碳布负载的缺氧型Na2Ti3O7纳米带阵列作为高性能柔性钠离子电池负极材料
张熙悦,黄雅兰,,吴树炜,曾银香,于明浩,程发良,卢锡洪,,*,童叶翔,*中山大学化学学院,生物无机和合成化学重点实验室,广州 5075
2南开大学,高级能源材料化学(教育部)重点实验室,天津 300071
3东莞理工学院,广东省先进纳米材料技术研究中心,广东 东莞 523808
1 Introduction
Continuous innovation in portable, wearable, and flexible electronics burns the further demands for flexible highperformance energy storage devices with good deformation tolerance1–3. With the similar electrochemical properties, more natural resources, lower price to lithium ion battery (LIB),sodium ion batteries (SIBs) have been boomed in recent years4,5. However, the larger Na+ionic radius (0.026 nm larger than Li+), and the relatively greater volume change in the process of Na+intercalation/extraction from the electrode materials generate a significant challenge to identification of a suitable negative electrode. Thus, one of the most challenges for SIBs is to explore stable anodes with high Na+storage capacity. Of the available anode materials, intercalation-type anode materials are capable to allow reversible ion intercalation/deintercalation and fast electron transfer, thus hold great promise to present satisfactory electrochemical performance6–10. Among them, titanium-based systems, as one of early 3d metal oxides that favor the insertion reactions, can be the attractively alternative one. Therefore, sodium titanate(Na2Ti3O7, NTO) is capturing increasing attention due to its unique zigzag layered framework, inherent chemical stability,abundant resources and environmental benignity11–16.Moreover, the low intercalation potential of the NTO (178 mAh·g−1at 0.3 V (vs Na+/Na)) that lies beyond the potential of Na dendrite growth17may guarantee the safety of the battery.However, NTO suffers from low electronic conductivity and poor structure stability, which severely triggers the full exploitation of its theoretical capacity (310 mAh·g−1) and compromises its cycling life17–26.
Toward these issues, considerable attempts have been devoted to the fabrication of various NTO nanostructures for SIBs. For instance, binder-free hydrogenated NTO nanoarrays on Ti foil achieved a reversible capacity of 227 mAh·g−127.Ultra-long NTO nanowires prepared by a hydrothermal method exhibited a stable discharge capacity of 211.9 mAh·g−1at 177 mA·g−1when used as SIBs anode28. N-doped carbon-coated NTO hollow spheres have been synthesized and showed a capacity of 210 mAh·g−1at 177 mA·g−129. However, the electrochemical performance of most current Na2Ti3O7 electrodes is yet below the expectation, which is due to their sluggish Na reaction kinetics as a result of the limited acceptable Na+active site and large bandgap of 3.7 eV23. In this regard, it remain a striking challenge and scientifically important to explore new effective method to design stable state-of-the-art NTO electrodes for SIBs.
In this work, we demonstrate that the electrochemical performance of NTO nanobelts can be significantly boosted by engineering oxygen vacancies, and their potential implementation as flexible anode for SIBs. Free-standing Na2Ti3O7nanobelts with oxygen vacancy were directly grown on carbon cloth (CC) through a simple hydrothermal and thermal reduction process (denoted as R-NTO/CC). The advanced three-dimensional (3D) textile electrode architecture with ordered configuration and nano-sized electrochemically active NTO enables short ion diffusion pathways and fast electron transport. The as-obtained R-NTO/CC depicted a remarkable areal specific capacity of 210.6 mAh·cm−2at 20 mA·cm−2. When the current density increased to 400 mA·cm−2,a high capacity of 69.7 mAh·cm−2was still retained, which is three times as high as bare NTO/CC. This 3D oxygen-deficient electrode could significantly promote Na+and electron transport, leading to remarkably improved electrochemical property. Furthermore, this material engineering holds great promise to modulating other electrodes and facilitate the large-scale implementation of high-performance and flexible SIBs.
