摄食DHA-磷脂酰丝氨酸对发育期小鼠体组织DHA水平的影响
吴 芳,王丹丹,温 敏,薛长湖,王玉明*
(中国海洋大学食品科学与工程学院,山东 青岛 266003)
摄食DHA-磷脂酰丝氨酸对发育期小鼠体组织DHA水平的影响
吴 芳,王丹丹,温 敏,薛长湖,王玉明*
(中国海洋大学食品科学与工程学院,山东 青岛 266003)
目的:比较研究断乳后干预二十二碳六烯酸-磷脂酰丝氨酸(docosahexaenoic acid-phosphatidylserine,DHA-PS)和DHA-甘油三酯(DHA-triglyceride,DHA-TG)对发育期ICR小鼠体组织DHA含量以及脂肪酸组成的影响。方法:母鼠孕期及哺乳期喂饲n-3多不饱和脂肪酸(n-3 polyunsaturated fatty acids,n-3 PUFAs)缺乏饲料,子代雄小鼠断乳后随机分为3 组,分别喂饲n-3 PUFAs缺乏饲料(n-3缺乏组)、DHA-TG饲料(DHA-TG组)、DHA-PS饲料(DHA-PS组),每组8 只,喂养2 周后检测小鼠脑皮质、精巢、肝脏、红细胞中脂肪酸组成和DHA含量。结果:补充DHA-PS和DHA-TG可以使发育期小鼠各组织中DHA含量显著增加(P<0.05),二十二碳五烯酸和花生四烯酸含量显著降低(P<0.05)。DHA-TG组精巢中DHA含量明显高于DHA-PS组;DHA-PS组肝脏TG、磷脂和红细胞中DHA水平显著高于DHA-TG组(P<0.05),而两组脑皮质DHA水平无显著性差异(P>0.05)。结论:膳食补充DHA-PS和DHA-TG均可明显提高发育期小鼠各组织中DHA水平,但二者的组织蓄积特性不同,DHA-PS可以更高效地提高小鼠肝脏和红细胞中DHA水平。
二十二碳六烯酸;二十二碳六烯酸-磷脂酰丝氨酸;甘油三酯;磷脂;小鼠
二十二碳六烯酸(docosahexaenoic acid,DHA,C22:6,n-3)是脑组织中含量最丰富的一种n-3多不饱和脂肪酸(n-3 polyunsaturated fatty acids,n-3 PUFAs),对脑部发育和功能十分重要[1-3]。研究表明,妊娠期和哺乳期n-3 PUFAs摄入不足会降低子代体内DHA水平,导致神经元可塑性改变,影响脑组织正常发育和功能,并增加成年期患抑郁与焦虑的风险[4-5]。脑胶质细胞和内皮细胞合成DHA能力有限,脑组织DHA蓄积主要来自肝脏利用亚麻酸(linoleic acid,LNA)合成及直接摄入,研究显示,单纯摄入LNA合成的DHA并不能满足脑对DHA的需求,因此,需要通过膳食直接摄取DHA[6]。