Fabrication of WO3/TiO2 Heterostructures for Efficiently Photocatalytic Gaseous Hydrocarbons Degradation: Origin of Photoactivity and Revisit the Role of WO3 Decoration①
WANG Dn PAN Xio-Yng WANG Gung-To YI Zhi-Guo
Fabrication of WO3/TiO2Heterostructures for Efficiently Photocatalytic Gaseous Hydrocarbons Degradation: Origin of Photoactivity and Revisit the Role of WO3Decoration①
WANG Dana, bPAN Xiao-Yanga②WANG Guang-TaobYI Zhi-Guoa②
a(350002)b(453007)
Efficient oxidation of gaseous small molecular hydrocarbons under mild conditions remains a significant but challenging task to date. Here we report that WO3decoration can obviously improve the performance of TiO2(P25) toward the photocatalytic oxidation of several small molecular hydrocarbons (C2H6, C3H8and C2H4) under simulated solar light irradiation. Among the WO3/TiO2heterostructures, the 10wt%WO3/TiO2nanocomposite shows the best photoactivities, which can efficiently oxidize C2H6, C3H8and C2H4within 15, 9 and 8 minutes, respectively under simulated sunlight with a light intensity of 200 mW/cm2. By strong contrast, a decreased photoactivity of TiO2by coupling with WO3is observed when investigating the performance of photocatalysts toward the degradation of methylene blue (MB) in liquid phase. The opposing effect of WO3decoration on the performance of TiO2is thoroughly investigated, and it is found that the improved photoactivities for gaseous hydrocarbon degradation is ascribed to the enhanced oxygen adsorption, resulting from WO3decoration rather than efficient charge separation within the WO3/TiO2heterostructures.
WO3, TiO2, photocatalytic, hydrocarbons;
1 INTRODUCTION
Air pollution caused by automobile exhaust is one of the major problems in urban areas[1]. Although most of the vehicles have been equipped with emission reduction systems[2], tailpipe emission from vehicles still contains high concentrations of light hydrocarbon pollutants (C2H6, C3H8, C2H4, etc.). Up to 80% of the emitted hydrocarbons is produced in the first 60 to 90 s following a cold-start because of the catalytic converter’s inability to oxidize hydro- carbons at low temperature (between 473 to 573 K)[3]These hydrocarbon pollutants in air have adverse effect on our health by reacting with NOunder solar light irradiation to form a more toxic photochemical smog[4]. Although thermocatalytic technique and adsorption method have been used to remove these pollutants, it still remains a challenge to effectively eliminate these hydrocarbon pollutants at ambient temperature because of their high C–H bond energy and weak molecule polarity[5, 6]. Therefore, it is necessary to develop an effective strategy to remove these hydrocarbon pollutants.
In recent years, tremendous efforts have been devoted to developing heterogeneous photocatalysts for hydrocarbon purification at room temperature[1-3, 6-9], which utilize sunlight as a green and free energy source[10-12]. By far the most studied material is TiO2, as it is considered to represent the most suitable photocatalyst, in view of its effectiveness and stabi- lity against photocorrosion[11, 13-20]. However, the application of TiO2photocatalyst is limited by its insufficient visible light absorption and low quantum efficiency, owing to its wide bandgap and fast charge recombination[11]. To deal with these problems, one effective strategy is to couple TiO2with narrow- band-gap semiconductors (CdS, Fe2O3, WO3, etc) with matched band structure[21-26].
Tungsten oxide (WO3) is a-type semiconductor with relatively small bandgap (g= 2.6 eV) and strong oxidizing power of photoexcited holes[27], which makes it a promising photocatalyst for hydro- carbon pollutant treatment. In addition, due to the matched band structure of TiO2and WO3, the photogenerated electrons of TiO2can be directly transferred to WO3[28], which was assumed to facile- tate the charge separation and thus may enhance the photoactivity of TiO2[29-31]. Under such assumption, the fabrication of WO3/TiO2nanocomposite has attracted much attention in the field of photo- catalysis[25, 28-33]. However, it seems there is paradox in the interpretation of the role of WO3decoration on the performance of TiO2as several studies have demonstrated that WO3decoration would result in decreased photoactivities of TiO2[26, 32], although electron transfer from TiO2to WO3is observed in WO3/TiO2nanocomposite[26]. Therefore, it is necessary to clarify the role of WO3decoration on the performance of TiO2and thus understand the origin of these inconsistent results.
In this work, WO3/TiO2nanocomposites are pre- pared by a wet-impregnation method, which shows much higher photoactivities than those of TiO2(P25) toward gas-phase degradation of C2H6, C3H8and C2H4. By strong contrast, a deactivation of TiO2photocatalyst by coupling with WO3is observed when testing photoactivities of the as-synthesized samples for methylene blue oxidation in liquid phase. Further investigation revealed that the entirely different role of WO3decoration on the performance of TiO2is attributed to the different reaction mechanisms involved in these reactions. In the gaseous hydrocarbon photooxidation, the enhancement of photoactivities of TiO2is attributed to the improved oxygen adsorption resulting from WO3decoration rather than enhanced charge separation by WO3/TiO2heterostructures.
2 EXPERIMENTAL
2. 1 Chemicals and materials
Degussa P25 was purchased from Hulls Cor- poration, Germany. Analytically pure ammonium tungstate hydrate ((NH4)10H2(W2O7)6), ammonium oxalate ((NH4)2C2O4),-butyl alcohol (C4H9OH) and methylene blue (C16H18ClN3S) were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals were used as received without further purification.
2. 2 Synthesis
To obtain the WO3/TiO2heterojunctions with different weight percentage of WO3, a modified impregnation method was employed. First, a certain amount (0.0579 g, 0.0956 g, 0.1222 g, 0.15 g, 0.1941 g, or 0.4714 g) of (NH4)10H2(W2O7)6was dissolved in 300 mL of deionized water with constant stirring to form a clear solution. Then 1 g of TiO2(P25) was added into the above solution and dispersed by ultrasonic treatment to obtainuniform suspension. After stirring for 12 h, the suspension was dried in a water bath at 363 K with constant stirring. After grinding in a mortar, the dried samples were then heat-treated in a muffle furnace at a rate of 5 K/min up to 723 K and dwelled for 90 minutes in air atmosphere.
2.3 Sample characterization
The crystalline structure of the sample was analy- zed by X-ray diffraction (XRD, Rigaku Miniflex II) equipment with Curadiation at a scan step of 0.02º. UV-visible diffuse reflectance spectra were collected by a Peking Elmer Lamda 900 UV/VIS/NIR spectrometer equipped with an integrating sphere. BaSO4was used as the reflec- tance standard. The morphology, composition and microstructure of the samples were investigated by high-resolution transmission electron microscopy (TEM, JEM-2010) with an energy-dispersive X-ray (EDX) analysis attachment. The X-ray photoelectron spectroscopy (XPS) was employed to characterize the chemical states of the sample. The surface areas of the samples were measured by a TriStar II 3020-BET/BJH surface area analyzer. In situ diffuse reflectance Fourier transform infrared spectroscopy (DRFTIS) studies were performed on a spectrometer Nexus FT-IR (Thermo Nicolet) by using a diffuse reflectance attachment equipped with a reaction chamber. 128 single beam spectra had been co-added at a resolution of 4 cm−1and the spectra were presented as Kubelka-Munk function referred to adequate background spectra. The background and samples spectra were taken (the average of accumulated 32 scans) over the frequency range of 4000~600 cm−1. O2temperature-programmed desorption measurements were performed on Micromeritics AutoChem II 2920 instrument con- nected to a MKS cirrus mass spectrum. Before measurement, the catalyst powder (0.1 g) was heated in a He flow and kept at 423 K for 60 min, and then cooled to 333 K and flowed with O2for 60 min. After that, the O2flow was replaced by He flow for 60 min to remove any un-adsorbed O2. The TPD results were recorded at a heating rate of 10 K/min. The O2desorbed was measured quantitatively by mass spectrum. The photoluminescence (PL) spectra of the photocatalysts were obtained by a Varian Cary Eclipse spectrometer with an excitation wavelength of 325 nm.
