氮杂环卡宾在三吡唑基硼酸阴离子为支撑配体的钌中心上的环金属化
于 谦,林 毅,聂 鹏,温庭斌
(厦门大学化学化工学院,福建厦门361005)
氮杂环卡宾(N-heterocyclic carbene,NHC)现已成为继有机膦配体之后的又一类重要配体[1-5].与传统的有机膦配体相比,NHC具有更强的σ-供电子能力,可与过渡金属形成更稳定的金属-卡宾σ键,所生成的配合物热稳定性高,对水和空气稳定;同时,NHC易于修饰,使得其空间和电子性质易于调节,结构更加多元化[1-2,5-7].这些独特的优势使得NHC在过渡金属催化和金属有机化学中得到了广泛的应用[1-4,8-10].而且在许多催化反应中,NHC配合物表现出比相应的膦配合物更高的催化活性,如钯催化的偶联反应[11-12]、钌催化的烯烃复分解反应[13]、铱催化的氢转移反应[14]等.不仅如此,NHC配体还可发生N-侧臂上取代基的C—H键活化而生成环金属化产物[15-17],这进一步丰富了NHC的反应性质.近年来,随着一些NHC环金属化配合物在催化[17-20]及发光材料[16,21-22]等领域的应用,NHC配体的环金属化引起人们越来越大的研究兴趣,特别是钌配合物中NHC环金属化方面的研究[18-20,23-25].

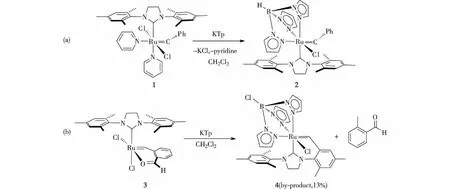
图1 已报道的同时包含Tp和NHC配体的两个钌配合物的合成[35-36] Fig.1The reported two ruthenium complexes including both Tp and NHC ligands[35-36]
鉴于TpRuCl(PPh3)2配合物在金属有机合成和催化反应中的重要作用以及NHC配体所表现出的优势,本课题组试图以TpRuCl(PPh3)2(5)和H2IMes(H)(OBut)(6)为原料,合成一个与多功能用途TpRuCl(PPh3)2类似的NHC配合物TpRuCl(NHC)(PPh3),以期在后续研究中以其为前体,开展TpRu(NHC)骨架配合物的金属有机化学研究.因此,本研究考察了TpRuCl(PPh3)2和NHC配体H2IMes的反应,但发现该反应未能分离得到预期产物TpRuCl(H2IMes)(PPh3) (A),而是生成了H2IMes配体环金属化的产物TpRu(H2IMes-H)(PPh3)(7) (其中H2IMes-H表示H2IMes的一个N-侧臂上邻位的甲基脱除了1个质子).分离得到的化合物7在二氯甲烷溶液中加热可解离PPh3配体,促成H2IMes-H配体进一步发生环金属化侧臂上苄基的C—H活化,经α-H消除转化为螯合的双卡宾配体H2IMes-2H,得到类似于化合物4的产物TpRuCl(H2IMes-2H) (8).
1 实验部分
1.1 仪器与试剂
除另有说明外,所有实验均在室温下、氮气(或氩气)气氛中采用标准的Schlenk技术操作.四氢呋喃、乙醚、正己烷、甲苯均为分析纯,使用前在氮气气氛中经钠-二苯甲酮除水后重蒸.二氯甲烷,分析纯,用前经氢化钙除水后重蒸.起始原料KTp[37]、TpRuCl(PPh3)2(5)[38]、H2IMes(H)(BF4)[24]和H2IMes(H)(OBut)(6)[24]根据文献中的方法合成,其他试剂均从Sigma-Aldrich、ACROS和Alfa-Aesar公司购买.
核磁共振(NMR)谱1H、31P{1H}、13C{1H}在Bruker AV400 (400 MHz)核磁共振仪上测定,1H-NMR和13C{1H}-NMR采用四甲基硅烷(TMS)为内标,31P{1H}-NMR采用85%(质量分数)H3PO4为内标.如无特别说明,操作温度为298 K.
X-射线单晶衍射在Oxford CCD衍射仪上收集数据,采用石墨单色化的Mo Kα射线 (λ=0.071 073 nm),电压50 kV,电流30 mA.