2 Experimental
2.1 Preparation of NTO/CC
Na2Ti3O7(NTO) was synthesized directly on carbon cloth(CC) by a simple hydrothermal reaction. Briefly, CC was first activated by 8 mol·L−1HNO3at 80 °C for 10 h refluxing under moderate stirring. After washing by distilled water and ethyl alcohol respectively for 10 min, a 2.5 cm × 3 cm CC was conducted in an aqueous solution of 20 mL 1 mol·L−1sodium hydroxide (NaOH), 0.43 mL 30% hydrogen peroxide (H2O2)and 0.22 mL Titanium(IV) isopropoxide (C12H28O4Ti, TIP).The solution was placed into a 30 mL Teflon-lined stainless steel autoclave, and then kept at 140 °C for 20 h. After hydrothermal reaction, the NTO nanobelt was uniformly grown on CC. The mass of NTO (0.41 mg·cm−2) was obtained by electronic scales (BT25S, 0.01 mg). The pristine NTO powder was prepared under the same conditions but without the addition of carbon cloth.
2.2 Preparation of R-NTO/CC
The NTO/CC was further thermally annealed at a temperature of 400 °C in mixed NH3and H2(5 : 95 by flow)atmosphere for 1 h using a ramp rate of 5 °C·min−1to obtain R-NTO/CC. The mass of R-NTO (0.41 mg·cm−2) was obtained by electronic scales (BT25S,0.01 mg).
2.3 Material Characterization
The microstructures and compositions of the electrode materials were analyzed using field-emission SEM (FE-SEM,JSM-6330F), transmission electron microscopy (TEM, FEI Tecnai G2F30) equipped with an EMSA/MAS energy dispersive spectroscope (EDS), Raman spectroscopy(Renishaw inVia), X-ray photoelectron spectroscopy (XPS,ESCALab250, Thermo VG) and X-ray diffractometry (XRD,D8 ADVANCE).
2.4 Electrochemical Measurement
All the half-cell tests were performed in standard CR2032-type coin cells and used sodium metal foils as counter electrodes. The R-NTO/CC and NTO/CC samples are directly acted as the working electrodes without conventional metal current collectors and any ancillary materials. All the cells were assembled in an Ar-filled glove box with a glass microfiber filter (Whatman GF/D) as the separator, and 1 mol∙L−1NaClO4in ethylene carbonate and diethyl carbonate (with volume ratio of 1 : 1) as electrolyte. A 2% (volume fraction) fluoroethylene carbonate was used as electrolyte additives. The charge/discharge cycles were performed at different current densities at room temperature. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were measured on an electrochemical workstation (CHI 1040c, Chenhua,Shanghai). The current densities and capacities were normalized by the geometric area of flexible electrode(constant 0.8 cm × 0.8 cm).
3 Results and discussion
3.1 Structure of R-NTO/CC
The Na2Ti3O7nanobelts with oxygen vacancy were characterized by scanning electron microscopy (SEM),transmission electron microscopy (TEM), and X-ray diffraction(XRD). Firstly, ultrathin NTO nanobelts arrays were uniformly grown on the glossy CC through a facile hydrothermal reaction(see the Experimental Section, Fig.S1, Supporting Information). And after thermal reduction process, R-NTO/CC was obtained without the morphologic change (Fig.1a). The randomly oriented nanobelts can form a unique 3D structure,which is of help in the penetration of electrolyte and the transmission of Na+/electrons. High-magnification SEM image(Fig.1b) further indicates that NTO nanobelts have an average diameter of about 50 nm and length up to 300 nm. TEM images(Fig.1c) are used to detail the structural properties of R-NTO scratched down from the CC substrate, which clearly shows that it consists of ultrathin nanobelts. Further elemental analysis confirms the homogeneous presence of Na, Ti, and O elements within the overall nanobelts (Fig.1d). Unlike the high-quality crystallinity