鱼油是膳食DHA补充的主要形式,市售鱼油主要有甘油三酯型DHA(DHA-triglyceride,DHA-TG)和乙酯型DHA(DHA-EE),而磷脂是脑内DHA的主要存在形式。目前,DHA-TG主要用于婴幼儿配方乳粉中,研究发现磷脂型DHA比DHA-TG更能有效地提高体组织中DHA水平[7]。磷脂酰丝氨酸(phosphatidylserine,PS)是脑磷脂中重要的组成成分,可以维持和改善脑功能、增强记忆和认知能力[8]。同时,神经系统中DHA主要富集于PS中,DHA的富集可以促进PS的生物合成和聚集[9]。膳食补充DHA-PS,不仅可提高脑中DHA含量,还可以同时提高PS水平[10],对认知障碍具有明显改善作用[11-12]。然而,膳食补充DHA-PS对发育期小鼠体组织脂肪酸组成的研究较少,本实验通过建立小鼠n-3 PUFAs缺乏模型,比较研究DHA-PS和DHA-TG对小鼠各组织DHA水平的影响,以期为婴幼儿配方乳粉的开发提供理论依据。
1 材料与方法
1.1 动物、材料与试剂
8 周龄雌、雄性ICR小鼠各30 只,SPF级,体质量(20±2) g(许可证号:SCXK(京)2007-0001),购自北京维通利华实验动物有限公司。
冷冻鱿鱼卵 威海博宇食品有限公司;TG型鱼油宜宾汇海源生物科技有限公司;实验试剂均为国产分析纯。
1.2 仪器与设备
LABORO-TA 4000型旋转蒸发仪 德国Heidolph公司;CP100MX型超速冷冻离心机 日本日立公司;HD-200p型加热器及氮吹设备 瑞士Blue Marlin公司;7820型气相色谱仪 美国Agilent科技公司。
1.3 方法
1.3.1 DHA-PS的制备
将冷冻的鱿鱼卵在室温解冻后匀浆,经真空冷冻干燥后打碎磨粉,提取鱿鱼卵总脂肪[13],经冷丙酮洗涤数次后获得DHA-磷脂粗脂。用硅胶柱层析法[14]纯化DHA-磷脂粗脂,即得DHA-PC。采用酶合成法[15]将DHA-PC转化为DHA-PS(原料中PS含量96.4%、PC含量3.57%)。
1.3.2 动物分组与饲养
雌鼠与雄鼠各30 只分开适应性喂养1 周后,按雌雄比1∶1合笼,隔天上午观察雌鼠阴栓,有阴栓的视为妊娠第0天的孕鼠,母代雌鼠在孕期和哺乳期均喂饲n-3 PUFAs缺乏饲料。将断乳时(出生第21天)雄性子代小鼠随机分为n-3 PUFAs缺乏组、DHA-TG组、DHA-PS组,每组8 只(仔鼠产自不同母鼠),分别喂食n-3 PUFAs缺乏饲料、含0.1% DHA-TG(质量分数,下同)和0.1% DHA-PS的饲料,饲养2 周。在AIN93G配方[16]的基础上配制各组饲料(表1),采用气相色谱法[17]测定3 组饲料的脂肪酸组成(表2)。