1.2 化合物的合成
1.2.1 TpRu(H2IMes-H)(PPh3)(7)
将10 mL甲苯加入到RuTpCl(PPh3)2(5)(0.20 g,0.23 mmol)和H2IMes(H)(OBut)(6)(0.30 g,1.00 mmol)的混合物中,80 ℃下搅拌4 h,冷却后过滤,将滤液减压浓缩至约1 mL,加入10 mL正已烷,析出黄色固体,用砂芯过滤,得到的固体用3×3 mL正已烷洗涤,真空下抽干,产量0.14 g,产率69%.1H-NMR (CDCl3,400.1 MHz):δ0.95 (s,3H,Me),1.35 (s,3H,Me),1.79 (d(unresolved),2JHH=13 Hz,1H,RuCH2),2.02 (s,3H,Me),2.09 (s,3H,Me),2.58 (s,3H,Me),2.64 (d(unresolved),2JHH=13 Hz,1H,RuCH2),3.46~3.53 (m,2H,NCH2),3.68 (m,1H,NCH2),4.57 (m,1H,NCH2),5.20 (t,3JHH=1.8 Hz,1H,Tp),5.89 (t,3JHH=1.8 Hz,1H,Tp),6.07 (s,1H,m-Ar),6.17 (t,3JHH=1.8 Hz,1H,Tp),6.21 (s,1H,m-Ar),6.32 (s,1H,m-Ar),6.40 (s,1H,m-Ar),7.12~6.72 (m,15H,PPh3),7.36 (s,br,1H,Tp),7.40 (s,br,1H,Tp),7.48 (d,3JHH=1.8 Hz,1H,Tp),7.60 (s,br,1H,Tp),7.61 (d,3JHH=1.8 Hz,1H,Tp),7.71 (s,1H,Tp);31P {1H}-NMR (CDCl3,162.0 MHz):δ57.4 (s,PPh3);13C{1H}-NMR (CDCl3,100.6 MHz):δ15.8,18.4 (s,Me),19.0 (d,JPC=7.8 Hz,RuCH2),21.0(s,Me×2),21.3(s,Me),50.5,53.3 (s,NCH2),104.3,104.8,106.1 (s,CH,Tp),126.7~151.3 (m,PPh3and Ar),234.6 (d,JPC=13 Hz,RuCN).元素分析得分子式C48H50BN8PRu,计算值(%):C 65.38,H 5.72,N 12.71;测定值(%):C 65.08,H 5.64,N 12.62.
将正己烷缓慢注入到化合物7的二氯甲烷溶液的上层,通过缓慢扩散得到黄色块状晶体.
1.2.2 TpRuCl(H2IMes-2H)(8)

将正己烷缓慢注入到化合物8的二氯甲烷溶液的上层,通过缓慢扩散得到绿色块状晶体.
1.3 晶体结构的解析
挑选适当的化合物7和8的晶体,在173 K下进行X-射线单晶衍射表征.全部强度数据均经过SADABS吸收校正.晶体结构采用SHELXS-97程序包解析,晶体结构由直接法解出.对全部非氢原子坐标及其各向异性热参数进行全矩阵最小二乘法修正(SHELXS-97程序包).化合物7晶体结构的不对称单元中包含1个二氯甲烷溶剂分子.化合物7·CH2Cl2和8的晶体学数据见表1,主要的键长及键角数据列于表2和3.晶体结构数据存于英国剑桥数据中心(Cambridge Crystallographic Data Centre,CCDC),编号分别为1543564 (7)和1543565 (8).
2 结果与讨论
2.1 产物的合成及结构表征
NHC配体H2IMes的叔丁氧基咪唑啉前体H2IMes(H)(OBut) (6)在80 ℃下加热可消除一分子叔丁醇得到NHC配体H2IMes[24],因此,通过TpRuCl(PPh3)3(5)与化合物6以摩尔比1∶4在甲苯溶液中于80 ℃下搅拌反应4 h后,经处理分离得到黄色固体产物TpRu(H2IMes-H)PPh3(7),产率69% (图2).如果反应中减少化合物6的投量,反应进行得缓慢且不完全.在考察化合物7的性质过程中,发现分离得到的化合物7在二氯甲烷中回流可转化为新的产物,反应10 h后,通过检测反应液的原位31P和1H-NMR谱,表明化合物7已完全反应,主产物为新生成的金属卡宾物种,同时有少量未知的副产物生成.经过硅胶柱层析分离,得到主产物TpRuCl(H2IMes-2H)(8),产率54% (图2).
化合物7经过X-射线单晶衍射表征,其晶体结构如图3所示.可以看出,化合物7中H2IMes配体取代了TpRuCl(PPh3)2的一个PPh3配体,并且其中一侧的N-侧臂上的均三甲基苯基中邻位的甲基发生环金属化形成了一个螯合的NHC配体H2IMes-H,并消除了一分子HCl.化合物7中的Ru金属中心的配位构型为一个扭曲的八面体.Ru1—C10的键长为0.200 9(3) nm,是典型的Ru—CNHC的键长,Ru1—C19的键长为0.212 9(3) nm,是典型的Ru—C单键的键长,与已报道的类似结构的化合物的相应键长十分接近.例如:化合物RuH(IMes-H)(CO)(PPh3)2(IMes=1,3-N,N-双(2,4,6-三甲基苯基)-咪唑-2-卡宾,IMes-H表示IMes脱除了1个质子)中的Ru—CNHC和Ru—Calkyl的键长分别为0.207 46(12)和0.214 29(12) nm[24];RuCl(H2IMes-H)(CO)(PCy3)中相应的两个键长分别为0.203 7(3)和0.209 7(2) nm[24];RuH(H2IMes-H)(CO)(PPh3)2中分别为0.204 5(2)和0.216 2(2) nm[39].