2. 4 Photocatalytic activity test
The photocatalytic oxidation of gaseous hydro- carbons was carried out in a homemade fixed-bed pyrex reactor of 450 ml capacity (see Fig. S1) and a flow-bed quartz reactor (28mm × 18mm × 1mm, see Fig. S2), respectively. All experiments were conducted at atmospheric pressure and room temperature. In a typical fixed-bed reaction: the as-obtained sample (0.2 g) was spread uniformly on the bottom of the reactor. Then, the reactor was flushed with N2repeatedly to remove water and CO2that were adsorbed on the catalyst and the inwall of reactor. Subsequently, 5 mL of O2and 90 μL of hydrocarbons were injected into the reactor by micro-syringe, respectively. The initial concentration of hy- drocarbons is 200 ppm (Volume). Prior to the irradiation, the reactor was kept in the dark for 2 h to ensure the establishment of an adsorption-desorption equilibrium between the photocatalyst and reactants. Then, the reactor was irradiated by a 300 W Xe lamp. The Xe spectrum is provided in Fig. S3. At a certain time interval, 4 mL of gas was sampled from the reactor and analyzed by a gas chromatography (GC9720 Fuli) equipped with a HP-Plot/U capillary column, a molecular sieve 13X column, a flame ionization detector (FID) and a thermal conductivity detector (TCD). The degradation percentage of hydrocarbons is indicated as C/C0. Here C is the concentration of hydrocarbons at certain reaction time, and C0is the initial concentration of hydro- carbons.
A typical flow-bed reaction was carried out as follows: the sample was placed in a quartz reactor, and then the mixed gas consisted of 78.9% N2and 21.1% O2, and small quantity of hydrocarbon gases (about 200 ppm) was flowed through the samples and analyzed directly by the gas chromatography (GC9720 Fuli). Before illumination, the flowing carrier gas was used to expel CO2and other species adsorbed on the surface of the catalysts. During the reaction, a 300 W Xe lamp was used to provide simulated solar light.
The methylene blue (MB) photooxidation in liquid phase was carried out as follows: 30 mg of pho- tocatalyst was dispersed into 60 mL of MB solution (20 ppm) in a quartz vial. The resulting suspension was stirred in the dark for 1 h to ensure the establishment of an adsorption-desorption equili- brium between the sample and reactant. Then the reaction system was irradiated by a 300 W Xe lamp (CEL-HXF300) system (800>>300 nm). As the reactions proceed, 3 mL of the suspension was taken at a certain time interval and was centrifuged to remove the catalyst. Afterwards, the residual amount of MB in the solution was analyzed on the basis of its characteristic optical absorption at 660 nm, using a UV/Vis/NIR sepectrophotometer (Perking Elmer Lambada 900) to measure the change of MB concentration with irradiation time based on Lambert-Beer’s law. The percentage of degradation is denoted as/C. Hereis the absorption of MB solution at each irradiation time interval of the main peak of the absorption spectrum, andCis the absorption of the initial concentration when the adsorption-desorption equilibrium was achieved.
3 RESULTS AND DISCUSSION
3. 1 Characterization of photocatalysts
The XRD patterns of the as-synthesized samples are shown in Fig. 1. For the blank WO3synthesized by calcination of ammonium tungstate hydrate, all the diffraction peaks can be indexed to the monoclinic phase (PDF # 71-2141) of WO3[34]. The commercial available TiO2(P25) consists of anatase and rutile phases. The presence of WO3in the 5wt%WO3/TiO2, 8wt%WO3/TiO2and 10wt%WO3/TiO2samples does not result in new XRD peaks, which may be ascribed to the even distribution and low WO3content in these samples[35]. When the addition ratio of WO3reaches 12%, 15% and 30%, besides the typical diffraction peaks of TiO2, additional peaks of WO3can be identified on these samples.

Fig. 1. XRD patterns of the blank WO3,TiO2 (P25) and WO3/TiO2 nanocompositeswith different WO3 weight ratios
The morphology and microstructure of the WO3/TiO2nanocomposite are investigated by TEM analysis. As shown in Fig. 3a and Fig. S5a, b, the average particle size of 10%WO3/TiO2nanocom- posite is determined to be 24 nm. The HRTEM image in Fig. 3b displays distinct lattice fringe of TiO2(101) facets (0.35 nm). In addition, the typical lattice spacing of 0.17 nm corresponding to the (–331) facet of WO3can also be identified in the HRTEM image. EDX analysis in Fig. 3c reveals the existence of Ti, W and O elements. Elemental mapping analysis (Fig. 3d-g) indicates that the Ti, W and O elements have uniform distribution, indicating that WO3is homogeneously decorated on the surface of TiO2.
