 minutes each), sections were coverslipped with Vectashield mounting solutioncontaining DAPI (Vector Laboratories, Burlingame CA, USA; #H-1200), and images were collected with a Nikon Eclipse Ti fluorescent microscope (Nikon Instruments Inc., Melville, NY, USA). BDA labeling was visualized using a streptavidin-568 antibody(ThermoFisher Scientific; #S11226, 1:200). The streptavidin antibody was diluted in 5% NGS with 0.3% TritonX-100 and applied overnight in 4°C. The next day the sections were washed and mounted as described above.
Data analysis
The area of a Schwann cell transplant was outlined in ImageJ(NIH, Bethesda MD, USA) by identifying GFP-positive areas; then the volume was calculated. A 50 μm2grid was then used to measure GFP-positive regions within the outlined areas. ImageJ identified a 50 μm2square as GFP-positive if there were more than 2 GFP-positive cells in each 50 μm2square. Cysts were identified and outlined using DAPI to identify areas with no cells. The GFAP-positive border was outlined around the injury site and the area within the border that was GFP-negative and GFAP-negative was defined as the lesion area, which included the cyst. For quantification of BDA-positive axons, 3 dorsoventral lines were drawn:(1) at the midpoint of the rostral/caudal line spanning the largest GFP-positive cell mass (the transplant epicenter); (2)500 μm rostral to the GFP-positive transplant border; and (3)500 μm caudal to the GFP-positive transplant border. The numbers of BDA-positive axons that crossed each line were counted and axon density was calculated by dividing the number of BDA-positive axons by the length of each dorsoventral line. To count the number of neurofilament-positive axons, 50 μm2grids were generated over the injury site and every sixth square in the GFP-positive region was quantified. Three sections were counted for each animal and 6–9 animals were counted for each group.
Statistics
All data are represented as mean ± standard error. The statistical significance comparing cyst volumes, lesion volumes,transplant volumes, and numbers of NF-positive axons was assessed using the unpairedt-test. The rest of the function results were compared using one-way ANOVA with repeated measures with the Tukey’spost hoctest. Numbers of BDA-positive axons were compared using two-way ANOVA with the Tukey’spost hoctest.T-test and two-way ANOVA were performed using GraphPad prism software v6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Gridwalk was assessed using the unpaired 2-tailedt-test with Welch’s correction. The BBB and von Frey tests were assessed using one-way ANOVA with repeated measures and Tukey’spost hoctests were performed using SPSS v24.0 (IBM Corp, Armonk, NY, USA).
Results
Macrophage depletion was observed in spleen tissue when clodronate was administered with a similar dosage (Figure 2B) compared to animals injected with liposomes containing PBS (Figure 2A).
Significant reduction in cyst and lesion volumes
Cyst volumeswere reduced by 4 fold (Figure 3C) in animals with the combinatory treatment (Figure 3Bvs. A).Cysts, identified by the absence of DAPI-positivecells, frequently surrounded the Schwann cell transplant in animals with Schwann cell transplants alone (Figure 3A), reducing Schwann cell-host cord interfaces. The lesion volume[defined as Schwann cell (GFP)-negative and astrocyte(GFAP)-negative, including the cysts] was reduced by 35%in the animals with the combinatory treatment (Figure 3D).The Schwann cell (GFP) transplant volumes (Figure 3E)were similar in animals with Schwann cell transplants alone(Figure 3A) and the combined treatment (Figure 3B).
Similar BDA-positive and NF-positive axon regeneration
BDA-positive axons, present in the Schwann cell transplant in animals with Schwann cell transplants alone (Figure 4A–C) and the combinatory treatment (Figure 4D–F), were not significantly different in number at the epicenter (Figure 4G). The total number of BDA-positive axons in the graft and host tissue at the epicenter of animals with Schwann cell transplants alone was significantly higher than in the graft in both groups and in host tissue in animals with the combined treatment (Figure 4G). The numbers of BDA-positive axons did not differ in both rostral and caudal spinal cord away from the injury between the groups (Figure 4H). However,the number of BDA-positive axons in the caudal spinal cord of animals with the combinatory treatment was significantly less than in the rostral spinal cord from both groups (Figure 4H). About 1/3 of the BDA-positive axons found rostrally were present in the cord caudal to the transplant (Figure 4H). When the area occupied by the axons was considered, the density showed no difference in the graft between groups (Figure 4I). However, density in the graft tissue of animals with Schwann cell transplants alone was significantly greater than in the host tissue of animals with the combinatory treatment (Figure 4I). The density in the caudal host cord was significantly lower than in the rostral host cord in animals with the combined treatment (Figure 4J).Robust NF-positive axon growth was observed in animals with Schwann cell transplants alone (Figure 5A–C) and the combined treatment (Figure 5D–F) in the epicenter. The NF-positive axon density in Schwann cell transplants did not differ between the groups (Figure 5G).