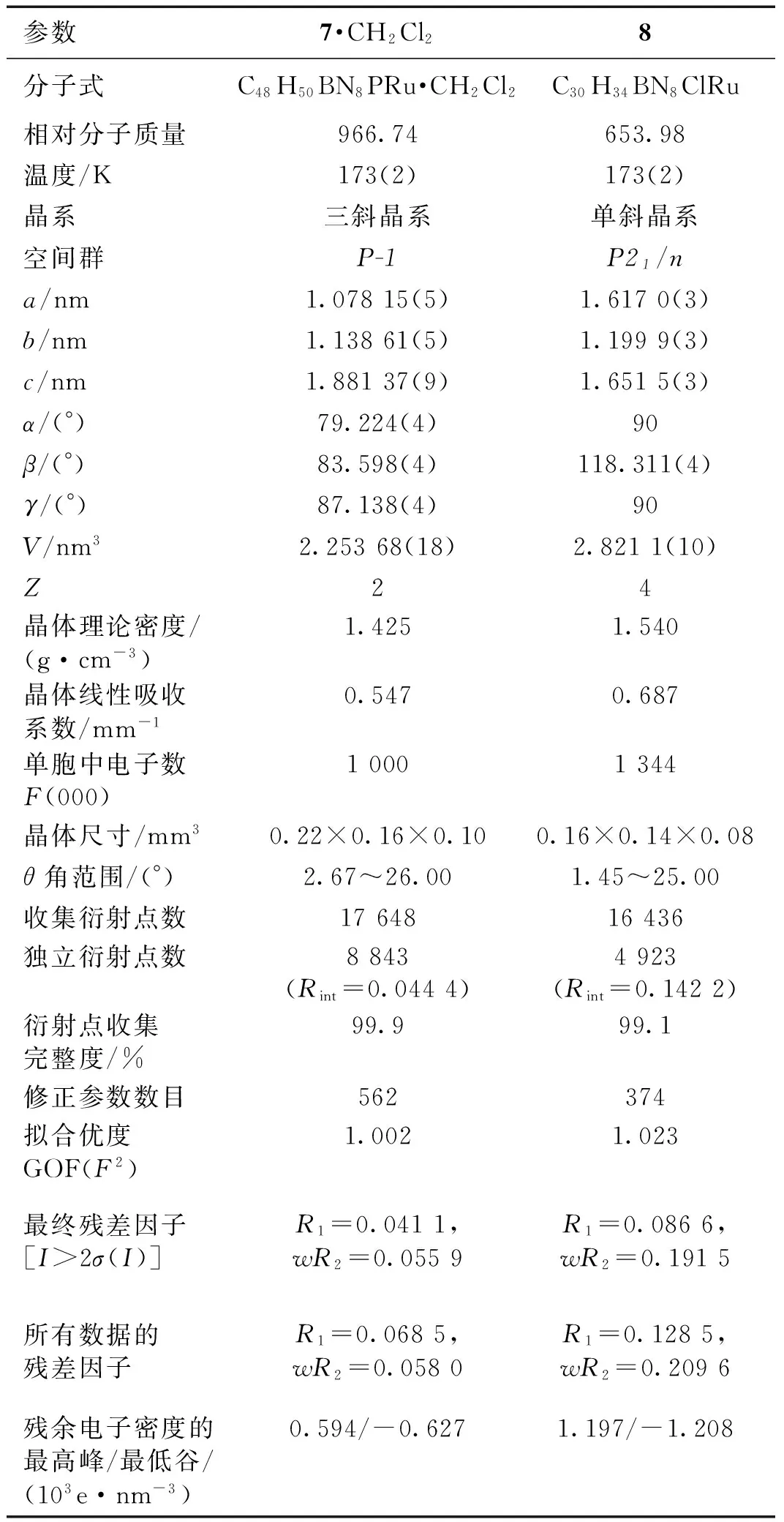
表1 化合物7·CH2Cl2和8的晶体学数据Tab.1 Crystal data and structure refinement of compounds 7·CH2Cl2 and 8
注:表中()内的数字为最后一位(或两位)有效数值的误差值,下同.
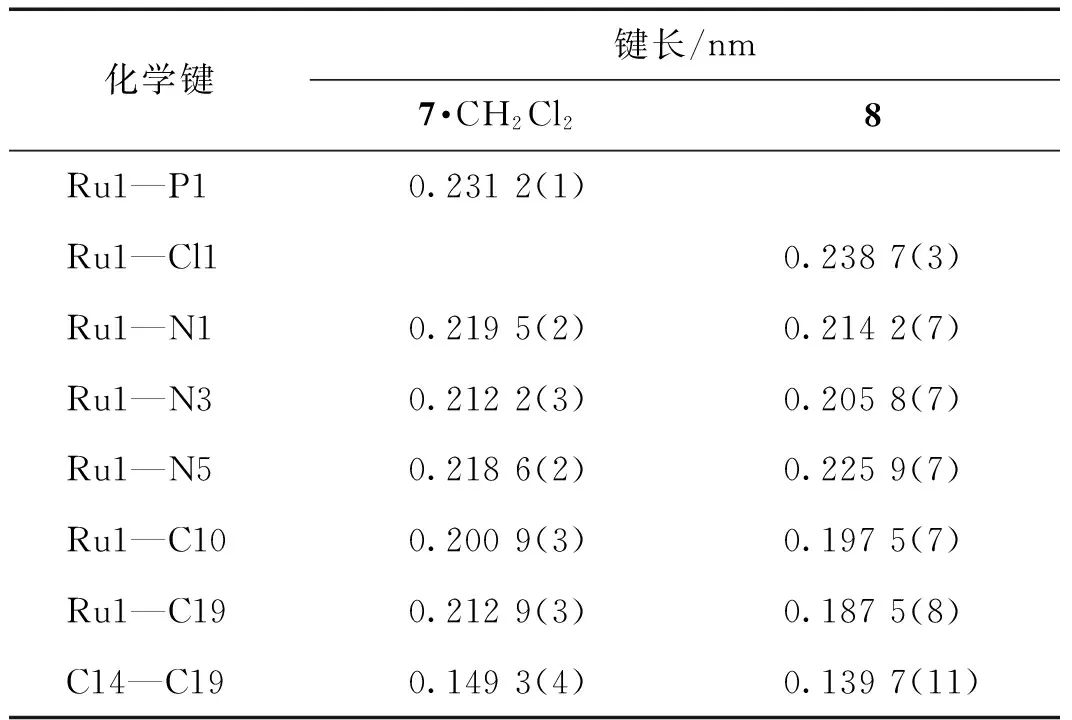
表2 化合物7·CH2Cl2和8的主要键长Tab.2 Selected bond lengths of compounds 7·CH2Cl2 and 8