structure, we can see the stacking faults existing in R-NTO (Fig.1e), which possibly arose due to the vacancy introduction. Moreover, Fig.1f shows the regionally distributed lattice fringes of the square region marked in Fig.1e. Lattice spacing of 0.20 and 0.34 nm were measured, corresponding to the (020) and (011) planes of Na2Ti3O7(JCPDS card No.31-1329) respectively. In addition, Fig.1g shows the XRD patterns of NTO/CC and R-NTO/CC. The XRD patterns of oxygen-deficient Na2Ti3O7powder (synthesized under similar conditions apart from the absence of CC substrate, denoted as R-NTO), Na2Ti3O7powder (denoted as NTO) and pure CC(Fig.S2, Supporting Information) was also collected for comparison. As versified, the reflections of R-NTO/CC excluding the peaks owning to CC could be indexed to the layered monoclinic Na2Ti3O7phase (JCPDS card No. 31-1329)21,25. Note that the R-NTO sample has broader diあraction peaks compared to NTO. It suggests that the crystallinity of NTO degrades after reduction, which could be due to the formation of defects30. To gain insight into the compositional evolution of Na2Ti3O7nanobelts upon thermally reduction process, Raman and X-ray photoelectron spectra(XPS) were collected for the NTO/CC and R-NTO/CC samples. Both the samples exhibit five peaks, which are indicative of different Raman scattering modes of the layered Na2Ti3O7(Fig.2a). In detail, the bands at about 279 cm−1is attributed to the Na―O―Ti bonds31, the peaks at about 447,647, and 821 cm−1are assigned to the triply and doubly coordinated oxygen bending and stretching vibration mode respectively, while the peak at about 917 cm−1corresponds to Ti―O stretching vibration involving non-bridging oxygen32–35.Likewise, XPS spectra were used to further probe the chemical states of the products. As observed, a weak N 1s XPS peak was detected for R-NTO/CC, indicating the small number of N dopant (Fig.S3, Supporting Information)36. Specifically, Fig.2c shows the normalized Ti 2p core level spectra of NTO/CC and R-NTO/CC. The two apparent peaks at about 465.41 eV (Ti 2p1/2) and 459.65 eV (Ti 2p3/2) match well with previously reported peaks of Ti4+. Importantly, the peak centers of both Ti 2p1/2and Ti 2p3/2of R-NTO/CC sample shifted positively toward low binding energy, suggesting the decrease of Ti valence state due to the partial replace of lattice O by N or hydroxyl groups after thermally reduction. Moreover, the fitted difference curve in Fig.2c shows the additional peaks centered at 464.6 and 459.3 eV, correlating with the characteristic Ti 2p doublets of Ti3+37,38, again revealing the introduction of oxygen vacancies in the R-NTO/CC. As shown in Fig.2d, the O 1s peaks were well deconvoluted into lattice O peak (centered at 530.5, 531.0, and 531.4 eV)39, and broad Ti―OH peak(centered at 532.7 eV)28,40. The R-NTO/CC sample exhibited apparently higher intensity in the Ti―OH peak when compared with that of NTO/CC sample, implying that hydroxyl groups were generated to replace the lattice O and endow R-NTO/CC electrode with the increased deficiency.

Fig.1 (a, b) SEM images, (c) TEM image, (d) elemental mapping images and (e, f) HRTEM images of the as-prepared R-NTO/CC sample.(g) XRD profiles of R-NTO, NTO, R-NTO/CC and NTO/CC samples.

Fig.2 (a) Raman spectra and (b) XPS survey spectra of the R-NTO/CC and NTO/CC samples. (c) Overlay of normalized Ti 2p core level XPS spectra of R-NTO/CC (red solid line) and NTO/CC (green dashed line), together with their difference spectrum (“R-NTO/CC” minus “NTO/CC”).
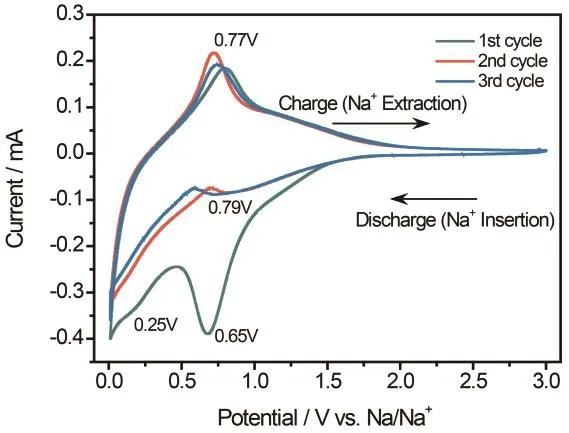
Fig.3 Representative cyclic voltammetry (CV) curves of R-NTO/CC obtained at a scan rate of 1 mV·s−1.
