炎症细胞在胃癌发展进程中所扮演的角色
崔 慧,惠起源
延安大学附属医院消化内科,陕西 延安 716000
炎症细胞在胃癌发展进程中所扮演的角色
崔 慧,惠起源
延安大学附属医院消化内科,陕西 延安 716000
胃癌作为消化道最常见的恶性肿瘤,对人类健康造成较大的危害。流行病学研究表明,炎症与胃癌的发生、发展关系密切,本文就炎症细胞与胃癌发生的相关机制作一概述。
炎症细胞;胃癌;相关性
胃癌作为全球第五大常见癌症,特别是在亚洲国家,尽管其发病率在近几十年有所下降,但仍是世界各地的主要健康问题。近年来,炎症细胞在胃癌发展中的作用逐渐被人们所重视,但其具体机制尚不明确,本文主要就中性粒细胞、血小板、淋巴细胞及巨噬细胞与胃癌的关系及其研究进展作一概述。
1 炎症与肿瘤的关系
19世纪60年代初期,Virchow在恶性肿瘤中发现了免疫细胞,并首次提出了肿瘤与炎症之间可能存在某种关联性。在此之前,我们对炎症的认识大多停留在其作为抵御外来病原体入侵的重要保护机制。不可否认,正常的急性炎症,持续时间很短,可帮助机体清除异源物质,但持续存在的慢性炎症反应,造成大量的炎性细胞浸润,这些炎性细胞分泌大量的细胞因子及活性介质,长期刺激下,引起DNA的损坏、细胞异常增殖及凋亡障碍。据推测,多达20%的癌症是通过慢性发作或持续感染引发的,所以炎症被认为是癌症的第七大特征[1]。炎症反应与肿瘤之间存在相互作用机制。首先,通过产生活性氧物质和促炎细胞因子,炎症可能缓慢地引起肿瘤发生,产生的癌细胞可以通过分泌细胞激素招募中性粒细胞和巨噬细胞[2],然后,这些被招募的细胞释放出其他分子,扩大反应。增长的肿瘤可能对正常组织造成物理损伤,释放损伤相关的分子模式(DAMP),形成一个利于肿瘤生长的炎性微环境[3],肿瘤诱导的炎症反应产生“雪球”效应,使肿瘤持续进展,然后炎症可以通过遗传进化促进肿瘤由低级别转变为恶化程度更高的状态,研究[4]表明,非甾体类抗炎药在一定程度上可以降低患者肿瘤的发生率。因而,重视感染因素在恶性肿瘤发病中的作用,对恶性肿瘤的预防、诊断及治疗等均有重要意义。
2 慢性炎症与胃癌的关系
胃癌发病机制复杂多样,是各种因素综合作用的结果。Warren和Marshall首次提出,消化性溃疡及慢性胃炎中存在螺旋杆菌。现代研究认为,持续的幽门螺杆菌(Helicobacter pylori,H.pylori)感染是胃癌发生的关键过程之一[5],通过H.pylori的长期感染,导致胃黏膜长期处于慢性炎症的状态,从而导致胃癌的发生、发展[6-7]。在胃黏膜、慢性炎症的发展过程中,特异性蛋白如Cag A功不可没[8],有学者[9]指出,H.pyloriCag A阳性的患者胃癌发病风险明显高于Cag A阴性患者。其他途径包括激活Toll-like感受器(TLR),NF-κB和环氧合酶-2(COX-2)-前列腺素E2(PGE2)途径[10],DNA损伤在含有蛋白酶和RONS的炎性环境中变得更加显著,除了对DNA直接破坏以外,表观遗传改变如DNA甲基化谷(DNA methylation valleys)的高甲基化已在文献[11]中有记录。H.pylori感染只是胃癌发生、发展进程中的一个诱因,炎症所导致的分子改变、遗传(不可逆变化的DNA序列)或表观遗传(DNA甲基化)改变才起到真正的主导作用,因此,在炎症形成的早期根除H.pylori是预防胃癌形成的有效举措。
3 不同炎症细胞参与肿瘤的发生机制
反映机体炎症状态的指标多种多样,包括各种炎症细胞及其分泌的炎症介质、C反应蛋白、降钙素原、红细胞沉降率等。外周血细胞计数可部分反映机体炎症状态,属于常规检查,易于获取且廉价。近几年的研究[12]表明,中性粒细胞与淋巴细胞比值(NLR)、血小板与淋巴细胞比值(PLR)、巨噬细胞等提示了机体肿瘤与机体免疫反应的相对状态,与肿瘤的发生、发展密切相关。作为反映全身炎症状态指标之一的NLR,已被用作肺癌、结直肠癌、肝癌、乳腺癌、肾癌等多种肿瘤的预后评估[13-17],显示出NLR可作为不同肿瘤中一种易获取的、廉价的生物学标志物,存在一定的学术价值。此外,大量研究[18-20]表明,PLR在多种恶性肿瘤预后中具有重要价值。下面分别就中性粒细胞、巨噬细胞、血小板及淋巴细胞参与肿瘤发生、发展过程的机制作如下阐述。