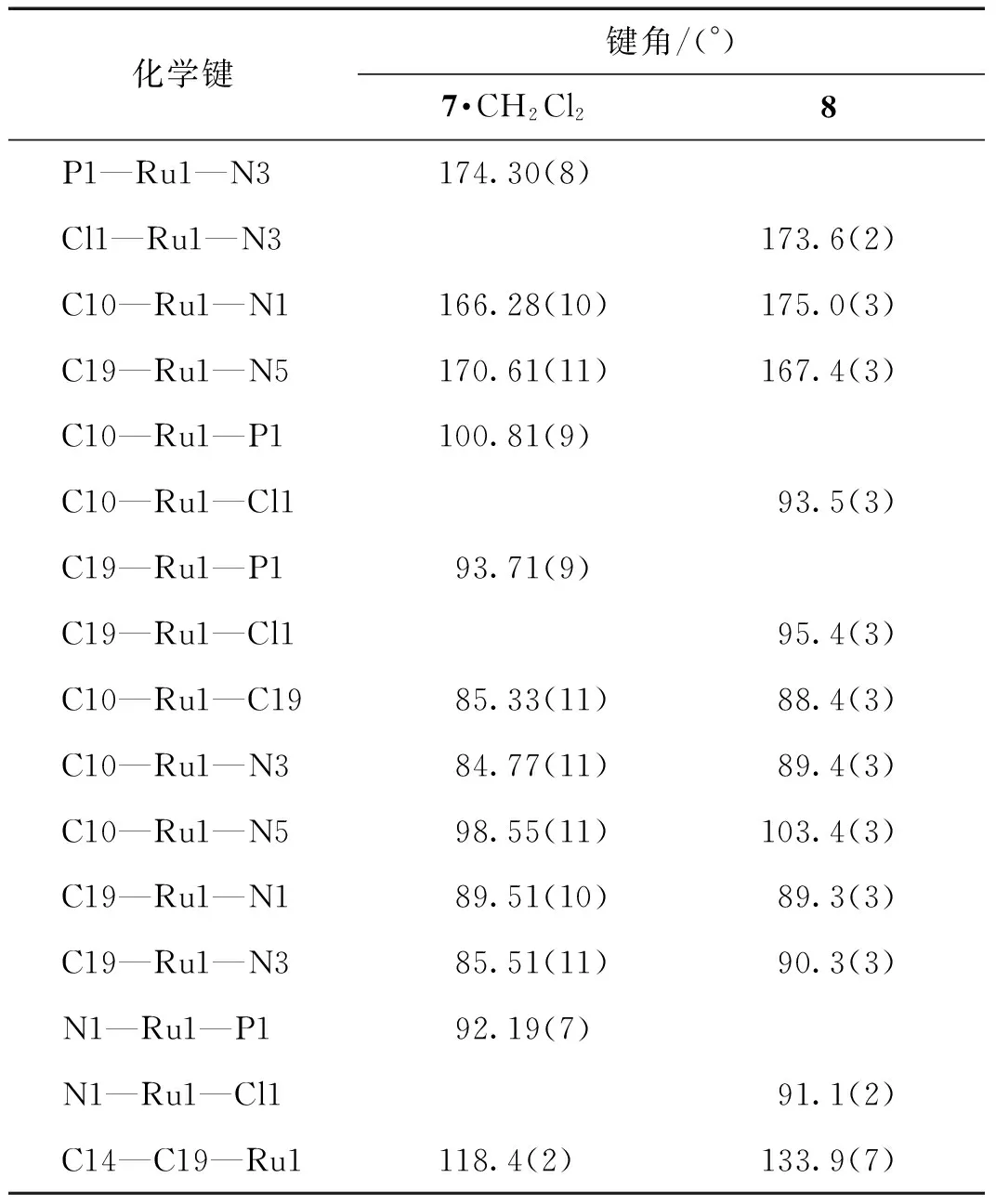
表3 化合物7·CH2Cl2和8的主要键角Tab.3 Selected bond angles of compounds 7·CH2Cl2 and 8
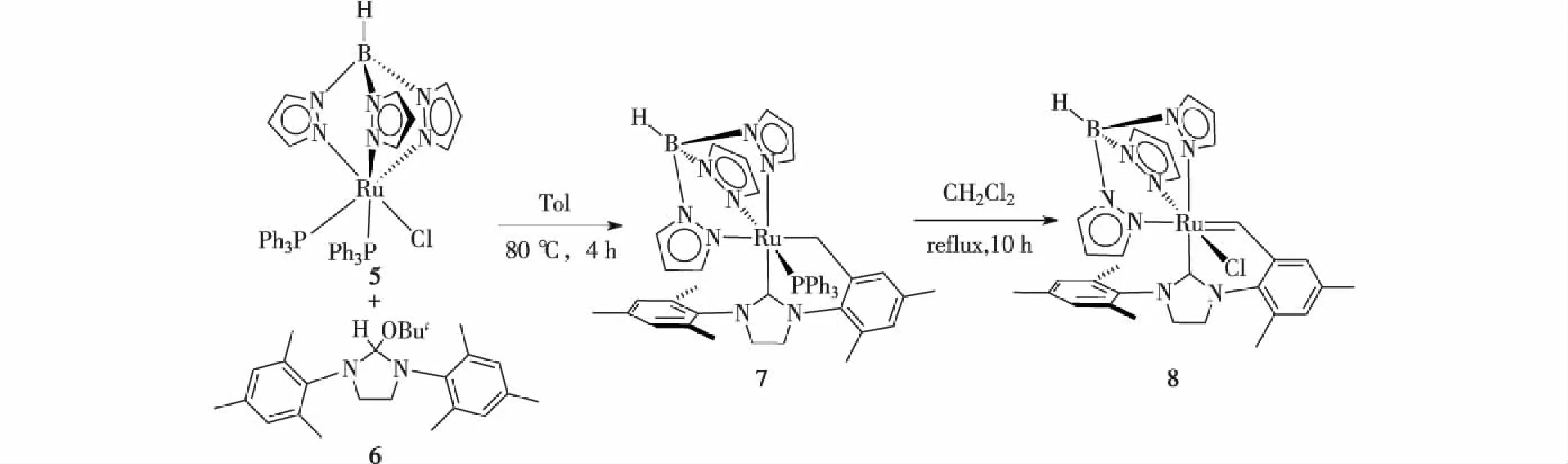
图2 化合物7和8的合成 Fig.2Synthesis of compounds 7 and 8
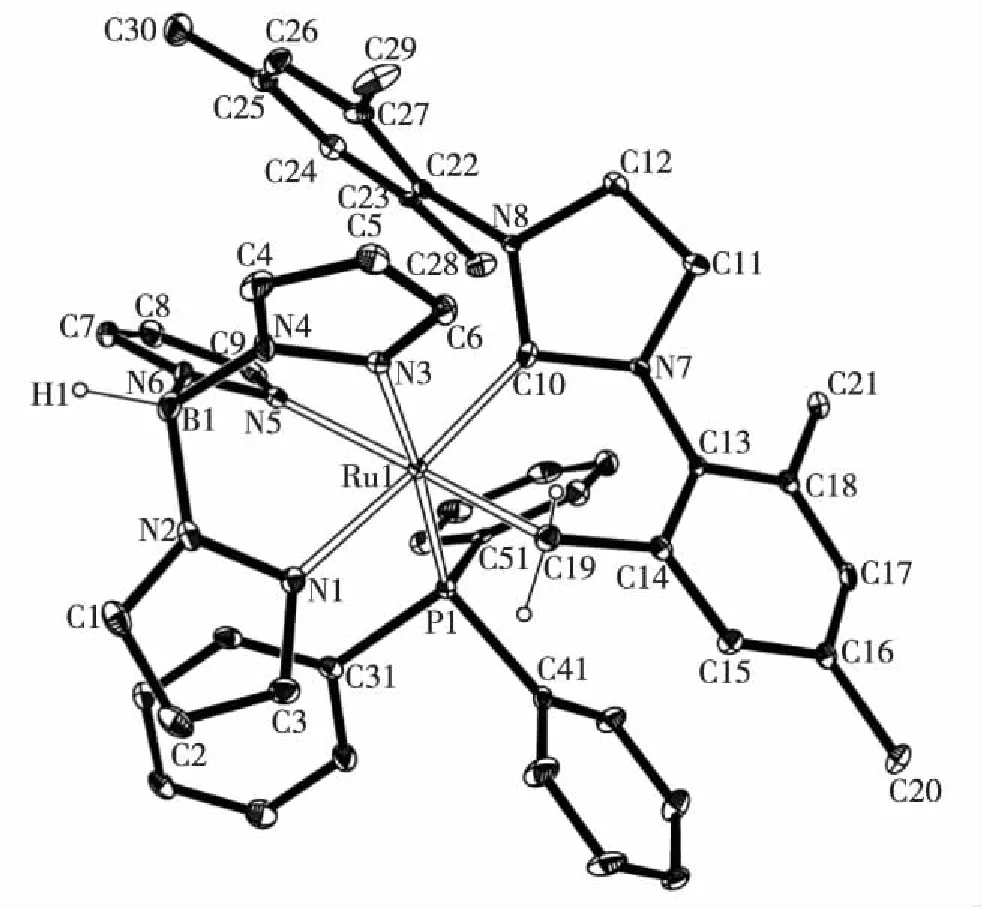
图3 化合物7的晶体结构 Fig.3Molecular structure of compound 7
化合物7的NMR数据与其晶体结构一致,其31P{1H}-NMR谱图在δ57.4处显示1个单峰信号.在1H-NMR谱图中,只观察到5组甲基的质子信号,发生环金属化的亚甲基上的2个质子由于所处化学环境不一样而表现出不同的化学位移,分别在δ1.79和2.64.NHC环上的2个亚甲基也存在类似情况,4个质子信号分别位于δ3.46~3.53 (m,2H,2个质子信号重叠),3.68 (m,1H),4.57 (m,1H).在13C{1H}-NMR谱图中,环金属化的亚甲基的碳信号位于δ19.0,NHC配体上的卡宾碳信号位于δ234.6.