Similar functional recoveries
Prior to injury, all rats scored 21 on the BBB scale, indicating no abnormalities in locomotion. One day following injury, all rats scored either a 0 or 1, indicating they had at most slight movement in the hip and/or knee and were all similarly injured. Over the following weeks, the rats regained locomotor ability with no distinction between the groups in either the BBB score (Figure 6A) or the subscore(Figure 6B). Similarly, rats performed equally well on the Gridwalk test; the number of hind paw slips or falls did not differ between groups (Figure 6C). Following injury, animals with Schwann cell transplants alone needed increased force to provoke a response that peaked at week 4 following transplantation compared to the baseline value (Figure 6D and E); the animals with the combinatory treatment were closer to their baseline scores than those receiving Schwann cell transplants alone (Figure 6D and E). By 8 weeks post transplantation, there was no difference between the groups.
Discussion
Previous studies indicated that macrophage depletion using liposome-encapsulated clodronate after SCI leads to improved pathology including smaller lesion size and increased axon growth in multiple species (Popovich et al., 1999; Zhu et al., 2014). Due to the translational potential of this pharmacological approach to macrophage depletion, we combined this approach with Schwann cell transplantation as a strategy to increase Schwann cell survival and axon growth into the graft. We predicted that the combined treatment would result in a larger Schwann cell graft and increased axon regeneration into the transplant. At 8 weeks after Schwann cell transplantation, there was a significant reduction in cyst and lesion volumes in the combined treatment group as compared to Schwann cell transplantation alone.However, these changes were not associated with improved Schwann cell survival, axon growth, or locomotor recovery,suggesting that although combining Schwann cell transplantation with macrophage depletion does improve histopathology of the injury site, its effect on axon growth and behavioral recovery is no better than what can be achieved with Schwann cell transplants alone.
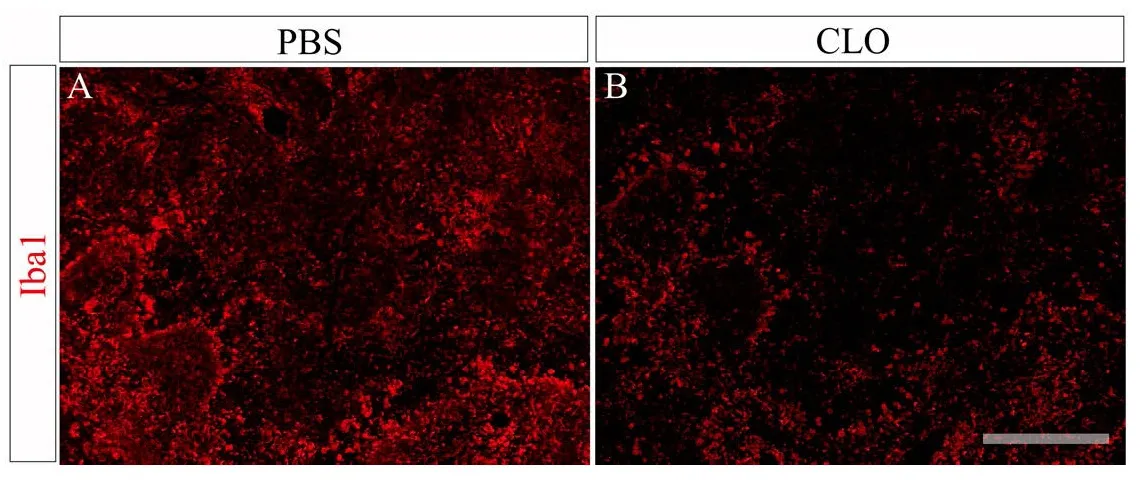
Figure 2 Fluorescent images of spleen tissue after staining with Iba1(red, macrophage/microglia) antibodies from an animal injected with liposomes containing PBS (A) or clodronate (B) at days 1, 3,and 6 and then perfused on day 7.

Figure 3 Fluorescent images from animals receiving Schwann cell transplants only (SC) (A) or the combinatory treatment (SC/CLO)(B) 8 weeks post transplantation to evaluate cyst, lesion, and transplant volumes.

Figure 4 Fluorescent images from animals with Schwann cell transplants only (SC) or the combinatory treatment (SC/CLO) to evaluate the number of BDA-positive axons.