3.1中性粒细胞作为血液中最丰富的白细胞类型,在机体受到炎症感染时,作为先头部队抵达炎症部位,杀灭病原微生物,中性粒细胞杀灭病原微生物的方式有三种:(1)吞噬作用:细菌或真菌被吞噬和消化的过程;(2)将细胞毒素脱颗粒到细胞外基质中;(3)以中性粒细胞外陷阱(NETs)的形式来杀灭微生物[21]。NETs是染色质、抗菌活性蛋白及多肽构成的网状陷阱样结构,其特有的DNA骨架可以网络微生物,限制其扩散及转移,使降解微生物的蛋白酶发挥充分的作用,且其在中性粒细胞死亡后依然发挥抗菌作用。但有研究[22]表明,这种“网状陷阱”里面的多种组分可以直接促进肿瘤细胞的增殖、远处转移及血管生成,且其可以锚定并包裹循环肿瘤细胞,为肿瘤细胞的远处转移提供活性蛋白丰富的微环境。有学者[23]认为,肿瘤细胞与NETs相互作用,转移性癌细胞通过“挟持”中性粒细胞激活包括NADDP氧化酶和PAD4的信号传导途径,促进NETs的形成。而形成的NETs可以将循环肿瘤细胞捕获并转移到其他位置[24],血管内NETs还可以增加局部血管通透性[25],这将使癌细胞更容易外渗。此外,中性粒细胞重塑肿瘤微环境,导致血管内皮生长因子、白介素-18和金属基质蛋白酶(MMP)家族成员的释放,促进肿瘤血管的生成及肿瘤的发生、发展[26-28]。中性粒细胞衍生的活性氧进一步降低细胞外基质的黏附促进性质,并通过NF-κB和STAT3的活化抑制肿瘤细胞凋亡,这些事件导致肿瘤进展加快,增加肿瘤细胞对周围组织的侵袭[29-30],基于上述,我们认为,中性粒细胞可以诱导肿瘤细胞生长和转移,并且肿瘤相关中性粒细胞的增加与癌症患者的较差预后相关。
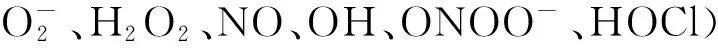
3.3血小板血小板是由意大利医师J.B比佐泽罗发现的从骨髓成熟的巨核细胞裂解脱落下来的形状不规则的、有膜无核的、具有生物活性的、可以在血管损伤后的止血过程中起重要作用的最小的血细胞。血小板增多也是炎症反应的一个重要组成部分,增多的血小板通过分泌炎症蛋白,如IL-6、TNF-α等,与肿瘤细胞转移相关[40-41]。此外,通过释放促进生长因子、趋化因子、促血管生成调节蛋白、蛋白水解酶和微粒的分泌因子,活化的血小板促进肿瘤细胞生长和侵袭[42]。BAMBACE等[43]研究表明,血小板可能通过产生血管生成因子,例如血小板衍生生长因子(PDGF)和血管内皮生长因子(VEGF)刺激肿瘤的产生和促进转移。NIESWANDT等[44]证实,血小板能够保护肿瘤细胞免于细胞溶解,并且保护肿瘤细胞使其逃避宿主免疫系统,且不易被识别,从而促使肿瘤细胞的增殖和扩散,通过整合素αⅡbβ3(糖蛋白Ⅱb/Ⅲa)桥连片段的表面屏蔽作为这种保护的主要机制。近年来有多项研究[45-46]报道,术前血小板计数和胃癌淋巴结转移率升高有关。而肿瘤细胞所产生的炎症因子IL-1、IL-3和IL-6等又可以促进巨核细胞增殖分化的作用,作为巨核细胞增殖产物的血小板也相应升高。血小板与肿瘤细胞为相互促进的关系。此外,高血小板数量将导致相对淋巴细胞减少,癌症患者将具有与淋巴细胞介导的细胞水平的抗肿瘤活性相关的低免疫应答。
3.4淋巴细胞研究[47]表明,淋巴细胞可以反映机体对抗肿瘤的能力,也可以在肿瘤早期减少转移和复发,通过在肿瘤发生开始时攻击和清除肿瘤细胞。与前面所提及的炎症细胞不同的是,淋巴细胞是主要的抗癌因子[48],在肿瘤防御中起重要作用,作为肿瘤特异性免疫反应的重要组成部分,通过诱导细胞毒性细胞死亡和细胞因子的产生介导宿主免疫应答,对肿瘤细胞起到特异性杀伤的作用,抑制肿瘤细胞增殖[49-50]。在肿瘤组织周围,有淋巴细胞浸润的患者可能比具有较少或无淋巴细胞浸润的患者预后更好[51]。研究[52]表明,当淋巴细胞与中性粒细胞两者共培养时,淋巴细胞可能被大量中性粒细胞抑制。在此之前,已有研究[53-54]表明,淋巴细胞减少在各种类型的癌症预后中均有价值。