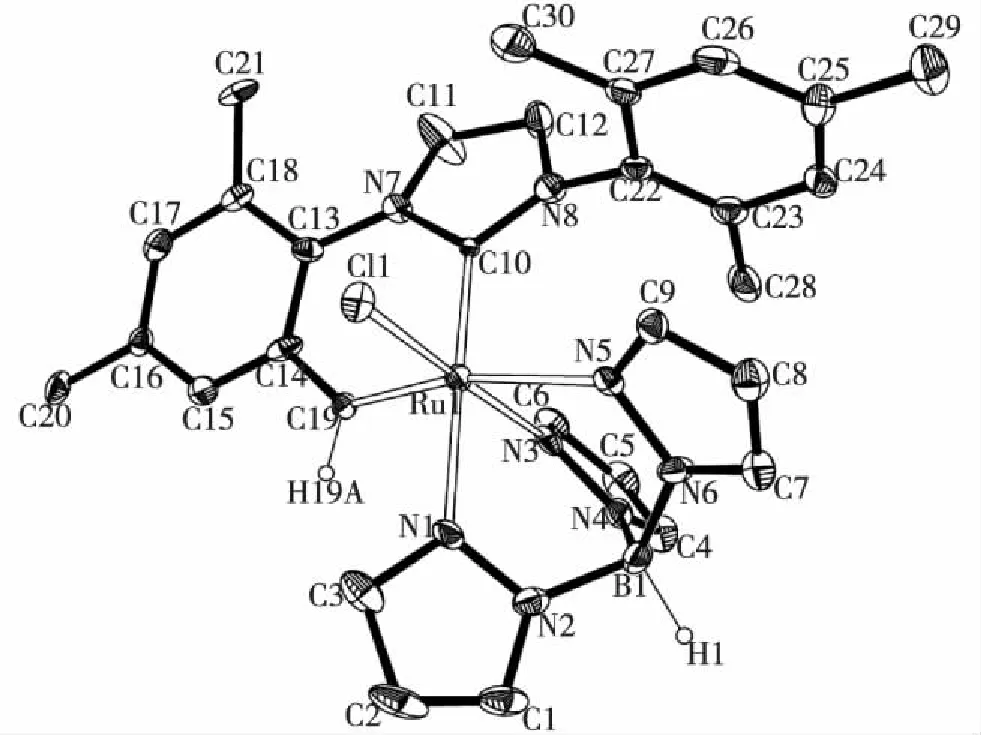
图4 化合物8的晶体结构 Fig.4Molecular structure of compound 8

2.2 反应机理
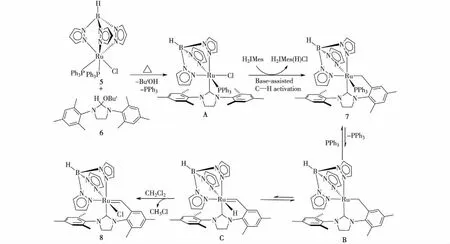
图5 生成化合物7和8的可能机理 Fig.5Proposed mechanism for the formation of compounds 7 and 8
上述两个反应中,生成TpRu(H2IMes-H)(PPh3) (7)和TpRuCl(H2IMes-2H) (8)的可能机理如图5所示.H2IMes(H)(OBut)(6)在加热条件下消除叔丁醇,首先产生的H2IMes与TpRuCl(PPh3)2(5)反应,取代一个PPh3配体,生成中间体TpRuCl(H2IMes)(PPh3) (A).由于反应体系中过量的H2IMes具有较强的碱性,可通过碱辅助的金属化-去质子化过程促进N-侧臂邻位上甲基的C—H键活化,并消除一分子HCl得到产物7.Hong等[40]报道了类似的通过体系中解离的PCy3作为碱促进的NHC配体N-侧臂芳基在钌中心的C—H键活化,而且与PCy3相比,H2IMes具有更强的碱性.本研究的实验结果也表明,如果反应中减少化合物6的投量,反应进行得缓慢且不完全.分离得到的化合物7在溶液中加热发生可逆地解离PPh3配体产生中间体B,16-电子的中间体B可发生苄基上可逆的α-H消除,转化为含Ru—H的苄卡宾中间体C,中间体C上的氢配体再和二氯甲烷溶剂发生H/Cl交换得到最终产物8.已有不少报道表明一些Ru—H化合物易和二氯甲烷发生H/Cl交换而转化为Ru—Cl化合物[36,41-42].本研究的实验过程中也发现,化合物5和6在溶液中加热反应时,即使在二氯甲烷存在下,也可使反应停留在产物7而不继续转化.不难理解,这是由于反应中解离下来的第一个PPh3配体存在于体系中,抑制了化合物7中PPh3配体的继续解离,使其难以发生后续反应.另外,分离得到的化合物7在C6D6中即使加热24 h也未见有明显反应,如果往溶液中加入二氯甲烷继续加热,经过10 h后即可完全反应,因此,后续发生的H/Cl交换可使溶液中产生的中间体C不断继续反应生成化合物8,也促使化合物7不断地转化为B,再生成C,最后推动反应得以完全.