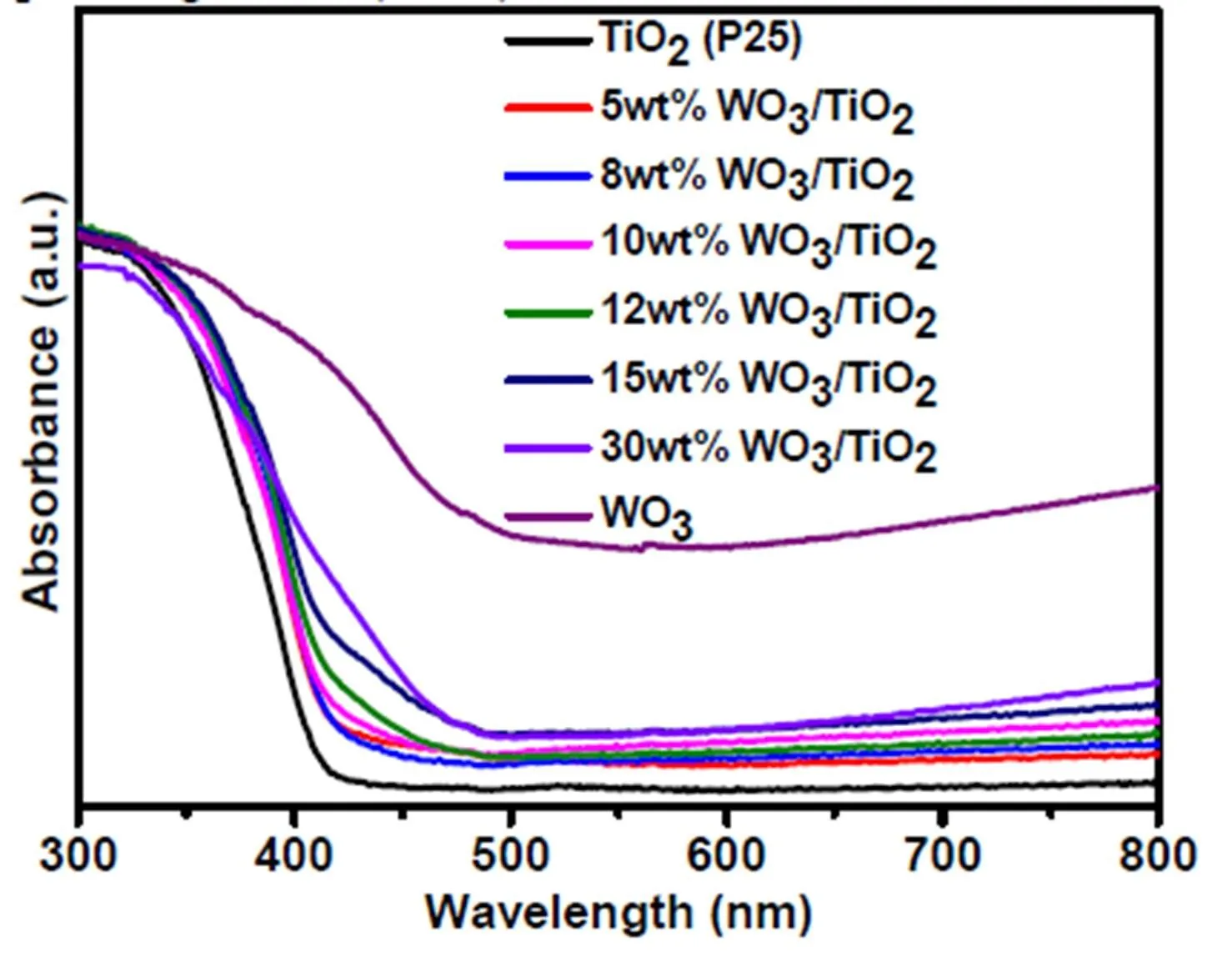
Fig. 2. UV-visible diffusive reflectance spectra of the blank WO3, TiO2(P25)and WO3/TiO2nanocomposites with different WO3weight ratios
Fig. 3. TEM (a) and HRTEM images (b) of the 10wt%WO3/TiO2nanocomposite; EDX spectrum of the 10wt%WO3/TiO2nanocomposite (c); high angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) image (d) and elemental mapping patterns of Ti (e), W (f) and O (g)
3.2 Photocatalytic degradation of hydrocarbons
The performance of WO3/TiO2nanocomposites is initially investigated by photocatalytic degradation of ethane (C2H6) under simulated solar light irradiation. Notably, C2H6is very stable because of its weak polarity as well as the inert C–C and C–H bonds[37]. As shown in Fig. 4a and b, WO3/TiO2nanocom- posites exhibit much higher photoactivities for C2H6oxidation than those of blank WO3and TiO2(P25). In particular, 10wt%WO3/TiO2demonstrates the highest C2H6oxidation rate among these samples, which is 5.3 times higher than that of blank TiO2(P25) (Table S1). This result indicates that the addition ratio of WO3is crucial to optimize the photoactivity of the nanocomposite. The highest photoactivity of 10wt%WO3/TiO2is in accordance with its highest surface area (Table S1). This result suggests that the surface area of catalyst can obviously affect the photoactivity, that is, the larger surface area of photocatalyst is beneficial for provi- ding more active sites for the reaction.
To examine the efficiency of WO3/TiO2nanocom- posite, we also investigated the photoactivities of 10wt%WO3/TiO2toward propane (C3H8) and ethylene (C2H4) degradation under simulated solar light irradiation (Fig. 4c-f). It is clearly shown that the blank WO3exhibits almost no photoactivities for the oxidation of these hydrocarbons. In contrast, 10wt%WO3/TiO2shows much higher photoactivities for C3H8and C2H4oxidation than those of the blank TiO2(P25), indicating the beneficial effect of WO3/TiO2heterostructure for achieving efficient photocatalytic performance. It is worth noting that both C3H8and C2H4can be efficiently removed within 10 minutes under the simulated solar light illumination (300<<800 nm). In addition, after 5 times recycling photocatalytic test, the performance of used sample remains similar to those of the fresh sample (Fig. S6-c).
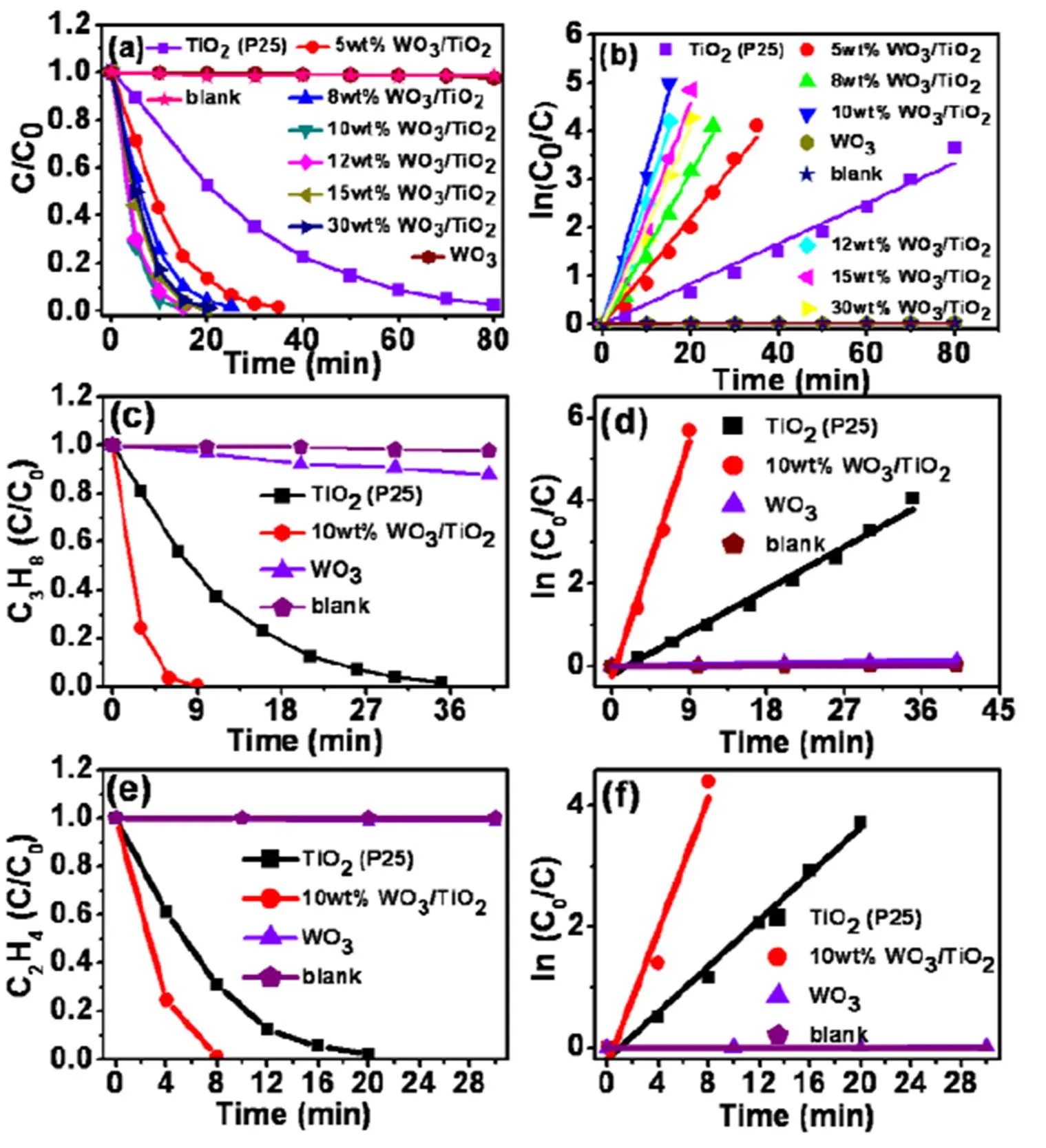
Fig. 4. Photocatalytic degradation of C2H6over blank WO3, TiO2(P25) and WO3/TiO2nanocomposites under simulated solar light illumination (300<<800 nm) (a); Pseudo-first-order kinetics analysis of photocatalytic reaction over the samples (b); Photocatalytic degradation of C3H8(c, d), C2H4(e, f) over the TiO2(P25), WO3and 10wt%WO3/TiO2nanocomposite under simulated solar light illumination (300<<800 nm)