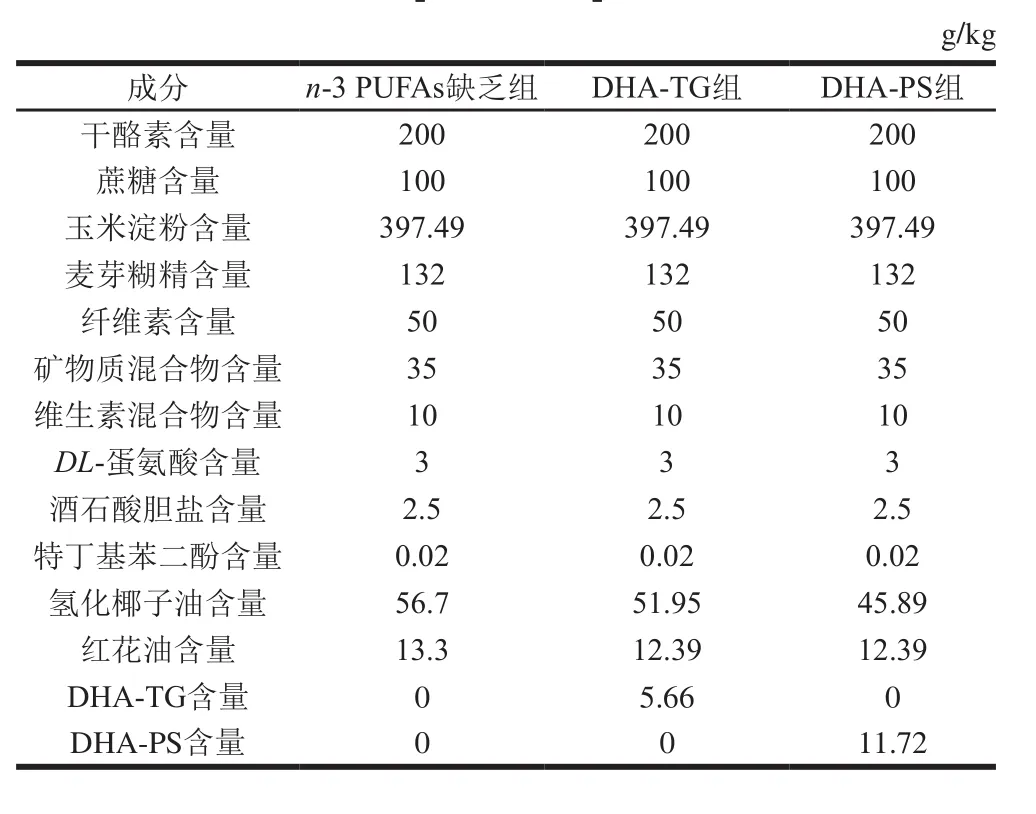
表1 各组饲料配方Table 1 Composition of experimental diets
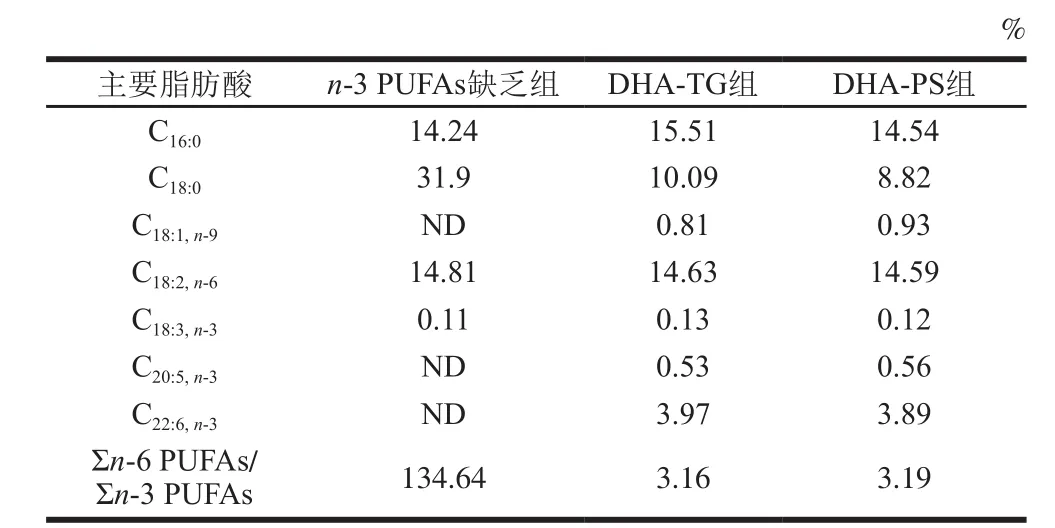
表2 各组饲料脂肪酸组成及含量Table 2 Fatty acid composition of experimental diets
小鼠自由摄食和饮水,在室温(23±2)℃、12 h明暗交替条件下喂养。每天更换饲料,测定并记录摄食量和体质量。在末次喂食后,禁食不禁水10 h,眼球取血处死,血液室温放置30 min后3 000 r/min离心20 min,获得血清和红细胞,取脑皮质、肝脏和精巢称质量后,于-80 ℃保存,备用。
1.3.3 各组织脂肪酸组成测定
[13]的方法提取红细胞、精巢、脑皮质和肝脏总脂质。用薄层色谱法[18]将肝脏总脂中的TG和磷脂分开。各脂质样品经皂化后,盐酸-甲醇法进行甲酯化,使用气相色谱仪进行脂肪酸组成分析:色谱柱选用INNOWax石英毛细管柱(30 m×320 μm,0.25 μm),分流比为20∶1,压力设定值为9.06 psi,流速为1.19 mL/min;柱温:起始温度为170 ℃,保持5 min,之后按照3 ℃/mn的速率升至210 ℃,然后在210 ℃保持30 min;检测器为氢火焰离子化检测器,进样口温度240 ℃,检测器温度250 ℃[16]。各组织脂肪酸含量以其占总脂肪酸含量的百分比表示。
1.4 数据统计分析
采用SPSS 11.0软件对数据进行分析,结果用 ±s表示,3 组间用Duncan’s多重比较分析,P<0.05为具有统计学意义上的差异。
2 结果与分析
2.1 DHA-TG和DHA-PS对小鼠生长指标的影响
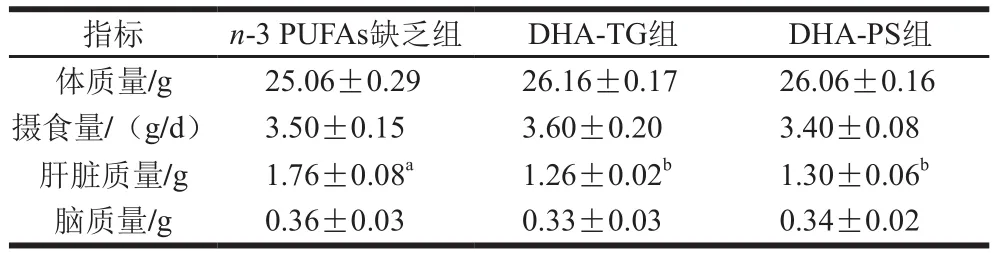
表3 DHA-TG和DHA-PS对小鼠生长指标的影响(n=8)Table 3 Effect of DHA-TG and DHA-PS on growth parameters in mice (n= 8)