3.2 Sodium storage performance
To evaluate the sodium storage capability, both the NTO and R-NTO grown on CC were directly tested without any additives in a coin-type with sodium foil as both the counter electrode and reference electrode. Specifically, the electrochemical sodium insertion property of R-NTO/CC electrode was firstly evaluated by cyclic voltammetry (CV)curves in a range from 0.01 to 3.00 V at a scan rate of 1 mV·s−1(Fig.3). The CV profile shows obvious redox peaks at 0.25,0.79 and 0.77 V, the characteristic of Na+insertion/ extraction in the sodium titanate lattice18,24,41–43. Meanwhile, there is a sharp peak around 0 V, which can be attributed to Na+intercalation into CC. However, the first cycle oxidation process has a big, prominent peak at around 0.65 V, which is associated with the severe formation of SEI layer. The rate performance of both the NTO/CC and R-NTO/CC electrodes were further assessed at various current densities ranging from 20 to 600 mA·cm−2(Fig.4a). And according to the chargedischarge cycles in Fig.S4 (Supporting Information), all the curves depicted appear similar shapes, indicating the high reversibility of the sodium ion intercalation/extraction process.Apparently, the R-NTO/CC electrode shows a remarkable areal specific capacity of 210.6 mAh·cm−2at 20 mA·cm−2, which is higher than that of the NTO/CC electrode (170.5 mAh·cm−2at 20 mA·cm−2). If we deduct contributions of carbon cloth, the CC supported R-NTO shows a maximum capacity calculated to be 256.8 mAh·g−1at 50 mA·g−1(based on the mass loading of 0.41 mg·cm−2), which is much higher than those of recently reported sodium titanate based electrodes, like Na2Ti6O13(147 mAh·g−1at 70 mA·g−1)44, Na2Ti2O4(OH)2 (150 mAh·g−1at 177 mA·g−1)10, Na2Ti3O7/N-doped carbon (210 mAh·g−1at 177 mA·g−1)29, hydrogenated Na2Ti3O7/Ti foil (227 mAh·g−1at 35.4 mA·g−1)27, Na2Ti3O7/carbon textile (110 mAh·g−1at 1 A·g−1)45and Na2Ti3O7/carbon cloth (211.9 mAh·g−1at 177 mA·g−1)17,46. When the current density increases to 400 mA·cm−2, a high capacity of 69.7 mAh·cm−2is still remained,while the NTO/CC electrode owns only 22.9 mAh·cm−2,revealing the improved rate capability of the R-NTO/CC electrode. When the current density finally return to 20 mA·cm−2, a reversible discharge capacity of 198.2 mAh·cm−2was reached by the R-NTO/CC electrode, indicating the outstanding tolerance for the fast sodium ion insertion/extraction. In addition to the initial cycle, all the charge/discharge processes exhibit nearly 100% Columbic efficiencies, which can be also confirmed by the charge-discharge curves (Fig.S5a, Supporting Information),further demonstrating the excellent sodium storage ability of R-NTO/CC electrode. Notably, to exclude the capacity contribution of CC substrate, CC was directly tested as SIB anode (Fig.S5b). Evidently, over a half of the calculated areal capacities of the R-NTO/CC electrode is substantially offered by Na2Ti3O7. Furthermore, the long-term cyclic stability of the NTO/CC and R-NTO/CC electrodes was also evaluated. As expected, a high discharge capacity of 78.9 mAh·cm−2was still retained for R-NTO/CC after 200 cycles (Fig.4b), which means a good capacity retention of 72%, outperforming that of NTO/CC electrode (26%). To reach a better understanding of the improved electrochemical performance of R-NTO/CC electrode, electrochemical impedance spectra (EIS) were carried out for both samples under a fully charged state. As shown in Fig.4c, the charge-transfer resistance (Rct), which can be reflected by the semicircles located at medium-frequency,remarkably decreased for R-NTO/CC when compared with that of NTO/CC. The value of Rctfor NTO/CC electrode (3533 Ω)is substantially larger than that of R-NTO/CC electrode (2041Ω), showing the enhanced diffusion of Na+in the R-NTO/CC.After a charge/discharge cycle at 200 mA·cm−2, the Rct of R-NTO/CC electrode is still much smaller than that of NTO/CC electrode. In addition, diffuse reflectance spectroscopy (DRS) was also conducted to gain insights into the influence of oxygen vacancies on the band gap of the NTO/CC and R-NTO/CC electrodes (Fig.S6, Supporting Information). Both samples show a drastic reflection in the ultraviolet range about 325 nm and 345 nm respectively, which are essentially in agreement with the corresponding valence-to-conduction band transitions of Na2Ti3O7. According to the formula of Kubelka–Munk function47:

where R, K, and S are stand for the reflection, absorption, and scattering coefficient, respectively. After Kubelka-Munk treatment of the DRS, Fig.4d gives the specific energy gap using the following relations when the material scatters in perfectly diffuse manner:

where hν is the photon energy, A1is a proportional constant and Eq.(3) was obtained by substituting Eq.(1) into Eq.(2)considering the S as constant with respect to wavelength. From Fig.4d, the calculated bandgap for R-NTO/CC is about 2.78 eV,smaller than that of NTO/CC (2.96 eV), again suggesting the enhanced conductivity of the R-NTO/CC48,49. And owing to the vacancy-hopping mechanism in Na2Ti3O723, Na+ion mainly transport along the energetically favorable trajectories with low energy barrier, which will facilitate the electrochemical reactions in sodium-ion batteries. Therefore,the superior electrochemical performance of R-NTO/CC is believed to be attributed to the rich accessible active sites and improved electrical conductivity in terms of the introduction of oxygen vacancy and 3D hierarchical electronic transport channels.