The photoactivites of 10wt%WO3/TiO2nanocom- posite for C2H6, C3H8and C2H4oxidation were further investigated in a flow mode under simulated solar light irradiation. In this mode, the sample was put in a quartz reactor with nitrogen-oxygen (20% O2/N2) as carrier gas and hydrocarbons (C2H6, C3H8or C2H4) as reactant gas. The flowing rate was kept at 10 ml/min. The intensity of the simulated sunlight on the catalyst surface was approximately 200 mW/cm2measured using a calibrated thermopile detector. Before light irradiation, CO2in the reaction system was removed by flowing carrier gas. As shown in Fig. 5a-c, when the lamp is turned on, the amount of hydrocarbons decreases rapidly. Simultaneously, the concentration of CO2increases quickly to a constant value. When the light is turned off, the concentration of CO2rapidly decreases to zero. Meanwhile, the amount of hydrocarbons comes back to a constant value. These results indicate that the hydrocarbons (C2H6, C3H8and C2H4) degradation is truly driven by a photocatalytic process. In addition, it is worth noting that the mineral ratios of C2H6, C3H8and C2H4are all determined to be ~100%, indicating that the 10wt%WO3/TiO2nanocomposite is highly efficient for complete oxidation of these hydrocarbon pollutants.
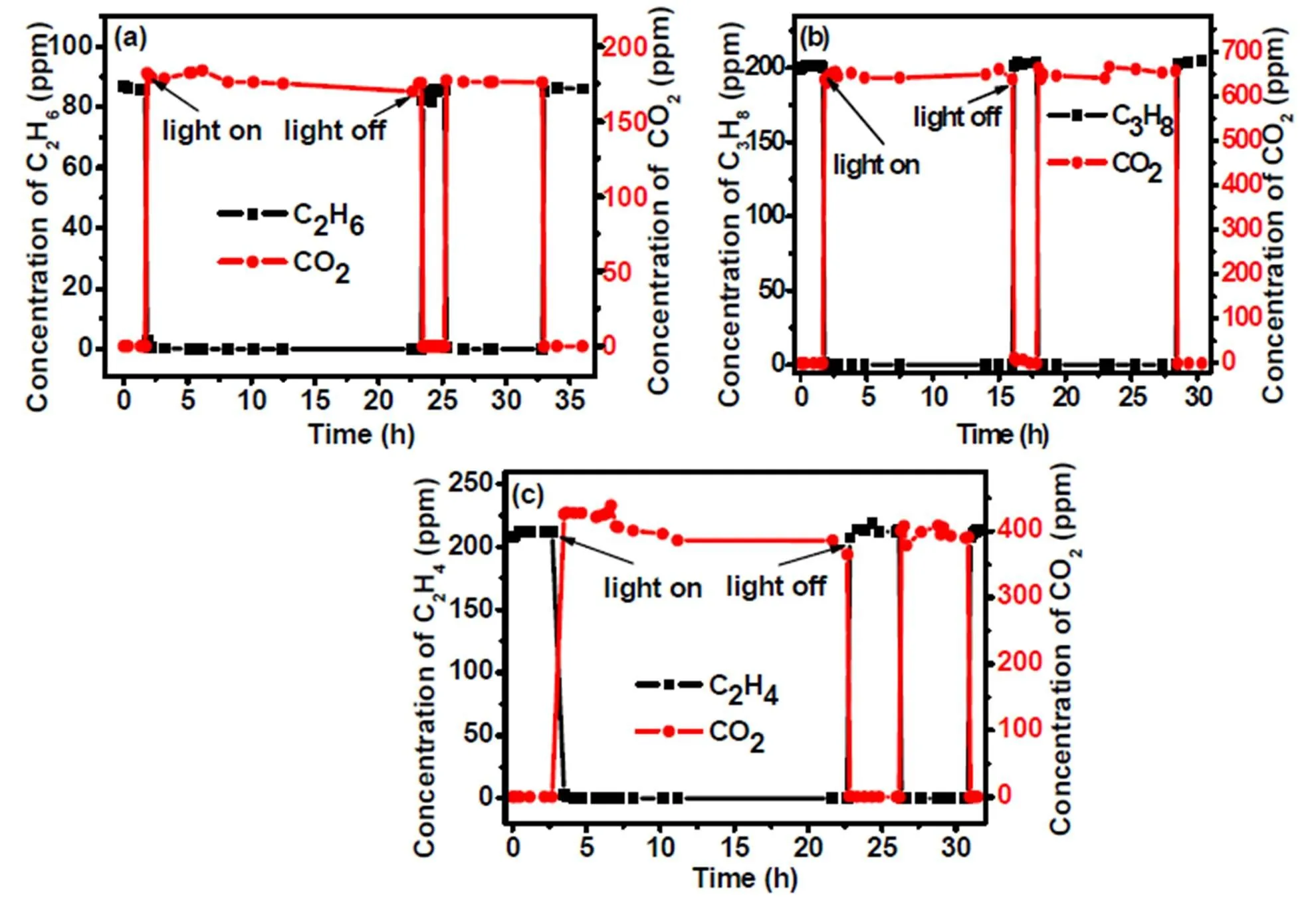
Fig. 5. Photocatalytic degradation of C2H6(a), C3H8(b) and C2H4(c) over 10wt%WO3/TiO2under simulated solar light irradiation in a flow mode
In situ diffuse reflectance Fourier transform infrared spectroscopy (DRFTIS) was utilized to fur- ther investigate possible intermediate species during the photocatalytic reactions. As shown in Fig. 6a-c, characteristic IR modes for the hydrocarbons (C2H6, C3H8and C2H4) can be clearly identified on the surface of 10wt%WO3/TiO2, indicating that these hydrocarbons are successfully adsorbed onto the surface of the catalysts. Under the simulated solar light irradiation, the intensity of the band assigned to(C–H) of C2H6, C3H8or C2H4decreases with increasing the irradiation time (Fig. 6a-c)[38], whereas the bands assigned to the characteristic modes of CO2at 2360 and 2340 cm-1gradually grow[39]. These results indicate that these hydrocarbons are oxidized to CO2during the photocatalytic reactions. In addition, the typical(C=O) adsorption bands at 1700~1730 cm-1for carbonyls appear under the simulated solar light irradiation, indicating that the intermediate species (carbonyl compounds) may form during the photocatalytic reaction[40].