如表3所示,各组小鼠体质量、摄食量均无显著差异(P>0.05)。实验测定了脑和肝脏质量,3 组小鼠脑质量无明显差异(P>0.05);与n-3 PUFAs缺乏组相比,DHA-TG组和DHA-PS组小鼠肝脏质量显著降低(P<0.05)。
2.2 DHA-TG和DHA-PS对小鼠红细胞脂肪酸组成的影响
表4显示,与n-3 PUFAs缺乏组相比,DHA-TG和DHA-PS组小鼠红细胞DHA含量分别升高了2.98倍和3.26 倍(P<0.05);二十二碳五烯酸(docosapentaenoic acid,DPA,C22:5,n-6)水平分别由2.13%下降至1.11%和0.30%(P<0.05);Σn-6 PUFAs/Σn-3 PUFAs分别下降了83.73%和85.08%(P<0.05)。
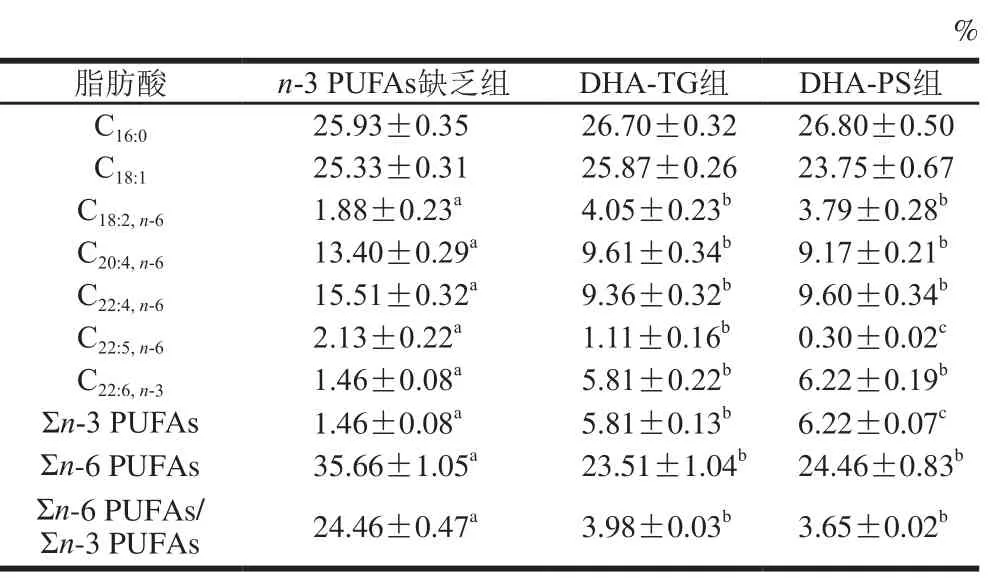
表4 小鼠红细胞脂肪酸组成及含量(n=8)Table 4 Fatty acid composition of lipids in red blood cells of mice (n= 8)
2.3 DHA-TG和DHA-PS对小鼠精巢脂肪酸组成的影响
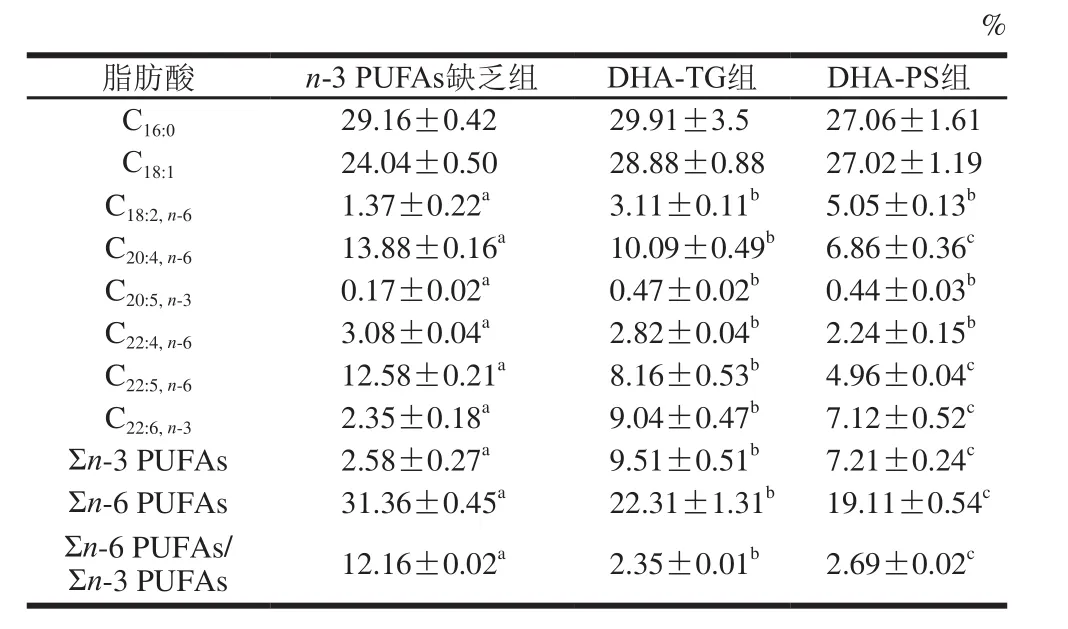
表5 小鼠精巢脂肪酸组成及含量(n=8)Table 5 Fatty acid composition of lipids in testis of mice (n= 8)
由表5可见,与n-3 PUFAs缺乏组相比,膳食补充DHA-TG和DHA-PS显著提高了小鼠精巢DHA水平(2.85、2.03 倍,P<0.05),而n-3 PUFAs缺乏组花生四烯酸(arachidonic acid,AA,C20:4,n-6)、DPA含量显著高于另外两组(P<0.05),DHA-TG组和DHA-PS组精巢Σn-6 PUFAs/Σn-3 PUFAs分别降低了80.67%和77.88%(P<0.05)。
2.4 DHA-TG和DHA-PS对小鼠肝脏TG和磷脂脂肪酸组成的影响
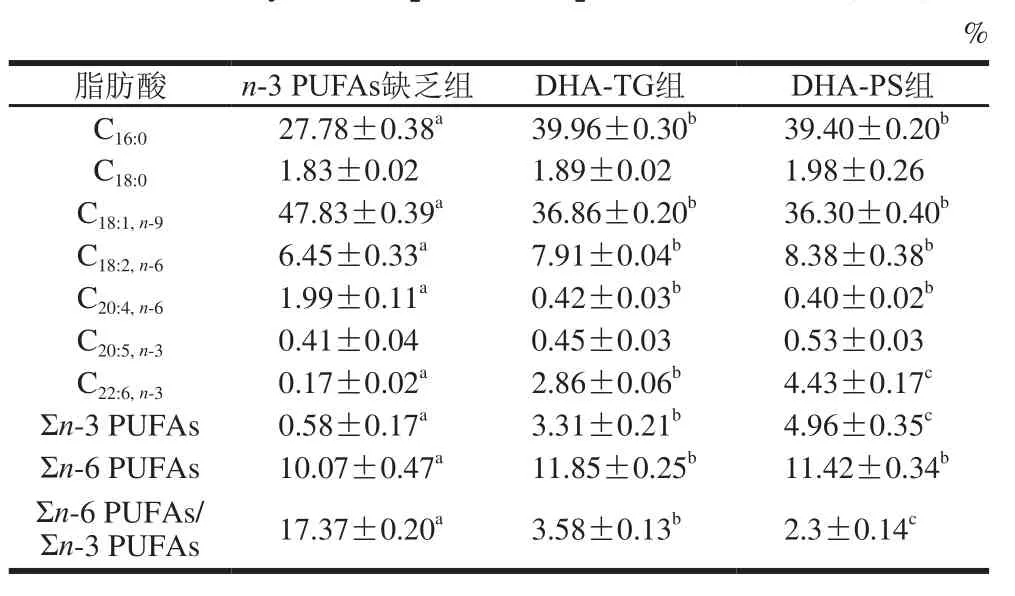
表6 小鼠肝脏TG组成及含量(n=8)Table 6 Fatty acid composition of lipids in liver of mice (n= 8)
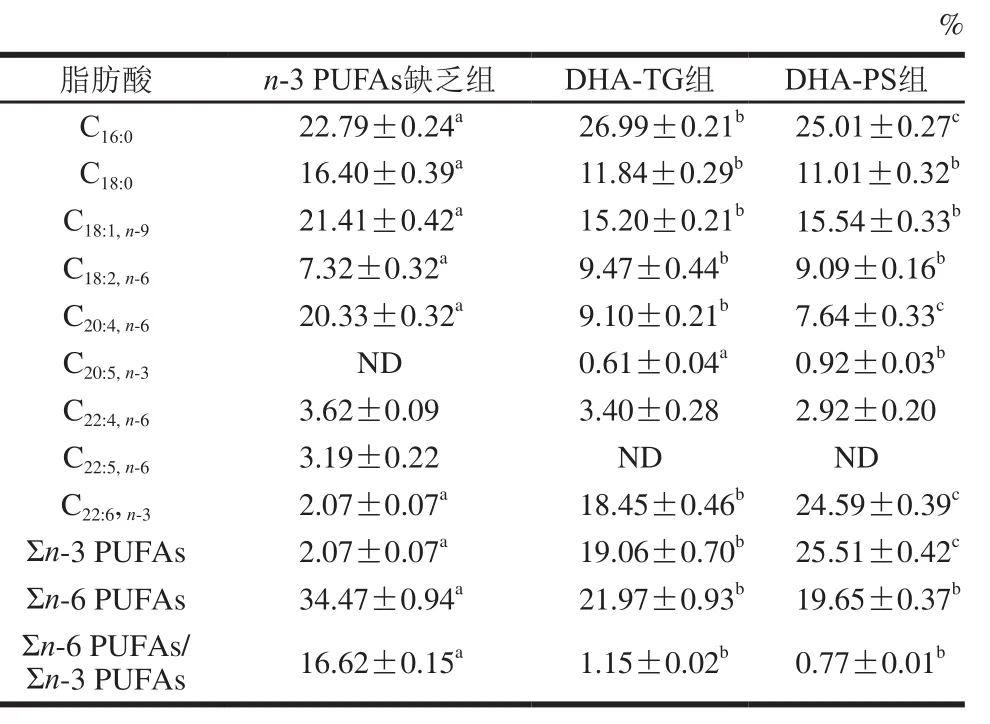