3 结 论
鉴于TpRuCl(PPh3)2配合物在金属有机合成和催化反应中的多种用途以及NHC配体所表现出的优势,本研究考察了TpRuCl(PPh3)2与NHC配体H2IMes的反应,试图合成一个和TpRuCl(PPh3)2类似的NHC配合物前体TpRuCl(H2IMes)(PPh3) (A).该反应未能分离得到预期产物A,而是发生了H2IMes配体一个N-侧臂上邻位甲基的C—H键活化,生成了H2IMes配体环金属化的产物TpRu(H2IMes-H)(PPh3) (7).分离得到的化合物7在二氯甲烷溶液中加热可解离PPh3配体,促使H2IMes-H配体进一步发生环金属化侧臂上苄基的C—H活化,经α-H消除转化为螯合的双卡宾配体H2IMes-2H配位的产物TpRuCl(H2IMes-2H) (8).虽然钌配合物中NHC配体因N-侧臂上取代基的C—H键活化而环金属化已有广泛的研究,但是环金属化后进一步发生α-H消除而生成螯合的双卡宾配位产物的例子却很罕见.在后续工作中将继续考察TpRuCl(PPh3)2与其他NHC配体的反应,并研究环金属化产物7和双卡宾螯合的钌配合物8的反应性质.
[1] HERRMANN W A.N-heterocyclic carbenes:a new concept in organometallic catalysis[J].Angew Chem Int Ed,2002,41(8):1290-1309.
[2] HAHN F E,JAHNKE M C.Heterocyclic carbenes:synthesis and coordination chemistry[J].Angew Chem Int Ed,2008,47(17):3122-3172.
[3] JONES W D.Diverse chemical applications of N-heterocyclic carbenes[J].J Am Chem Soc,2009,131(42):15075-15077.
[4] POYATOS M,MATA J A,PERIS E.Complexes with poly(N-heterocyclic carbene) ligands:structural features and catalytic applications[J].Chem Rev,2009,109(8):3677-3707.
[5] HOPKINSON M N,RICHTER C,SCHEDLER M,et al.An overview of N-heterocyclic carbenes[J].Nature,2014,510(7506):485-496.
[6] DRÖGE T,GLORIUS F.The measure of all rings:N-hete-rocyclic carbenes[J].Angew Chem Int Ed,2010,49(39):6940-6952.
[8] COLACINO E,MARTINEZ J,LAMATY F.Preparation of NHC-ruthenium complexes and their catalytic activity in metathesis reaction[J].Coord Chem Rev,2007,251(5/6):726-764.
[9] DRAGUTAN V,DRAGUTAN I,DELAUDE L,et al.NHC-Ru complexes:friendly catalytic tools for manifold chemical transformations[J].Coord Chem Rev,2007,251(5/6):765-794.
[10] DIEZ-GONZALEZ S,MARION N,NOLAN S P.N-he-terocyclic carbenes in late transition metal catalysis[J].Chem Rev,2009,109(8):3612-3676.
[11] HILLIER A C,GRASA G A,VICIU M S,et al.Catalytic cross-coupling reactions mediated by palladium/nucleophilic carbene systems[J].J Organomet Chem,2002,653(1/2):69-82.
[12] MARION N,NAVARRO O,MET J,et al.Modified (NHC)Pd(allyl)Cl (NHC=N-heterocyclic carbene) complexes for room-temperature Suzuki-Miyaura and Buchwald-Hartwig reactions[J].J Am Chem Soc,2006,128(12):4101-4111.
[13] TRNKA T M,GRUBBS R H.The development of L2X2Ru=CHR olefin metathesis catalysts:an organometallic success story[J].Acc Chem Res,2001,34(1):18-29.
[14] HANASAKA F,FUJITA K,YAMAGUCHI R.Synthesis of new cationic Cp*Ir N-heterocyclic carbene complexes and their high catalytic activities in the oppenauer-type oxidation of primary and secondary alcohols[J].Organometallics,2005,24(14):3422-3433.
[15] SCOTT N M,NOLAN S P.Stabilization of organometallic species achieved by the use of N-heterocyclic carbene (NHC) ligands[J].Eur J Inorg Chem,2005(10):1815-1828.
[16] ALBRECHT M.Cyclometalation using d-block transition metals:fundamental aspects and recent trends[J].Chem Rev,2010,110(2):576-623.
[18] BURLING S,PAINE B M,NAMA D,et al.C—H activation reactions of ruthenium N-heterocyclic carbine complexes:application in a catalytic tandem reaction involving C—C bond formation from alcohols[J].J Am Chem Soc,2007,129(7):1987-1995.
[19] HARTUNG J,DORNAN P K,GRUBBS R H.Enantio selective olefin metathesis with cyclometalated ruthenium complexes[J].J Am Chem Soc,2014,136(37):13029-13037.
[20] MA C,AI C,LI Z,et al.Synthesis and alkyne insertion reactions of NHC-based cyclometalated ruthenium(Ⅱ) complexes[J].Organometallics,2014,33(19):5164-5172.
[21] YANG C,MEHMOOD F,LAM T L,et al.Stable luminescent iridium(Ⅲ) complexes with bis(N-heterocyclic carbene) ligands:photo-stability,excited state properties,visible-light-driven radical cyclization and CO2reduction,and cellular imaging[J].Chem Sci,2016,7(5):3123-3136.