Figure 5 Fluorescent images from animals with Schwann cell transplants only (SC) or the combined treatment(SC/CLO) to evaluate the number NF-positive axons.

Figure 6 Functional assessment using the BBB locomotor, von Frey filament sensory, and Gridwalk tests.
Schwann cell transplantation alone after contusion injury reduces cyst size but does not usually prevent their formation (Takami et al., 2002). Macrophages have been shown to contribute to secondary tissue damage, neuronal loss and demyelination in the first 2 weeks after SCI (Popovich et al.,1999; Nishio et al., 2009; David and Kroner, 2011). Therefore, Schwann cells were transplanted 1 week after injury,to encounter a less hostile environment for the transplanted cells and to provide some neuroprotection (Fortun et al.,2009) to reduce secondary damage after SCI. Earlier studies showed that clodronate treatment without Schwann cell transplants after a contusive SCI reduced the cyst volumes compared to injury only animals (Popovich et al., 1999). An assessment of the injury site here revealed that the combined treatment resulted in a four-fold reduction in cyst volume compared to Schwann cell transplants alone. In our study,the significant reduction in cyst size suggested there might be a combinatory effect from both clodronate treatment and Schwann cell transplants. Despite the reduced cyst volume,the volume occupied by the GFP-positive Schwann cells did not differ between groups, suggesting that Schwann cell survival was not improved.
Decrease in cyst volume was observed by Popovich et al. (1999) when only clodronate was administered without Schwann cell transplants after SCI. In the same study, there was also an increase of uncharacterized tissue matrix in clodronate treated animals. A similar tissue matrix increase also could have occured in the animals receiving the combined treatment and could have contributed to the less dramatic decrease in lesion volume observed in our study.
Analysis of the combined treatment effect on Schwann cell graft size may have been limited by the large time gap between cessation of the clodronate treatment and the time the animals were perfused (approximately 7 weeks). A macrophage depletion only group was not included because the goal of our study was to find combinatorial treatments to improve Schwann cell transplantation. Macrophage depletion was observed in the spleen when the same dose was given to naïve animals at days 1, 3, 6 and then perfused at day 7.The lesion volumes that included the cavity and degenerated tissue seemed to be smaller in animals with the combined treatment, suggesting that more tissue was spared compared to animals with Schwann cell transplants alone.
BDA-positive and NF-positive axons were counted in the transplant to evaluate the combinatorial effect of Schwann cell transplants and macrophage depletion on axon regeneration. Robust growth of NF-positive axons was observed into the transplant in both groups. Medium-chain NF is a neuron-specific intermediate filament that increases axon diameter, facilitating axon conduction (Jacomy et al., 1999).It is present in all axons with the exception of those of granule cells in the cerebellar cortex (Shaw et al., 1981).
BDA was injected into the ventro-lateral white matter of the spinal cord at T5 8 weeks following transplantation to label reticulospinal and propriospinal tracts (Reed et al., 2009). These tracts were targeted due to their relatively greater affinity for the Schwann cell transplant (Hill et al.,2001; Chau et al., 2004; Kanno et al., 2014). Although this quantification method does not distinguish between axon sparing, sprouting, or regeneration in the contiguous host tissue, axon regeneration into the Schwann cell graft is suggested because the graft was initially devoid of axons (Sharp et al., 2012; Tuszynski and Steward, 2012). The numbers of BDA-positive axons in the graft or host tissue around the epicenter or caudal to the graft were not altered by the combination of Schwann cell transplants and depletion of macrophages. The combined treatment did not improve axon growth significantly into the lesion site or the spared tissue as previously described (Popovich et al., 1999). However,the main difference from the previous study was the addition of Schwann cell transplants. Schwann cells transplanted alone into a contusive injury promote axon growth into the lesion (Takami et al., 2002). Abundant yet no difference in NF-positive or BDA-positive axon growth into the lesion site between the groups suggests that the Schwann cell transplant created a celling effect on axon growth into the implant, thus playing a more significant role than macrophage depletion alone on axon regeneration.
After contusion injury, cavity formation results in narrowing of the spinal cord tissue around the epicenter that could lead to an increase in axon density (Ek et al., 2012).As the cyst volume was significantly greater in the Schwann cell transplant alone animals, axon density could have been higher than in animals with the combined treatment. Also,the overall cyst area and axon density are easier to quantify in transverse tissue sections. However, in order to evaluate the continuity of transplanted Schwann cells in the lesion and their host-transplant interfaces, the tissue samples were processed here into sagittal sections. Evaluating sagittal sections would provide limited information on lateral axon distribution unless all serial sections across the width of the spinal cord were evaluated. The evaluation of 3 sections around the epicenter, where similar numbers of NF-positive axons were observed between the groups, may explain the lack of significant difference between the groups.