表7 小鼠肝脏磷脂组成及含量(n=8)Table 7 Fatty acid composition of phospholipid in liver tissues of mice (n= 8)
对肝脏中TG(表6)和磷脂(表7)组成分析可知,肝脏中主要的单不饱和脂肪酸是C18:1,TG中饱和脂肪酸以C16:0为主,磷脂中饱和脂肪酸主要是C16:0和C18:0。膳食补充DHA-TG和DHA-PS可显著提高肝脏DHA水平,TG中DHA分别增加了15.82 倍和25.06倍(P<0.05),磷脂中DHA含量分别增加了7.91、10.88 倍(P<0.05)。同时,膳食补充DHA-TG和DHA-PS,肝脏TG中AA分别降低了78.89%、79.90%,磷脂中AA分别降低了55.24%、62.42%,Σn-6 PUFAs/Σn-3 PUFAs显著下调(P<0.05)。
2.5 DHA-TG和DHA-PS对小鼠脑皮质脂肪酸组成的影响

表8 小鼠脑皮质脂肪酸组成及含量(n=8)Table 8 Fatty acid composition of lipids in cerebral cortex of mice (n= 8)
脑皮质脂肪酸组成分析结果显示(表8),DHA是脑内主要的n-3 PUFAs。膳食补充DHA-TG与DHA-PS后,脑皮质DHA含量分别升高1.17 倍与1.21 倍(P<0.05)。同时,膳食补充DHA-TG与DHA-PS后,脑皮质Σn-6 PUFAs显著下调(P<0.05),其中DPA含量分别下降了53.49%和48.28%,AA含量分别下降了15.66%和16.45%。
3 讨 论
孕期及哺乳期母体饮食中n-3 PUFAs水平会影响子代脑和外周组织中DHA含量[19-20]。本研究发现孕期与哺乳期缺乏n-3 PUFAs,其子代脑皮质、肝脏、精巢、红细胞DHA含量较低,Σn-6 PUFAs/Σn-3 PUFAs较高,膳食补充DHA-TG和DHA-PS,均可显著提高各组织DHA含量(P<0.05),但二者在各组织的蓄积效率存在差异。
研究表明,DHA-PS比DHA-TG能更高效地提高组织中DHA水平[21-22]。本研究发现,膳食补充DHA-TG和DHA-PS,均能显著提高肝脏磷脂与TG中DHA含量,降低AA和DPA等n-6 PUFAs的水平,且DHA-PS对肝脏DHA补充效果明显优于DHA-TG。膳食摄入的脂肪进入血浆后会逐渐进入红细胞膜中,并在一系列脂肪酶和血浆脂质共同作用下缓慢代谢,因此,红细胞膜脂肪酸组成能够更可靠地反映机体一段时间内的脂质代谢状况[23]。