[22] ALABAU R G,EGUILLOR B,ESLER J,et al.CCC-Pincer-NHC osmium complexes:new types of blue-green emissive neutral compounds for organic light-emitting devices (OLEDs)[J].Organometallics,2014,33(19):5582-5596.
[23] LIU H J,RAYNAUD C,EISENSTEIN O,et al.Cyclometalated N-heterocyclic carbine complexes of ruthenium for access to electron-rich silylene complexes that bind the Lewis acids CuOTf and AgOTf[J].J Am Chem Soc,2014,136(32):11473-11482.
[24] TRNKA T M,MORGAN J P,SANFORD M S,et al.Synthesis and activity of ruthenium alkylidene complexes coordinated with phosphine and N-heterocyclic carbine ligands[J].J Am Chem Soc,2003,125(9):2546-2558.
[25] JAZZAR R F,MACGREGOR S A,MAHON M F,et al.C—C and C—H bond activation reactions in N-heterocyclic carbine complexes of ruthenium[J].J Am Chem Soc,2002,124(18):4944-4945.
[26] TROFIMENKO S.Recent advances in poly(pyrazolyl)borate (scorpionate) chemistry[J].Chem Rev,1993,93(3):943-980.
[27] TROFIMENKO S.Scorpionates:genesis,milestones,prognosis[J].Polyhedron,2004,23(2/3):197-203.
[28] PAULO A,CORREIA J,CAMPELLO M,et al.A short ride on scorpionates:from d- to f-elements[J].Polyhedron,2004,23(2/3):331-360.
[29] SLUGOVC C,SCHMID R,KIRCHNER K.Hydridotris(pyrazolyl)borate ruthenium complexes:properties and applications[J].Coord Chem Rev,1999,185/186:109-126.
[30] BECKER E,PAVLIK S,KIRCHNER K.The organometallic chemistry of group 8 tris(pyrazolyl)borate com-plexes[J].Adv Organomet Chem,2008,56:155-197.
[31] CROSSLEY I R.The organometallic chemistry of group 9 poly(pyrazolyl)borate complexes[J].Adv Organomet Chem,2008,56:199-321.
[32] BAJO S,ESTERUELAS M A,LPEZ A M,et al.Osmium-acyl decarbonylation promoted by Tp-mediated allenylidene abstraction:a new role of the Tp ligand[J].Organometallics,2014,33(15):4057-4066.
[33] CASTRO-RODRIGO R,ESTERUELAS M A,LPEZ A M,et al.Preparation,spectroscopic characterization,X-ray structure,and theoretical investigation of hydride-,dihydrogen-,and acetone-OsTp complexes:a hydridotris(pyrazolyl)borate-cyclopentadienyl comparison[J].Organometallics,2007,26(18):4498-4509.
[34] LIU R S.Catalytic transformations of terminal alkynes by cationic tris(1-pyrazolyl)borate ruthenium catalysts:versatile chemistry via catalytic allenylidene,vinylidene,and π-alkyne intermediates[J].Synlett,2008,6:801-812.
[35] SANFORD M S,LOVE J A,GRUBBS R H.A versatile precursor for the synthesis of new ruthenium olefin metathesis catalysts[J].Organometallics,2001,20(25):5314-5318.
[36] BURTSCHER D,PERNER B,MEREITER K,et al.Peculiarities of the reaction of (SPY-5-34)-dichloro-(κ2(C,O)-2-formylbenzylidene)(1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene)ruthenium with potassium hydridotris(pyrazolyl)borate[J].J Organomet Chem,2006,691(24/25):5423-5430.
[37] TROFIMENKO S.Poly(1-pyrazolyl)borates,their transition-metal complexes,and pyrazaboles[C]∥PARRY R W.Inorganic synthesis volume Ⅻ.New York:McGRAW-HILL Book Company,1970:99-109.
[38] HILL A F,WILTON-ELY J D E T.Chlorohydro-tris(pyrazol-1-yl)borato-bis(triphenylphosphine)ruthenium(Ⅱ) RuCl[К3-HB(pz)3](PPh3)2} (pz=pyrazol-1-yl) [C]∥COUCOUVANIS D.Inorganic synthesis volume 33.New York:John Wiley & Sons,Inc.,2002:206-208.
[39] ABDUR-RASHID K,FEDORKIW T,LOUGH A J,et al.Coordinatively unsaturated hydridoruthenium(Ⅱ) complexes of N-heterocyclic carbenes[J].Organometallics,2004,23(1):86-94.
[40] HONG S H,CHLENOV A,DAY M W,et al.Double C—H activation of an N-heterocyclic carbine ligand in a ruthenium olefin metathesis catalyst[J].Angew Chem Int Ed,2007,46(27):5148-5151.
[41] FREEMAN S T N,LEMKE F R,HAAR C M,et al.Effect of ancillary ligation on the relative bond disruption enthalpies of Ru—H and Ru—Cl bonds in Cp(PR3)2RuX (PR3=PMe3,PMe2Ph,PMePh2,PPh3;X=H,Cl)[J].Organometallics,2000,19(23):4828-4833.
[42] LIU Z,XU J,RUAN W,et al.A half-sandwich 1,2-azaborolyl ruthenium complex:synthesis,characterization,and evaluation of its catalytic activities[J].Dalton Trans,2013,42(33):11976-11980.