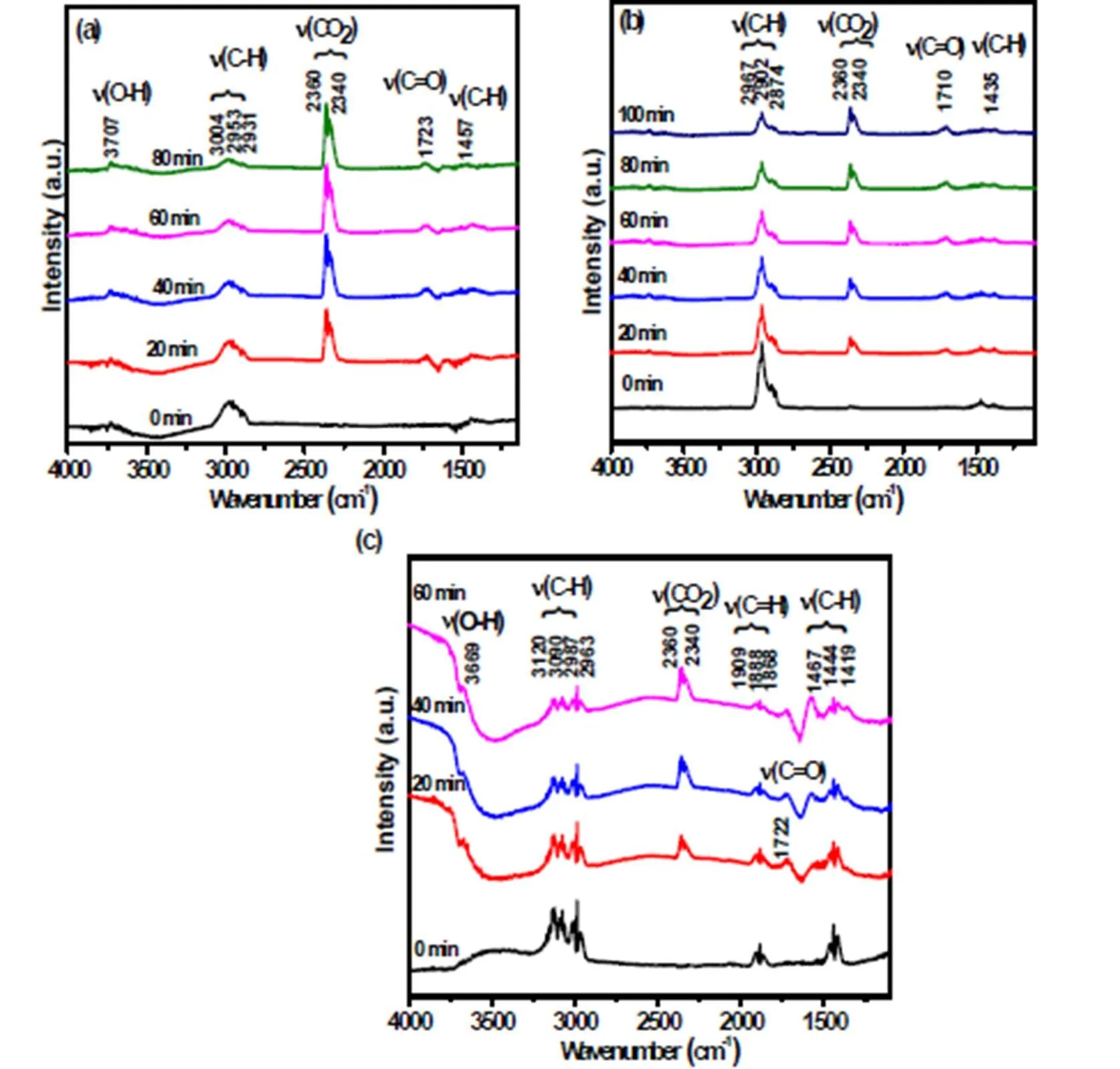
Fig. 6. In situ diffuse reflectance Fourier transform infrared spectroscopy (DRFTIS)spectra on 10wt%WO3/TiO2during the photocatalytic C2H6(a), C3H8(b), C2H4(c)degradation under simulated solar light irradiation (300<<800 nm)
3. 3 Origin of the enhanced catalytic activity of WO3/TiO2
To understand the origin of the enhanced pho- tocatalytic activity of WO3/TiO2heterostructure, pho- toluminescence (PL) analysis was utilized to inves- tigate the charge separation process. As shown in Fig. 7a, 10wt%WO3/TiO2nanocomposite shows similar PL intensity to that of TiO2(P25), suggesting that WO3decoration does not promote the charge separa- tion in TiO2, although the charge transfer from TiO2to WO3is thermodynamically favorable. Even if the charge transfer from TiO2to WO3is successful, the electrons transferring from TiO2would be trapped at the W5+sites, thus they cannot be efficiently transferred to the O2molecules (Fig. 7b)[28], which is evidenced by XPS analysis. As shown in Fig. S7a, the XPS peak of W 4for the fresh 10wt%WO3/TiO2nanocomposite is symmetric, indicating fully coordinated W6+ions. However, a shoulder 1.1 eV at the right of the main W 4peak appears after the photocatalytic reactions, indicating the creation of W (V) species on the surface (Fig. S7b)[34]. These W (V) species are stable even when exposed to air for several days, suggesting that molecular oxygen is difficult to oxidize the W (V) species. Therefore, the enhanced photoactivity of WO3/TiO2nanocomposite cannot be explained by the constantly employed scenario that involves charge separation by forming WO3/TiO2heterojunction. Notably, the creation of W (V) species on the surface of WO3/TiO2nanocom- posite does not have harmful effect on the perfor- mance of the photocatalyst, since no deactivation is observed on the WO3/TiO2nanocomposite (Fig. S6a-c).