本研究发现,膳食补充DHA-PS和DHA-TG,前者可以更高效地提高红细胞中DHA水平,这与Ramprasath等[24]的实验结果一致。膳食补充DHA可以改变精巢脂肪酸组成并调节睾酮生成,影响精子浓度与活力,与生殖能力相关[25],本研究发现,膳食补充DHA-TG和DHA-PS均显著提高小鼠精巢DHA水平(P<0.05),且DHA-TG效果较佳,这表明DHA的分子形式不同,其在精巢中的蓄积效率存在差异性。
已有研究报道,磷脂型AA比TG型AA在脑部具有更高的蓄积效率[26],DHA-PS对脑组织的补充效率也明显优于DHA-TG,Graf[27]和Lagarde[28]等发现低脂饮食条件下,磷脂型DHA在成年大鼠脑部蓄积效率显著高于DHA-TG。Batetta等[29]发现,高脂饮食条件下分别喂食大鼠磷脂型DHA和DHA-TG,前者可以更高效地提高脑部磷脂中DHA含量。Liu Lei等[30]通过新生小猪短期喂养模型发现,膳食补充DHA-PC比DHA-TG更高效地提高脑组织中DHA含量。本研究通过在小鼠孕期与哺乳期喂食n-3 PUFAs缺乏饲料,使子鼠脑内DHA含量明显降低,n-3 PUFAs缺乏组小鼠脑内DHA含量为7.17%,断乳时补充DHA-TG与DHA-PS两周,脑皮质DHA含量显著提高至15.59%和15.85%,但是二者无明显差异。这可能由于断乳时小鼠还处于发育期,仍需要摄取足量DHA以满足脑组织正常的生长发育,此时膳食补充DHA-TG与DHA-PS,脑组织快速摄取DHA,2 周后,脑内DHA含量已经趋于饱和。因此,本实验结果尚不能判断DHA-TG与DHA-PS对脑内DHA补充效率是否存在差异,需要进一步实验验证。
综上所述,膳食补充DHA-PS和DHA-TG均可明显提高生长发育期小鼠各组织中DHA水平,但二者蓄积效果不同,DHA-PS对于红细胞、肝脏中DHA水平的蓄积效果好于DHA-TG。
参考文献:
[1] RYAN A S, ASTWOOD J D, GAUTIER S, et al. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies[J]. Prostaglandins, Leukotrienes and Essential Fatty Acids, 2010, 82(4/5/6): 305-314. DOI:10.1016/j.plefa.2010.02.007.