Fig.4 (a) Rate capacity of NTO/CC and R-NTO/CC and corresponding Coulombic efficiency. (b) Cycling performance collected for NTO/CC and R-NTO/CC at 200mA cm−2. (c) EIS spectra of the R-NTO/CC and NTO/CC before and after cycle. (d) Specific energy gap after Kubelka-Munk treatment of the diffuse reflectance spectroscopy (DRS).
4 Conclusions
In summary, flexible free-standing oxygen-deficient NTO nanobelt arrays were successfully grown on CC through a simple hydrothermal process and thermal reduction process.The R-NTO/CC electrode yield a remarkable areal specific capacity of 210.6 mAh·cm−2at 20 mA·cm−2, which is three times as high as bare NTO/CC electrode. The enhanced electrochemical performance can be attributing to the advanced 3D array architecture, reduced energy band gap, improved charge transport and increased electronic conductivity. This work not only demonstrates a simple method to elaborately improve the electrochemical property for NTO as anode for SIBs, but also provides much needed inspiration to modulating other electrodes and facilitate the large-scale implementation of high-performance electrochemical energy storage systems.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) Xiao, Y. M.; Wu, J. H.; Yue, G. T.; Lin, J. M.; Huang, M. L.; Fan,L. Q.; Lan, Z. Acta Phys. -Chim. Sin. 2012, 28 (3), 578. [肖尧明,吴季怀, 岳根田, 林建明, 黄妙良, 范乐庆, 兰章. 物理化学学报, 2012, 28 (3), 578.] doi: 10.3866/PKU.WHXB201201032
(2) Xia, K. L.; Jian, M. Q.; Zhang, Y. Y. Acta Phys. -Chim. Sin. 2016,32 (10), 2427. [夏凯伦, 蹇木强, 张莹莹. 物理化学学报, 2016,32 (10), 2427.] doi: 10.3866/PKU.WHXB201607261
(3) Zhuang, L. Acta Phys. -Chim. Sin. 2017, 33, 655. [庄林. 物理化学学报, 2017, 33, 655.] doi: 10.3866/PKU.WHXB201703093
(4) Huang, Z. L.; Wang, L. P.; Mou, C. X.; Li, J. Z. Acta Phys. -Chim.Sin. 2014, 30, 1787. [黄宗令, 王丽平, 牟成旭, 李晶泽. 物理化学学报, 2014, 30, 1787.] doi: 10.3866/PKU.WHXB20140852
(5) Xu, J.; Yang, D. Z.; Liao, X. Z.; He, Y. S.; Ma, Z. F. Acta Phys. -Chim.Sin. 2015, 31, 913. [许婧, 杨德志, 廖小珍, 何雨石, 马紫峰.物理化学学报, 2015, 31, 913.]doi: 10.3866/PKU.WHXB201503162
(6) Zhang, W.; Liu,Y.; Chen, C.; Li, Z.; Huang, Y.; Hu, X. Small 2015,11 (31), 3822. doi: 10.1002/smll.201500783
(7) Lamuel David, R. B.; Singh, G. ACS Nano 2014, 8 (2), 1759.doi: 10.1021/nn406156b
(8) Yuan, S.; Huang, X. D.; Ma, H.; Wang, M. F.; Zhang, X. Adv.Mater. 2014, 26 (14), 2273. doi: 10.1002/adma.201304469
(9) Wang, X.; Li, Y.; Gao, Y.; Wang, Z.; Chen, L. Nano Energy 2015,13, 687. doi: 10.1016/j.nanoen.2015.03.029