Both the BBB scores and subscores did not show significant improvement in our combinatory treatment. Animals receiving clodronate only in another study showed an improvement in BBB subscores at 4 weeks post injury, the longest time point assessed (Popovich et al., 1999). The BBB scores and subscores at the same time point in our study were similar. The comparable improvement of the animal over time may be due to the Schwann cells that promote similar axon growth in both groups. Similarly, differences between the groups were not observed in the number of foot falls or slips on the Gridwalk. Our combinatory treatment targeting improvement of axon regeneration into the graft was based on previous studies (Popovich et al., 1999; Bamber et al., 2001);no additional treatment was provided for promoting axon regeneration into the caudal host/Schwann cell graft interface and beyond the transplant. Therefore, a significant improvement of functional recovery was not expected in our study.At 4 weeks following transplantation, however, only animals with Schwann cell transplants alone required a stimulatory force significantly higher than their baseline scores, suggesting that the animals with the combined treatment were not as hypoesthetic as the animals with Schwann cell transplants alone. As time progressed after withdrawing clodronate treatments at 18 days post contusion, more force was required to elicit a response. This suggested that if clodronate treatment had been administered until end point, the animals might have remained less hypoesthetic than animals with Schwann cell transplants alone. It would be of interest to study the injury site at 4 weeks post-transplantation histologically to assess sensory axon regeneration through the graft.
The combined treatment of macrophage depletion and Schwann cell transplantation after SCI significantly reduced the cyst and lesion volumes compared to Schwann cell transplants alone. However, these changes were not associated with improved Schwann cell survival, axon growth, or locomotor recovery, suggesting that although combining Schwann cell transplantation with macrophage depletion does improve histopathology of the injury site, the effect on axon growth and behavioral recovery is no better than what can be achieved with Schwann cell transplantation alone.Based on these results, combining macrophage depletion with Schwann cell transplantation does not seem to be a viable option for therapeutic treatment after SCI.
Author contributions:LHF and YSL designed and performed the experiments, analyzed the data, and wrote the manuscript. JKL and MBB also participated in designing the experiments and in revising the manuscript.
Conflicts of interest:None declared.
Financial support:NINDS R01NS09923(MBB), R01NS081040(JKL), The Miami Project to Cure Paralysis, and the Buoniconti Fund.
Research ethics:The institutional Animal Care and Use Committee (IACUC)of the University of Miami approved all animal procedures (IACUC protocol#15-079).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommer-cial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W (2017) Cell transplantation therapy for spinal cord injury. Nat Neurosci 20:637-647.
Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM (2001) Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur J Neurosci 13:257-268.
Basso DM (2004) Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma 21:395-404.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1-21.
Blits B, Oudega M, Boer GJ, Bartlett Bunge M, Verhaagen J (2003) Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience 118:271-281.
Blits B, Kitay BM, Farahvar A, Caperton CV, Dietrich WD, Bunge MB (2005)Lentiviral vector-mediated transduction of neural progenitor cells before implantation into injured spinal cord and brain to detect their migration, deliver neurotrophic factors and repair tissue. Restor Neurol Neurosci 23:313-324.
Bunge MB, Monje PV, Khan A, Wood PM (2016) From transplanting Schwann cells in experimental rat spinal cord injury to their transplantation into human injured spinal cord in clinical trials. In: Progress in Brain Research. Elsevier, Amsterdam, The Netherlands.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55-63.
Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM (2004)Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J 18:194-196.
Chen A, Xu XM, Kleitman N, Bunge MB (1996) Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp Neurol 138:261-276.
Coyne TM, Marcus AJ, Woodbury D, Black IB (2006) Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells 24:2483-2492.
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388-399.
Ek CJ, Habgood MD, Dennis R, Dziegielewska KM, Mallard C, Wheaton B,Saunders NR (2012) Pathological changes in the white matter after spinal contusion injury in the rat. PLoS One 7:e43484.
Enomoto M, Bunge MB, Tsoulfas P (2013) A multifunctional neurotrophin with reduced affinity to p75NTR enhances transplanted Schwann cell survival and axon growth after spinal cord injury. Exp Neurol 248:170-182.
Follenzi A, Naldini L (2002) HIV-based vectors. Preparation and use. Methods Mol Med 69:259-274.
Fortun J, Hill CE, Bunge MB (2009) Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neurosci Lett 456:124-132.
Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD (2005)Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci 25:1169-1178.
Golden KL, Pearse DD, Blits B, Garg MS, Oudega M, Wood PM, Bunge MB(2007) Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp Neurol 207:203-217.