[2] PATERNITI I, IMPELLIZZERI D, PAOLA R D, et al. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: in-vivo and in-vitro studies[J]. Journal of Neuroinf l ammation, 2014, 11(1): 1-3. DOI:10.1186/1742-2094-11-6.
[3] GÓDORKACSÁNDI A, FELSZEGHY K, RANKY M, et al.Developmental docosahexaenoic and arachidonic acid supplementation improves adult learning and increases resistance against excitotoxicity in the brain[J]. Acta Physiologica Hungarica, 2013, 100(2): 186-96.DOI:10.1556/APhysiol.100.2013.005.
[4] LAFOURCADE M, LARRIEU T, MATO S, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions[J]. Nature Neuroscience, 2011, 14(3): 345-50. DOI:10.1038/nn.2736.
[5] HOEIJMAKERS L, LUCASSEN P J, KOROSI A. The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function[J]. Frontiers in Molecular Neuroscience, 2014, 7(1): 3-8.
[6] INNIS S M. Dietary (n-3) fatty acids and brain development[J].Journal of Nutrition, 2007, 137(4): 855-859.
[7] GHASEMIFARD S, HERMON K, TURCHINI G M, et al. Metabolic fate (absorption, β-oxidation and deposition) of long-chain n-3 fatty acids is affected by sex and by the oil source (krill oil or fish oil)in the rat[J]. British Journal of Nutrition, 2015, 114(5): 684-692.DOI:10.1017/S0007114515002457.
[8] PEPEU G, PEPEU I M, AMADUCCI L. A review of phosphatidylserine pharmacological and clinical effects. is phosphatidylserine a drug for the ageing brain?[J]. Pharmacological Research, 1996, 33(2): 73-80.DOI:10.1006/phrs.1996.0013.
[9] GUO M, STOCKERT L, AKBAR M, et al. Neuronal specific increase of phosphatidylserine by docosahexaenoic acid[J]. Journal of Molecular Neuroscience, 2007, 33(1): 67-73. DOI:10.1007/s12031-007-0046-z.
[10] OHKUBO T, TANAKA Y. Administration of DHA-PS to aged mice was suitable for increasing hippocampal PS and DHA ratio[J]. Journal of Oleo Science, 2010, 59(5): 247-253. DOI:10.5650/jos.59.247.
[11] WEN M, DING L, ZHANG L Y, et al. DHA-PC and DHA-PS improved Aβ1-40 induced cognitive deficiency uncoupled with an increase in brain DHA in rats[J]. Journal of Functional Foods, 2016,22: 417-430. DOI:10.1016/j.jff.2016.02.004.
[12] LEE B, SUR B J, HAN J J, et al. Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats[J]. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2010, 34(6):1085-1093. DOI:10.1016/j.pnpbp.2010.05.031.
[13] FOLCH J, LEES M, SLOANE STANLEY G H. A simple method for the isolation and purif i cation of total lipids from animal tissue[J].Journal of Biological Chemistry, 1957, 226(1): 497-509.
[14] 李金章. 甘油酯型鱼油的制备及活性研究[D]. 青岛: 中国海洋大学,2011: 22-23.
[15] HOSOKAWA M, SHIMATANI T, KANADA T, et al. Conversion to docosahexaenoic acid-containing phosphatidylserine from squid skin lecithin by phospholipase D-mediated transphosphatidylation[J].Journal of Agricultural and Food Chemistry, 2000, 48(10): 4550-4554.DOI:10.1021/jf991186s.
[16] DEMAR J C, MA K Z, BELL J M, et al. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids[J]. Journal of Neurochemistry, 2004, 91(5): 1125-1137. DOI:10.1111/j.1471-4159.2004.02789.x.
[17] 刘艳青, 李兆杰, 楼乔明, 等. 皱纹盘鲍内脏脂质分析[J]. 水产学报,2012, 36(6): 989-992.
[18] HAMMOND E W. Chromatography for the analysis of lipids. chapter 3.thin layer chromatography[M]. Boca Raton: CRC Press. 1993: 21-24.
[19] 樊超男, 田春雨, 夏露露, 等. 不同年龄阶段小鼠脑聚集二十二碳六烯酸及脂肪酸去饱和酶的变化[J]. 中国儿童保健杂志, 2012, 20(8):709-712.
[20] 付慧聪, 高亚兵, 樊超男, 等. 孕期和哺乳期n-3多不饱和脂肪酸对子代小鼠成年脑神经发生及凋亡的影响[J]. 中国体视学与图像分析,2015, 20(2): 161-169.
[21] RAMPRASATH V R, EYAL I, ZCHUT S, et al. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fi sh oil[J]. Lipids in Health and Disease, 2013, 12(1): 133-138. DOI:10.1186/1476-511X-12-178.
[22] ULVEN S M, KIRKHUS B, LAMGLAIT A, et al. Metabolic effects of krill oil are essentially similar to those of fi sh oil but at lower dose of EPA and DHA, in healthy volunteers[J]. Lipids, 2011, 46(1): 37-46.DOI:10.1007/s11745-010-3490-4.