(10) Zhang, Y.; Guo, L.; Yang, S. Nanoscale 2015, 7, 14618.doi: 10.1039/C5NR03076E
(11) Senguttuvan, P.; Rousse, G.; Seznec, V.; Tarascon J. M.; Palacín,M. R. Chem. Mater. 2011, 23, 4109. doi: 10.1021/cm202076g
(12) Chen, C.; Wen, Y.; Hu, X.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan,B.; Huang, Y. Nat. Commun. 2015, 6, 6929.doi: 10.1038/ncomms7929
(13) Naeyaert, P. J. P.; Avdeev, M.; Sharma, N.; Yahia, H. B.; Ling, C.D. Chem. Mater. 2014, 26, 7067. doi: 10.1021/cm5035358
(14) Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Adv. Mater. 2016,28, 2259. doi: 10.1002/adma.201504412
(15) Liao, J. Y.; Manthiram, A. Nano Energy 2015, 18, 20.doi: 10.1016/j.nanoen.2015.09.014
(16) Doeff, M. M.; Cabana, J.; Shirpour, M. J. Inorg. Organomet.Polym. Mater. 2013, 24, 5. doi: 10.1007/s10904-013-9977-8
(17) Rousse, G.; Arroyo-de Dompablo, M. E.; Senguttuvan, P.;Ponrouch, A.; Tarascon, J. M.; Palacín, M. R. Chem. Mater. 2013,25, 4946. doi: 10.1021/cm4032336
(18) Dong, S.; Shen, L.; Li, H.; Nie, P.; Zhu, Y.; Sheng, Q.; Zhang, X.;J. Mater. Chem. A 2015, 3, 21277. doi: 10.1039/C5TA05714K
(19) Andersson, S.; Wadsley, A. D. Acta Cryst. 1961, 14, 1245.doi: 10.1107/S0365110X61003636
(20) Xu, L.; Xia, J.; Wang, L.; Qian, J.; Li, H.; Wang, K.; Sun, K.; He,M. Chem. Eur. J. 2014, 20, 2244. doi: 10.1002/chem.201304312
(21) Wang,W.; Yu, C.; Lin, Z.; Hou, J.; Zhu, H.; Jiao, S. Nanoscale 2013, 5, 594. doi: 10.1039/C2NR32661B
(22) Zou, W.; Li, J.; Deng, Q.; Xue, J.; Dai, X.; Zhou, A.; Li, J. Solid State Ionics 2014, 262, 192. doi: 10.1016/j.ssi.2013.11.005
(23) Pan, H.; Lu, X.; Yu, X.; Hu, Y. S.; Li, H.; Yang, X. Q.; Chen, L.Adv. Energy Mater. 2013, 3, 1186. doi: 10.1002/aenm.201300139
(24) Wang, W.; Yu, C.; Liu, Y.; Hou, J.; Zhu, H.; Jiao, S. RSC. Adv.2013, 3, 1041. doi: 10.1039/C2RA22050D
(25) Yin, J.; Qi, L.; Wang, H. ACS. Appl. Mater. Interfaces 2012, 4,2762. doi: 10.1021/am300385r
(26) Yan, Z.; Liu, L.; Shu, H.; Yang, X.; Wang, H.; Tan, J.; Zhou, Q.;Huang, Z.; Wang, X. J. Power Sources 2015, 274, 8.doi: 10.1016/j.jpowsour.2014.10.045
(27) Fu, S.; Ni, J.; Xu, Y.; Zhang, Q.; Li, L. Nano Lett. 2016, 16, 7.doi: 10.1021/acs.nanolett.6b01805
(28) Li, Z.; Shen, W.; Wang, C.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y.J. Mater. Chem. A 2016, 4, 17111. doi: 10.1039/C6TA08416H
(29) Xie, F.; Zhang, L.; Su, D.; Jaroniec, M.; Qiao, S. Z. Adv. Mater.2017, doi: 10.1002/adma.201700989.