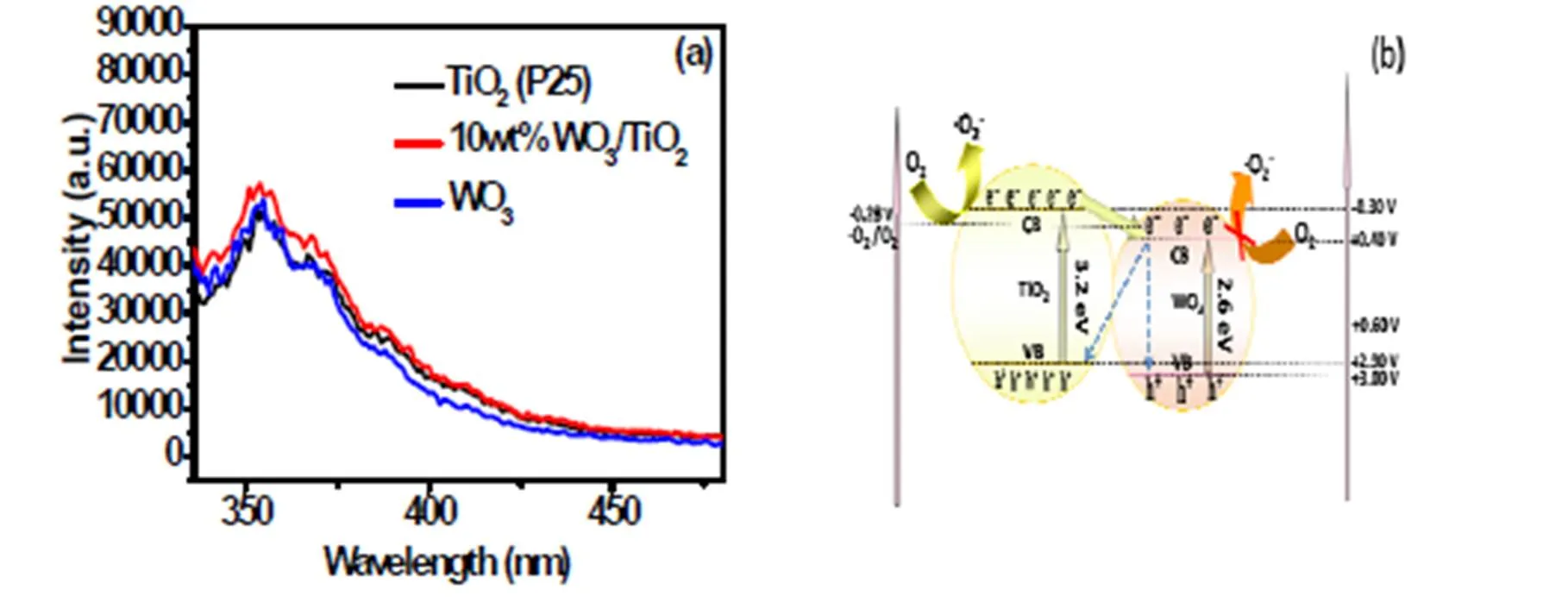
Fig. 7. Photoluminescence spectra of the TiO2(P25), WO3and 10wt%WO3/TiO2nanocomposite (a);proposed charge transfer process over WO3/TiO2nanocomposite under simulated solar light irradiation (b)
In previous studies, it was shown that WO3is much more acidic than TiO2[25], which has higher affinity for chemical species having unpaired electrons[25]. Therefore, the adsorption of oxygen with unpaired electrons on the catalyst surface is investigated by temperature programmed desorption (TPD), as shown in Fig. 8. As compared to TiO2(P25), the O2desorption peak of 10wt%WO3/TiO2shows higher intensity and is shifted to higher temperature. These results indicate that WO3decoration facilitates more oxygen adsorption and enhances the interaction between oxygen and the catalyst surface. Since molecular oxygen is the pre- dominant oxidant during the photocatalytic degra- dation of gaseous hydrocarbons (Fig. S8), the enhan- ced oxygen adsorption can improve the photoactivity of TiO2.
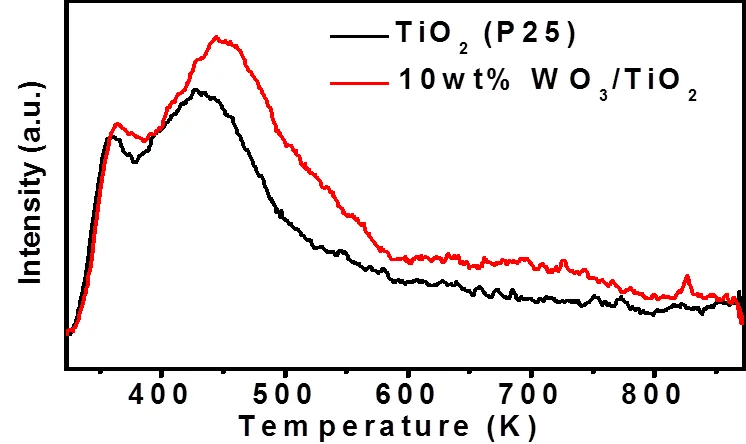
Fig. 8. O2temperature programmed desorption profiles of TiO2(P25) and 10wt%WO3/TiO2
In addition, the enhanced visible light absorption by WO3decoration (Fig. 2) may also contribute to the enhancement of the photoactivities of WO3/TiO2nanocomposite. To study the possible contributions of enhanced visible light absorption on the perfor- mance of photocatalyst, we also investigated the performance of WO3/TiO2nanocomposite under UV and visible light irradiation. As shown in Fig. 9a-c, the 10wt% WO3/TiO2nanocomposite shows obvious photoactivities towards the degradation of C2H6, C3H8and C2H4under visible light irradiation. However, the visible light photoactivities of 10wt% WO3/TiO2are much lower than that of 10wt% WO3/TiO2under UV and simulated solar light irradiation. Moreover, it is found that the UV light photoactivities of the sample are similar to the photoactivity of 10wt% WO3/TiO2under simulated solar light irradiation. These results suggest that the enhanced visible light absorption by WO3decoration does facilitate activation of the WO3/TiO2nanocomposite under visible light irradiation, but it does not have an obvious influence on the perfor- mance of the photocatalyst under simulated solar light irradiation.
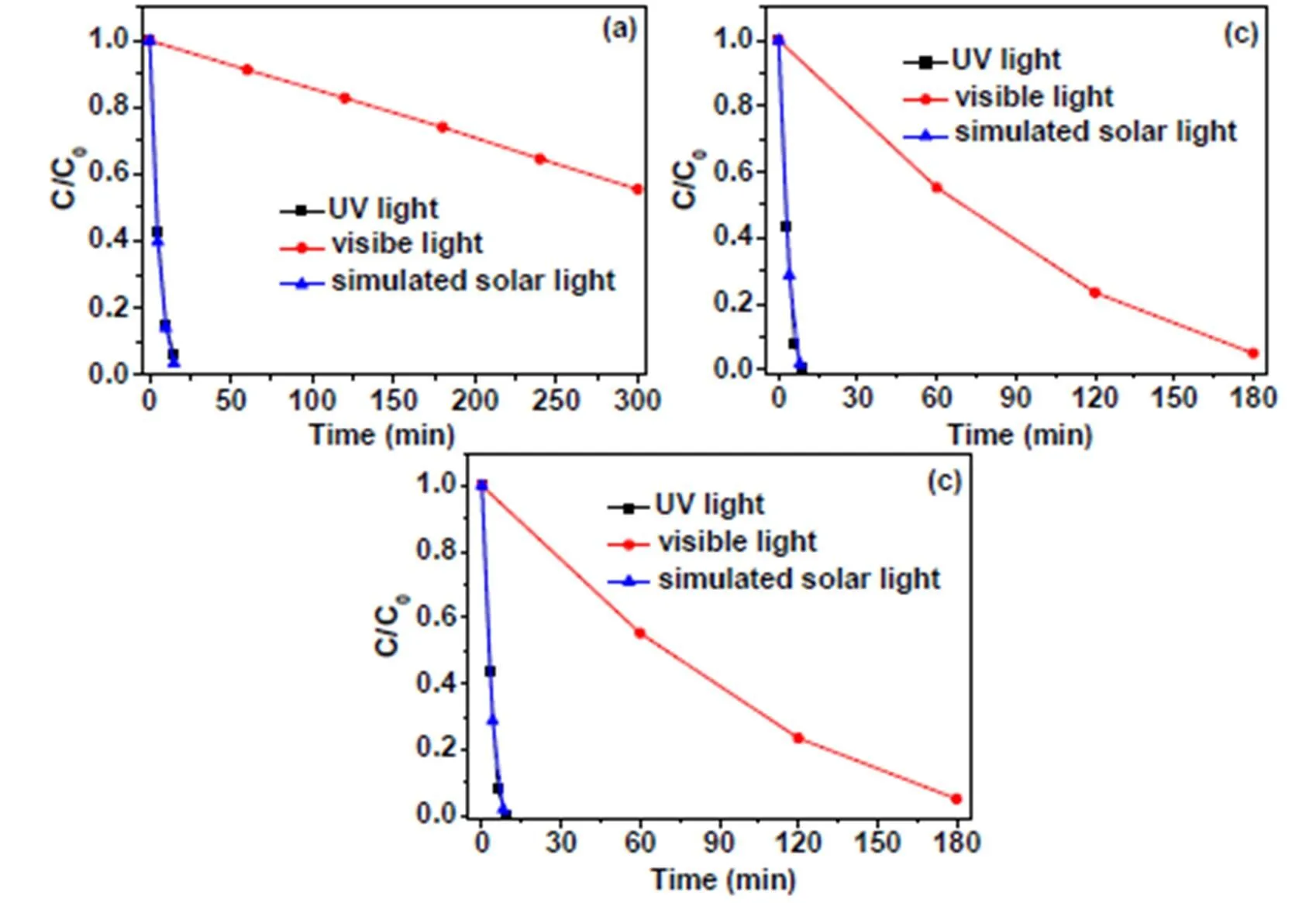
Fig. 9. Photocatalytic degradation of C2H6(a), C3H8(b) and C2H4(c) over the 10wt%WO3/TiO2nanocomposite under UV light (300<<380 nm), visible light (420<<800 nm)and simulated solar light (300<<800 nm) illumination
Notably, an obviously decreased photoactivity of TiO2(P25) by coupling with WO3is observed on the WO3/TiO2nanocomposites toward photocatalytic degradation of methylene blue in liquid phase (Fig. S9). To understand this phenomenon, con- trolled experiments were further conducted. As shown in Fig. S10, the addition of ammonium oxalate (AO) scavenger for photoexcited holes signi- ficantly suppresses the photocatalytic reac- tion[41]. A similar and obvious inhibition phenome- non for photocatalytic reaction is also observed when the scavenger of-butyl alcohol (TBA) for hydroxyl radicals is added[41]. These results indicate that the photogenerated holes and hydroxyl radicals play important roles during the liquid phase methylene blue photodegradation. By contrast, when the reaction system is saturated with N2to expel the dissolved oxygen in the solution, the photoactivity of 10wt%WO3/TiO2is almost not changed, indicating that molecular oxygen is not the primary oxidant during the liquid phase reaction. Therefore, the enhanced oxygen adsorption caused by WO3decora- tion has few effects on the methylene blue photo- degradation. Since the photoexcited electrons in the conduction band of WO3can be hardly scavenged by molecular oxygen, these electrons would recombine with the photogenerated holes or reduce W6+to form W5+(Fig. 7b). As a result, the photoactivity of WO3/TiO2is lower than pure TiO2(P25), which is consistent with the previous report[32].