[23] BANDARRA N M, MONTEIRO M, MARTÍNEZ J A, et al.Erythrocyte membrane fatty acid incorporation as a marker of fi sh diet in young overweight Europeans[J]. Journal of Aquatic Food Product Technology, 2007, 16(4): 3-11.
[24] RAMPRASATH V R, EYAL I, ZCHUT S, et al. Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil[J].Lipids in Health and Disease, 2015, 14(1): 142.
[25] WALKER W H. Molecular mechanisms of testosterone action in spermatogenesis[J]. Steroids, 2009, 74(7): 602-607. DOI:10.1016/j.steroids.2008.11.017.
[26] WIJENDRAN V, HUANG M C, DIAU G Y, et al. Eff i cacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates[J]. Pediatric Research,2002, 51(3): 265-72. DOI:10.1203/00006450-200203000-00002.
[27] GRAF B A, DUCHATEAU G S M J E, PATTERSON A B, et al. Age dependent incorporation of14C-DHA into rat brain and body tissues after dosing various14C-DHA-esters[J]. Prostaglandins Leukotrienes and Essential Fatty Acids, 2010, 83(2): 89-96.
[28] LAGARDE M, BERNOUD N, BROSSARD N, et al.Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain[J]. Journal of Molecular Neuroscience, 2001, 16(2/3): 201-204. DOI:10.1385/JMN:16:2-3:201.
[29] BATETTA B, GRIINARI M, CARTA G, et al. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inf l ammatory mediators in obese Zucker rats[J]. Journal of Nutrition,2009, 139(8): 1495-1501.
[30] LIU Lei, BARTKE N, VAN DAELE H, et al. Higher efficacy of dietary DHA provided as a phospholipid than as a triglyceride for brain DHA accretion in neonatal piglets[J]. Journal of Lipid Research, 2014,55(3): 531-539.
Effect of Dietary DHA-Phosphatidylserine on DHA Level of Body Tissues in Developing Mice
WU Fang, WANG Dandan, WEN Min, XUE Changhu, WANG Yuming*
(College of Food Science and Engineering, Ocean University of China, Qingdao 266003, China)
Objective: To investigate the comparative effects of post-weaning dietary docosahexaenoic acidphosphatidylserine (DHA-PS) and DHA-triglyceride (DHA-TG) on DHA concentration and fatty acid composition of lipids in various tissues of developing mice. Methods: ICR female mice were fed n-3 polyunsaturated fatty acids (PUFAs)def i cient diet during maternal pregnancy and lactation, and the weanling (3-week old) ICR-strain male mouse pups were randomly assigned to three groups, which were fed three different types of diets including n-3 PUFAs def i ciency (n-3 PUFAs Def group), DHA-TG (DHA-TG group) and DHA-PS (DHA-PS group), respectively. After 2 weeks of feeding, all mouse pups were sacrif i ced, and cortex, testis, liver, and red blood cells were harvested for detecting DHA level and fatty acid composition of lipids. Results: Compared to the n-3 Def group, the concentration of DHA in developing mouse tissues in the DHA-TG and DHA-PS groups was signif i cantly increased, whereas the concentrations of docosapentaenoic acid (DPA) and arachidonic acid (AA) were decreased. The DHA-PS group exhibited signif i cantly higher DHA levels in erythrocytes, liver triglyceride and phospholipid but lower DHA level in testis compared with the DHA-TG group. There was no difference in DHA level in cortex between the two groups. Conclusion: Dietary supplementation of DHA-PS and DHA-TG signif i cantly increased the level of DHA in the tissues of developing mice, but resulted in different accumulation patterns of DHA and DHA-PS more effectively increased DHA contents in the liver and erythrocytes.
docosahexaenoic acid; docosahexaenoic acid-phosphatidylserine; triacylglycerol; phospholipid; mice
10.7506/spkx1002-6630-201801020
TS218
A
1002-6630(2018)01-0131-05
吴芳, 王丹丹, 温敏, 等. 摄食DHA-磷脂酰丝氨酸对发育期小鼠体组织DHA水平的影响[J]. 食品科学, 2018, 39(1):
131-135. DOI:10.7506/spkx1002-6630-201801020. http://www.spkx.net.cn
2016-10-20
国家自然科学基金面上项目(31371757);国家自然科学基金重点项目(31330060)
吴芳(1993—),女,硕士研究生,研究方向为水产化学与分子营养。E-mail:fangwuouc@163.com
*通信作者简介:王玉明(1973—),男,教授,博士,研究方向为食品营养。E-mail:wangyuming@ouc.edu.cn
WU Fang, WANG Dandan, WEN Min, et al. Effect of dietary DHA-phosphatidylserine on DHA level of body tissues in developing mice[J]. Food Science, 2018, 39(1): 131-135. (in Chinese with English abstract)
10.7506/spkx1002-6630-201801020. http://www.spkx.net.cn