4 展望
随着治疗手段的不断进步(包括手术、放疗、化疗、靶向治疗),胃癌的发病率在近几十年有所下降,但其仍是世界各地的主要健康问题,较低的5年生存率使得研究者们不断地探寻一种可靠的生物标志物来预测其发生与发展,这些炎症相关指标包括中性粒细胞与淋巴细胞比值、血小板计数、巨噬细胞以其方便、快速、可重复性的优势成为近年来研究的热点,但目前多数研究为回顾性分析,且各个指标的界值仍有待商榷,未来需要大量研究以拟定标准化临界值,有待于我们进行大样本、多中心、前瞻性的研究来为临床提供更充足的理论依据,综合评判其对胃癌患者的预后影响,以便临床更好地应用。
[1] MANTOVANI A, ALLAVENA P, SICA A, et al. Cancer-related inflammation [J]. Nature, 2008, 454(7203): 436-444.
[2] BONAVITA E, GALDIERO M R, JAILLON S, et al. Phagocytes as corrupted policemen in cancer-related inflammation [J]. Adv Cancer Res, 2015, 128: 141-171.
[3] WANG D, DUBOIS R N. Immunosuppression associated with chronic inflammation in the tumor microenvironment [J]. Carcinogenesis, 2015, 36(10): 1085-1893.
[4] GARCIA-ALBENIZ X, CHAN A T. Aspirin for the prevention of colorectal cancer [J]. Best Pract Res Clin Gastroenterol, 2011, 25(4-5): 461-472.
[5] SUBHASH V V, HO B. Inflammation and proliferation-a causal event of host response to Helicobacter pylori infection [J]. Microbiology, 2015, 161(6): 1150-1160.
[6] SANTOS J C, RIBEIRO M L. Epigenetic regulation of DNA repair machinery in Helicobacter pylori-induced gastric carcinogenesis [J]. World J Gastroenterol, 2015, 21(30): 9021-9037.
[7] COVER T L. Helicobacter pylori diversity and gastric cancer risk [J]. MBio, 2016, 7(1): e01869-e01915.
[8] SUZUKI N, MURATA-KAMIYA N, YANAGYA K, et al. Mutual reinforcement of infl ammation and carcinogenesis by the Helicobacter pylori CagA oncoprotein [J]. Sci Rep, 2015, 5: 10024.
[9] LEMKE L B, GE Z, WHARY M T, et al. Concurrent Helicobacter bilis infection in C57BL/6 mice attenuates proinflammatory H.pylori-induced gastric pathology [J]. Infect Immun, 2009, 77(5): 2147-2158.