4 CONCLUSION
In conclusion, our results suggest that the influe- nce of WO3decoration on the performance of TiO2is strongly dependent on the mechanism of photo- catalytic reactions. For different photocatalytic reac- tions, the WO3decoration may have opposite effects on the performance of TiO2. For gaseous hydrocar- bon degradation (C2H6, C3H8and C2H4), molecular oxygen is the predominant oxidant. Since WO3decoration could enhance the oxygen adsorption, the photoactivities of TiO2are enhanced by WO3decora- tion, whereas for MB degradation in liquid phase, photogenerated holes and hydroxyl radicals are the primary oxidants. Under such a condition, the elec- trons on the conduction band of WO3would either recombine with the photogenerated holes or react with W6+to form W5+and thus decrease the perfor- mance of TiO2.
(1) Hüsken, G.; Hunger, M.; Brouwers, H. J. H. Experimental study of photocatalytic concrete products for air purification.2009, 44, 2463-2474.
(2) Enterkin, J. A.; Setthapun, W.; Elam, J. W.; Christensen, S. T.; Rabuffetti, F. A.; Marks, L. D.; Stair, P. C.; Poeppelmeier, K. R.; Marshall, C. L. Propane oxidation over Pt/SrTiO3nanocuboids.2011, 1, 629-635.
(3) Heck, R. M.; Farrauto, R. J. Automobile exhaust catalysts.2001, 221, 443-457.
(4) Li, Y.; Cai, Y.; Chen, X.; Pan, X.; Yang, M.; Yi, Z. Photocatalytic oxidation of small molecule hydrocarbons over Pt/TiO2nanocatalysts.2016, 6, 2760-2767.
(5) Choudhary, T. V.; Banerjee, S.; Choudhary, V. R. Catalysts for combustion of methane and lower alkanes.2002, 234, 1-23.
(6) Schmale, J.; Shindell, D.; von Schneidemesser, E.; Chabay, I.; Lawrence, M. Air pollution: clean up our skies.2014, 515, 335-337.
(7) Chen, X.; Huang, X.; Yi, Z. Enhanced ethylene photodegradation performance of g-C3N4–Ag3PO4composites with direct Z-scheme configuration.2014, 20, 17590-17596.
(8) Keller, N.; Ducamp, M. N.; Robert, D.; Keller, V. Ethylene removal and fresh product storage: a challenge at the frontiers of chemistry. Toward an approach by photocatalytic oxidation.2013, 113, 5029-5070.
(9) Long, P.; Zhang, Y.; Chen, X.; Yi, Z. Fabrication of YBi1-xVO4solid solutions for efficient C2H4photodegradation.2015, 3, 4163-4169.
(10) Fox, M. A.; Dulay, M. T. Heterogeneous photocatalysis.1993, 93, 341-357.
(11) Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: present situation and future approaches.2006, 9, 750-760.
(12) Kubacka, A.; Fernández-García, M.; Colón, G. Advanced nanoarchitectures for solar photocatalytic applications.2011, 112, 1555-1614.
(13) Zheng, Z.; Huang, B.; Meng, X.; Wang, J.; Wang, S.; Lou, Z.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. Metallic zinc-assisted synthesis of Ti3+self-doped TiO2with tunable phase composition and visible-light photocatalytic activity.2013, 49, 868-870.
(14) Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2nanocrystals leads to high photocatalytic efficiency.2011, 133, 16414-16417.
(15) Pei, Z.; Ding, L.; Feng, W.; Weng, S.; Liu, P. Defect self-doped TiO2for visible light activity and direct noble metal anchoring.2014, 16, 21876-21881.
(16) Zhu, Q.; Peng, Y.; Lin, L.; Fan, C. M.; Gao, G. Q.; Wang, R. X.; Xu, A. W. Stable blue TiO2-xnanoparticles for efficient visible light photocatalysts.2014, 2, 4429-4437.
(17) Umezawa, N.; Ye, J. Role of complex defects in photocatalytic activities of nitrogen-doped anatase TiO2.2012, 14, 5924-5934.
(18) Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-situ-reduced synthesis of Ti3+self-doped TiO2/g-C3N4heterojunctions with high photocatalytic performance under LED light irradiation.2015, 7, 9023-9030.