(30) Lu, X.; Wang, G.; Xie, S.; Shi, J.; Li, W.; Tong, Y.; Li, Y. Chem.Commun. 2012, 48, 7717. doi: 10.1039/C2CC31773G
(31) Chen, C.; Wang, J.; Zhao, Q.; Wang, Y.; Chen, J. ACS. Energy Lett. 2016, 1, 1165. doi: 10.1021/acsenergylett.6b00515
(32) Zhang, Y.; Guo, L.; Yang, S. Chem. Commun. 2014, 50, 14029.doi: 10.1039/C4CC06451H
(33) M, K. H.; Miyaji, F.; Kokubo, T.; Nakamura, T. J. Mater. Sci.Mater. Med., 1997, 8, 341. doi: 10.1023/A:1018524731409
(34) Ma, K. F. R.; Sasaki, T.; Osada, M.; Bando, Y. J. Phys. Chem. B 2005, 109, 6210. doi: 10.1021/jp044282r
(35) Dylla, A. G.; Xiao, P.; Henkelman, G.; Stevenson, K. J. J. Phys.Chem. Lett. 2012, 3(15), 2015. doi: 10.1021/jz300766a
(36) Liu, C.; Sun, T.; Wu, L.; Liang, J.; Huang, Q.; Chen, J.; Hou,W.Appl. Catal. B: Environ. 2015, 170–171, 17.doi: 10.1016/j.apcatb.2015.01.026
(37) Tang,Y.; Tao, J.; Zhang, Y.; Wu, T.; Tao, H.; Bao, Z. Acta Phys. -Chim. Sin. 2008, 24, 2191. [汤育欣, 陶杰, 张焱焱, 吴涛,陶海军, 包祖国. 物理化学学报, 2008, 24, 2191.]doi: 10.1016/S1872-1508(08)60082-0
(38) Li, X.; Liu, S. Acta Phys. -Chim. Sin. 2008, 24, 2019.doi: 10.1016/S1872-1508(08)60079-0
(39) Ko, J. S.; Doan-Nguyen, V. V.; Kim, H. S.; Muller, G. A.; Serino,A. C.; Weiss, P. S.; Dunn, B. S. ACS. Appl. Mater. Interfaces 2017,9, 1416. doi: 10.1021/acsami.6b10790
(40) Zhan, X.; Shirpour, M. Chem. Commun. 2017, 53, 204.doi: 10.1039/C6CC08901A
(41) Ho, C. K.; Li, C. Y. V.; Chan, K. Y. Ind. Eng. Chem. Res., 2016,55, 10065. doi: 10.1021/acs.iecr.6b01867
(42) Rudola, A.; Saravanan, K.; Mason, C. W.; Balaya, P. J. Mater.Chem. A 2013, 1, 2653. doi: 10.1039/C2TA01057G
(43) Ge,Y.; Jiang, H.; Zhu, J.; Lu, Y.; Chen, C.; Hu, Y.; Qiu, Y.; Zhang,X. Electrochim. Acta 2015, 157, 142.doi: 10.1016/j.electacta.2015.01.086
(44) Rudola, A.; Saravanan, K.; Devaraj, S.; Gong, H.; Balaya, P. Chem.Commun. 2013, 49, 3. doi: 10.1039/C3CC44381G
(45) Dong, S.; Shen, L.; Li, H;. Pang, G.; Dou, H.; Zhang, X. Adv.Funct. Mater. 2016, 26, 3703. doi: 10.1002/adfm.201600264
(46) Xu, X.; Yan, M.; Tian, X.; Yang, C.; Shi, M.; Wei, Q.; Xu, L.; Mai,L. Nano Lett. 2015, 15, 3879. doi: 10.1021/acs.nanolett.5b00705
(47) Zhang, Z. J.; Feng, A.; Sun, X. Y.; Guo, K.; Man, Z. Y.; Zhao, J. T.J. Alloy. Compd. 2014, 592, 73. doi:10.1016/j.jallcom.2013.12.211
(48) Yang, Q.; Chen, L.; Hu, C.; Wang, S.; Zhang, J.; Wu, W. J. Alloy.Compd. 2014, 612, 301. doi: 10.1016/j.jallcom.2014.05.193
(49) Gu, Y.; Su, X.; Du, Y.; Wang, C. Appl. Surf. Sci. 2010, 256, 5862.doi: 10.1016/j.apsusc.2010.03.065