[10] ECHIZEN K, HIROSE O, MAEDA Y, et al. Inflammation in gastric cancer: interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways [J]. Cancer Sci, 2016, 107(4): 391-397.
[11] ABU-REMAILEH M, BENDER S, RADDATZ G, et al. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer [J]. Cancer Res, 2015, 75(10): 2120-2130.
[12] POLLARD J W. Tumour-educated macrophages promote tumour progression and metastasis [J]. Nat Rev Cancer, 2004, 4(1): 71-78.
[13] SARRAF K M, BELCHER E, RAEEVSKY E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer [J]. J Thorac Cardiovasc Surg, 2009, 137(2): 425-428.
[14] LI M X, LIU X M, ZHANG X F, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis [J]. Int J Cancer, 2014, 134(10): 2403-2413.
[15] MANO Y, SHIRABE K, YAMASHITA Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis [J]. Ann Surg, 2013, 258(2): 301-305.
[16] AZAB B, BHATT V R, PHOOKAN J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients [J]. Ann Surg Oncol, 2012, 19(1): 217-224.
[17] PICHLER M, HUTTERER G C, STOECKIGT C, et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients [J]. Br J Cancer, 2013, 108(4): 901-907.
[18] SHIMADA H, TAKIGUCHIN, KAINUMA O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer [J]. Gastric Cancer, 2010, 13(3): 170-176.
[19] KWON H C, KIM S H, OH S Y, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer [J]. Biomarkers, 2012, 17(3): 216-222.
[20] ASHER V, LEE J, INNAMAA A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer [J]. Clin Transl Oncol, 2011, 13(7): 499-503.
[21] BRANZK N, PAPAYANNOPOULOS V. Molecular mechanisms regulating NETosis in infection and disease [J]. Seminars Immunopathol, 2013, 35(4): 513-530.
[22] HOUGHTON A M, RZYMKIEWICZ D M, JI H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth[J]. Nat Med, 2010, 16(2):219-223.
[23] PARK J, WYSOCKI R W, AMOOZGAR Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps [J]. Sci Transl Med, 2016, 8(361): 361ra138.
[24] COOLS-LARTIGUE J, SPICER J, MCDONALD B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis [J]. J Clin Invest, 2013, pii: 67484.
[25] KOLACZKOWSKA E, JENNE C N, SUREWAARD B G, et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature [J]. Nat Commun, 2015, 6: 6673.
[26] WU T, LI Y, LU J, et al. Increased MMP-21 expression is associated with poor overall survival of patients with gastric cancer [J]. Med Oncol, 2013, 30(1): 323.
[27] JABLONSKA E, PUZEWSKA W, GRABOWSKA Z, et al. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer [J]. Cytokine, 2005, 30(3): 93-99.
[28] ARDI V C, KUPRIYANOVA T A, DERYUGINA E I, et al. Human neutrophils uniquely release TIMP-Free MMP-9 to provide a potent catalytic stimulator of angiogenesis [J]. Proc Natl Acad Sci U S A, 2007, 104(51): 20262-20267.
[29] XIONG H, DU W, WANG J L, et al. Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer [J]. J Mol Med (Berl), 2012, 90(9): 1037-1046.
[30] DUMITRU C A, LANG S, BRANDAU S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression [J]. Semin Cancer Biol, 2013, 23(3): 141-148.
[31] LAVIN Y, WINTER D, BLECHER-GONEN R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment [J]. Cell, 2014, 159(6): 1312-1326.
[32] 杨继乐, 张莉, 王莉. 单核-巨噬细胞的分化和功能研究进展[J]. 细胞与分子免疫学杂志, 2014, 30(11): 1213-1216.
[33] DING P, WANG W, WANG J, et al. Expression of tumor-associated macrophage in progression of human glioma [J]. Cell Biochem Biophys, 2014, 70(3): 1625-1631.
[34] HERWIG M C, BERGSTROM C, WELLS J R, et al. M2/M1 ratio of tumor associated macrophages and PPAR-gamma expression in uveal melanomas with class 1 and class 2 molecular profiles [J]. Exp Eye Res, 2013, 107: 52-58.
[35] MACARTHUR M, HOLD G L, EL-OMAR E M. Inliammation and cancer Ⅱ. role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy [J]. Am J Physiol Gastrointest Liver Physiol, 2004, 286(4): G515-G520.
[36] MAEDA H, AKAIKE T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer [J]. Biochemistry (Mosc), 1998, 63(7): 854-865.
[37] LEACH S A, THOMPSON M, HILL M. Bacterially catalysed N-nitrosation reactions and their relative importance in the human stomach [J]. Carcinogenesis, 1987, 8(12): 1907-1912.
[38] FEDERICO A, MORGILLO F, TUCCILLO C, et al. Chronic inflammation and oxidative stress in human carcinogenesis [J]. Int J Cancer, 2007, 121(11): 2381-2386.
[39] GREENHOUGH A, SMARTT H J, MOORE A E, et al. The COX-2/PGE 2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment [J]. Carcinogenesis, 2009, 30(3): 377-386.
[41] TESFAMARIAM B. Involvement of platelets in tumor cell metastasis [J]. Pharmacol Ther, 2016, 157: 112-119.
[42] ALEKSANDROVA K, BOEING H, NÖTHLINGS U, et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer [J]. Hepatology, 2014, 60(3): 858-871.
[43] BAMBACE N M, HOLMES C E. The platelet contribution to cancer progression [J]. J Thromb Haemost, 2011, 9(2): 237-249.
[44] NIESWANDT B, HAFNER M, ECHTENACHER B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets [J]. Cancer Res, 1999, 59(6):1295-1300.
[45] SHODA K, KOMATSU S, ICHIKAWA D, et al. Thrombocytosis associated with poor prognosis in patients with gastric cancer [J]. Gan to Kagaku Ryoho, 2015, 42(12): 1980-1982.
[46] LI F X, WEI L J, ZHANG H, et al. Significance of thrombocytosis in clinicopathologic characteristics and prognosis of gastric cancer [J]. Asian Pac J Cancer Pre, 2014, 15(16): 6511-6517.
[47] ZHANG L, CONEJO-GARCIA J R, KATSAROS D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer [J]. N Engl J Med, 2003, 348(3): 203-213.
[48] QUIGLEY D A, KRISTENSEN V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells [J]. Mol Oncol, 2015, 9(10): 2054-2062.
[49] CHOCHI K, ICHIKURA T, MAJIMA T, et al. The increase of CD57+ T cells in the peripheral blood and their impaired immune functions in patients with advanced gastric cancer [J]. Oncol Rep, 2003, 10(5): 1443-1448.
[50] STOCKMANN C, SCHADENDORF D, KLOSE R, et al. The impact of the immune system on tumor: angiogenesis and vascular remodeling [J]. Front Oncol, 2014, 4: 69.
[51] MARTINET L, GARRIDO I, FILLERON T, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer [J]. Cancer Res, 2011, 71(17): 5678-5687.
[52] PETRIE H T, KLASSEN L W, KAY H D. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes [J]. J Immunol, 1985, 134(1): 230-234.
[53] FOGAR P, SPERTI C, BASSO D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome [J]. Pancreas, 2006, 32(1): 22-28.
[54] BLAKE-MORTIMER J S, SEPHTON S E, CARLSON R W, et al. Cytotoxic T lymphocyte count and survival time in women with metastatic breast cancer [J]. Breast J, 2004, 10(3): 195-199.
Rolesofinflammatorycellsinprogressionofgastriccancer
CUI Hui, HUI Qiyuan
Department of Gastroenterology, Yan’an University Affiliated Hospital, Yan’an 716000, China
Gastric cancer as the most common tumor of the digestive tract, causes great harm to human health. Epidemiological studies have shown that inflammation is closely related to the development and progression of gastric cancer. The mechanism of inflammatory cells and gastric cancer were reviewed in this paper.
Inflammatory cells; Gastric cancer; Correlation
崔慧,硕士,研究方向:胃癌及其癌前病变。E-mail:734576054@qq.com
惠起源,教授,主任医师,硕士研究生导师,研究方向:胃癌及其癌前病变。E-mail:qyhui@163.com
10.3969/j.issn.1006-5709.2017.12.002
R735.2
A
1006-5709(2017)12-1327-04
2017-08-24
陈香宇)