(19) Xiang, Q.; Yu, J.; Wang, W.; Jaroniec, M. Nitrogen self-doped nanosized TiO2sheets with exposed {001} facets for enhanced visible-light photocatalytic activity.2011, 47, 6906-6908.
(20) Liu, G.; Yang, H. G.; Wang, X.; Cheng, L.; Lu, H.; Wang, L.; Lu, G. Q.; Cheng, H. M. Enhanced photoactivity of oxygen-deficient anatase TiO2sheets with dominant {001} Facets.2009, 113, 21784-21788.
(21) Liu, S.; Zhang, N.; Tang, Z. R.; Xu, Y. J. Synthesis of one-dimensional CdS@TiO2core-shell nanocomposites photocatalyst for selective redox: the dual role of TiO2shell.2012, 4, 6378-6385.
(22) Zhang, N.; Zhang, Y.; Pan, X.; Yang, M. Q.; Xu, Y. J. Constructing ternary CdS-Graphene-TiO2hybrids on the flatland of graphene oxide with enhanced visible-light photoactivity for selective transformation.2012, 116, 18023-18031.
(23) Lou, Z.; Li, F.; Deng, J.; Wang, L.; Zhang, T. Branch-like hierarchical heterostructure (Fe2O3/TiO2): a novel sensing material for trimethylamine gas sensor.2013, 5, 12310-12316.
(24) DeKrafft, K. E.; Wang, C.; Lin, W. Metal-organic framework templated synthesis of Fe2O3/TiO2nanocomposite for hydrogen production.2012, 24, 2014-2018.
(25) Papp, J.; Soled, S.; Dwight, K.; Wold, A. Surface acidity and photocatalytic activity of TiO2, WO3/TiO2, and MoO3/TiO2photocatalysts.1994, 6, 496-500.
(26) Tada, H.; Kokubu, A.; Iwasaki, M.; Ito, S. Deactivation of the TiO2photocatalyst by coupling with WO3and the electrochemically assisted high photocatalytic activity of WO3.2004, 20, 4665-4670.
(27) Zheng, H.; Ou, J. Z.; Strano, M. S.; Kaner, R. B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured tungsten oxide – properties, synthesis, and applications.2011, 21, 2175-2196.
(28) Zhao, D.; Chen, C.; Yu, C.; Ma, W.; Zhao, J. Photoinduced electron storage in WO3/TiO2nanohybrid material in the presence of oxygen and postirradiated reduction of heavy metal ions.2009, 113, 13160-13165.
(29) Paramasivam, I.; Nah, Y. C.; Das, C.; Shrestha, N. K.; Schmuki, P. WO3/TiO2nanotubes with strongly enhanced photocatalytic activity.2010, 16, 8993-8997.
(30) Smith, W.; Zhao, Y. Enhanced photocatalytic activity by aligned WO3/TiO2two-layer nanorod arrays.2008, 112, 19635-19641.
(31) Ismail, A. A.; Abdelfattah, I.; Helal, A.; Al-Sayari, S. A.; Robben, L.; Bahnemann, D. W. Ease synthesis of mesoporous WO3-TiO2nanocomposites with enhanced photocatalytic performance for photodegradation of herbicide imazapyr under visible light and UV illumination.2016, 307, 43-54.
(32) Dozzi, M. V.; Marzorati, S.; Longhi, M.; Coduri, M.; Artiglia, L.; Selli, E. Photocatalytic activity of TiO2-WO3mixed oxides in relation to electron transfer efficiency.2016, 186, 157-165.
(33) Song, K. Y.; Park, M. K.; Kwon, Y. T.; Lee, H. W.; Chung, W. J.; Lee, W. I. Preparation of transparent particulate MoO3/TiO2and WO3/TiO2films and their photocatalytic properties.2001, 13, 2349-2355.
(34) Wang, G.; Ling, Y.; Wang, H.; Yang, X.; Wang, C.; Zhang, J. Z.; Li, Y. Hydrogen-treated WO3nanoflakes show enhanced photostability.2012, 5, 6180-6187.
(35) Martín, C.; Solana, G.; Rives, V.; Marcì, G.; Palmisano, L.; Sclafani, A. Physico-chemical properties of WO3/TiO2systems employed for 4-nitrophenol photodegradation in aqueous medium.1997, 49, 235-243.
(36) Pan, J. H.; Lee, W. I. Preparation of highly ordered cubic mesoporous WO3/TiO2films and their photocatalytic properties.2006, 18, 847-853.
(37) Brigden, C. T.; Poulston, S.; Twigg, M. V.; Walker, A. P.; Wilkins, A. J. J. Photo-oxidation of short-chain hydrocarbons over titania.2001, 32, 63-71.
(38) Hägglund, C.; Kasemo, B.; Österlund, L.reactivity and FTIR study of the wet and dry photooxidation of propane on anatase TiO2.2005, 109, 10886-10895.
(39) Khatri, R. A.; Chuang, S. S. C.; Soong, Y.; Gray, M. Carbon dioxide capture by diamine-grafted SBA-15: a combined fourier transform infrared and mass spectrometry study.2005, 44, 3702-3708.
(40) van der Meulen, T.; Mattson, A.; Österlund, L. A comparative study of the photocatalytic oxidation of propane on anatase, rutile, and mixed-phase anatase-rutile TiO2nanoparticles: role of surface intermediates.2007, 251, 131-144.
(41) Pan, X.; Xu, Y. J. Defect-mediated growth of noble-metal (Ag, Pt, and Pd) nanoparticles on TiO2with oxygen vacancies for photocatalytic redox reactions under visible light.2013, 117, 17996-18005.
7 June 2017;
23 November 2017
10.14102/j.cnki.0254-5861.2011-1748
① Supported by the National Key Project on Basic Research (No. 2013CB933203), the Strategic Priority Research Program of the Chinese Academy
of Sciences (No. XDB20000000), the National Natural Science Foundation of China (No. 21607153, 21373224 and 21577143), the Natural Science Foundation of Fujian Province (No. 2015J05044), and the Frontier Science Key Project of the Chinese Academy of Sciences (QYZDB-SSW-JSC027)
②Pan Xiao-Yang, E-mail: xypan@fjirsm.ac.cn; Yi Zhi-Guo, E-mail: zhiguo@fjirsm.ac.cn
- 结构化学的其它文章
- Synthesis, Crystal Structure and Photoluminescent Property of a New Zn(II) Complex Based on 3,4-Bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole①
- Transitional Area of Ce4+ to Ce3+ in SmxCayCe1-x-yO2-δ with Various Doping and Oxygen Vacancy Concentrations: A GGA + U Study①
- A New Dinuclear Zinc Polymer Based on 3-Methoxy-2-hydroxybenzaldehyde:Synthesis, Structure, Spectral Characterization and Hirshfeld Surface Analysis①
- Two Copper Complexes Based on Pyrazole- 3-carboxylic Acid as Heterogeneous Catalysts for Highly Selective Oxidation of Alkylbenzenes①
- Synthesis, Crystal Structure and Cytotoxic Activities of Oxazolidin-2-one Derivatives①
- Crystal Structures, Thermal Behaviors and Biological Activities of Acylhydrazone Compounds Containing Pyrazine Rings and Halogen Atoms①