Guest J, Santamaria AJ, Benavides FD (2013) Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr Opin Organ Transplant 18:682-689.
Guest JD, Rao A, Olson L, Bunge MB, Bunge RP (1997) The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp Neurol 148:502-522.
Hill CE, Beattie MS, Bresnahan JC (2001) Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol 171:153-169.
Hill CE, Moon LD, Wood PM, Bunge MB (2006) Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia 53:338-343.
Jacomy H, Zhu Q, Couillard-Despres S, Beaulieu JM, Julien JP (1999) Disruption of type IV intermediate filament network in mice lacking the neurofilament medium and heavy subunits. J Neurochem 73:972-984.
Kanno H, Pressman Y, Moody A, Berg R, Muir EM, Rogers JH, Ozawa H, Itoi E, Pearse DD, Bunge MB (2014) Combination of engineered schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J Neurosci 34:1838-1855.
Kunkel-Bagden E, Dai HN, Bregman BS (1993) Methods to assess the development and recovery of locomotor function after spinal cord injury in rats.Exp Neurol 119:153-164.
Meijs MF, Timmers L, Pearse DD, Tresco PA, Bates ML, Joosten EA, Bunge MB, Oudega M (2004) Basic fibroblast growth factor promotes neuronal survival but not behavioral recovery in the transected and Schwann cell implanted rat thoracic spinal cord. J Neurotrauma 21:1415-1430.
Menei P, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB (1998)Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci 10:607-621.
Nishio Y, Koda M, Hashimoto M, Kamada T, Koshizuka S, Yoshinaga K, Onodera S, Nishihira J, Okawa A, Yamazaki M (2009) Deletion of macrophage migration inhibitory factor attenuates neuronal death and promotes functional recovery after compression-induced spinal cord injury in mice. Acta Neuropathol 117:321-328.
Okano H, Ogawa Y, Nakamura M, Kaneko S, Iwanami A, Toyama Y (2003)Transplantation of neural stem cells into the spinal cord after injury. Semin Cell Dev Biol 14:191-198.
Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB (2004) cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med 10:610-616.
Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, Golden K, Kitay BM, Blits B, Wood PM, Bunge MB (2007) Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia 55:976-1000.
Plant GW, Bates ML, Bunge MB (2001) Inhibitory proteoglycan immunoreactivity is higher at the caudal than the rostral Schwann cell graft-transected spinal cord interface. Mol Cell Neurosci 17:471-487.
Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT (1999)Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol 158:351-365.
Reed WR, Shum-Siu A, Whelan A, Onifer SM, Magnuson DS (2009) Anterograde labeling of ventrolateral funiculus pathways with spinal enlargement connections in the adult rat spinal cord. Brain Res 1302:76-84.
Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M, Firouzi M (2008) Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes.Neurosci Lett 443:46-50.
Saberi H, Firouzi M, Habibi Z, Moshayedi P, Aghayan HR, Arjmand B, Hosseini K, Razavi HE, Yekaninejad MS (2011) Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J Neurosurg Spine 15:515-525.
Sharp KG, Flanagan LA, Yee KM, Steward O (2012) A re-assessment of a combinatorial treatment involving Schwann cell transplants and elevation of cyclic AMP on recovery of motor function following thoracic spinal cord injury in rats. Exp Neurol 233:625-644.
Shaw G, Osborn M, Weber K (1981) An immunofluorescence microscopical study of the neurofilament triplet proteins, vimentin and glialfibrillary acidic protein within the adult rat brain. Eur J Cell Biol 26:68-82.
Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB (2002)Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci 22:6670-6681.
Tuszynski MH, Steward O (2012) Concepts and methods for the study of axonal regeneration in the CNS. Neuron 74:777-791.
Williams RR, Henao M, Pearse DD, Bunge MB (2015) Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant 24:115-131.
Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB (1995) A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol 134:261-272.
Zhou XH, Ning GZ, Feng SQ, Kong XH, Chen JT, Zheng YF, Ban DX, Liu T,Li H, Wang P (2012) Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow-up. Cell Transplant 21 Suppl 1:S39-47.
Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR, Lee JK (2014) Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol Dis 74:114-125.
- 中国神经再生研究(英文版)的其它文章
- Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease
- Alpha-7 nicotinic acetylcholine receptor agonist treatment in a rat model of Huntington’s disease and involvement of heme oxygenase-1
- Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice
- Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imaging study
- Intracerebroventricularly-administered 1-methyl-4-phenylpyridinium ion and brain-derived neurotrophic factor affect catecholaminergic nerve terminals and neurogenesis in the hippocampus, striatum and substantia nigra
- Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease

