季节性模拟条件下中缅树鼩内分泌激素调节作用的研究
朱万龙, 谢 静, 蔡金红, 王政昆
(云南师范大学 生命科学学院 云南省高校西南山地生态系统动植物生态适应进化及保护重点实验室, 昆明 650500)
季节性模拟条件下中缅树鼩内分泌激素调节作用的研究
朱万龙, 谢 静, 蔡金红, 王政昆
(云南师范大学 生命科学学院 云南省高校西南山地生态系统动植物生态适应进化及保护重点实验室, 昆明 650500)
为了阐明内分泌激素在中缅树鼩体重调节中的作用,模拟夏季和冬季环境,测定了其血清瘦素含量、胰岛素含量和甲状腺素含量。结果表明:中缅树鼩的血清瘦素水平在模拟冬季环境中显著降低;胰岛素水平与瘦素水平呈显著的正相关,说明中缅树鼩胰岛素能够正向调节和刺激瘦素的分泌;模拟冬季环境显著增加甲状腺素T3含量,从而增强其产热能力,而模拟夏季环境对其影响不大,呈现出降低的趋势,在一定程度上减弱了产热。以上结果说明中缅树鼩在季节性变化过程中会通过调整内分泌激素的含量来调节其能量代谢。
中缅树鼩;内分泌激素;体重调节
小型哺乳动物体重的季节性波动主要受到其能量摄入和能量支出的影响[1-2]。随着体重的变化,动物的血清瘦素含量也表现出显著的季节波动[3],而且这种季节波动与其能量代谢密切相关[4],如动物在冬季降低瘦素含量,与此同时其体重、体脂重量和摄入能在冬季也显著降低[2]。然而,低温可以使实验大鼠和小鼠血清瘦素显著降低,而其摄入能显著增加[5-6]。不同动物其血清瘦素对摄入能的调节方式不同,暗示着瘦素对动物体重调节机制可能是不一样的[7]。
研究表明瘦素与胰岛素之间存在相互关系,胰岛素可增加瘦素的mRNA表达,从而增加瘦素的浓度,即瘦素水平与胰岛素水平相关。低温使得小型哺乳动物的产热能力增加[8],其重要的生理基础之一就是甲状腺功能状态或甲状腺激素的含量发生了变化[9],如长爪沙鼠能耐受极端的高低温环境[10],其产热能力也随环境温度的急剧变化而改变,其甲状腺激素的含量也发生了改变[11]。
中缅树鼩为东洋界特有的小型哺乳动物,目前关于其季节性变化过程中内分泌激素的调节作用还没有报道。本研究对中缅树鼩在模拟冬季和夏季环境中血清瘦素、胰岛素、甲状腺素等激素含量进行测定,最终来阐明中缅树鼩的内分泌激素对其体重调节的作用。
1 材料和方法
1.1 动物来源
实验动物捕自云南省昆明市禄劝县附近灌丛中(海拔1679 m),动物捕回后室温单笼饲养,定点喂以由玉米面、奶粉、白糖以适当比例混合制得熟食,添加少许水,每隔一天加喂苹果、梨等水果适量。实验动物均为非繁殖期成年个体,饲养地点为云南师范大学生命科学学院动物饲养房。动物适应一个月后进行实验,挑选成年雌性中缅树鼩(n=70),分为两大组。一组驯化于冬季模拟环境(5℃,光照为8 L:16D)28 d,于0、7、14、21、28 d处死动物(n=7);另一组驯化于夏季模拟环境(25℃,光照为16 L:8D)28 d,于0、7、14、21、28 d处死动物(n=7)。动物处死后,分离血清,测定其激素含量。每组动物实验前体重差异不显著。
1.2 内分泌激素的测定
将动物处死,取血,4℃静置1 h,于4℃、4000 r/min离心30 min,吸取上层血清置于-80℃低温冰箱内保存。血清瘦素、甲状腺激素、促甲状腺激素、睾酮及褪黑激素含量分别采用对应的放射免疫分析试剂盒(美国Linco 公司生产) 到昆明医学院第二附属医院进行测定。
1.3 数据分析
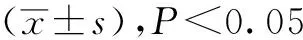
2 结果
2.1 体重、血清瘦素含量和胰岛素含量
中缅树鼩在模拟冬季环境中,体重显著增加(F=2.23,P<0.05),而血清瘦素含量则显著降低(F=5.21,P<0.01, 图1);在模拟夏季环境中,中缅树鼩体重显著下降 (F=1.95,P<0.05),血清瘦素浓度差异不显著(P>0.05, 图2)。血清胰岛素含量在模拟冬季环境中显著降低,胰岛素含量与瘦素水平呈显著正相关(r=0.54,P<0.05, 图3)。
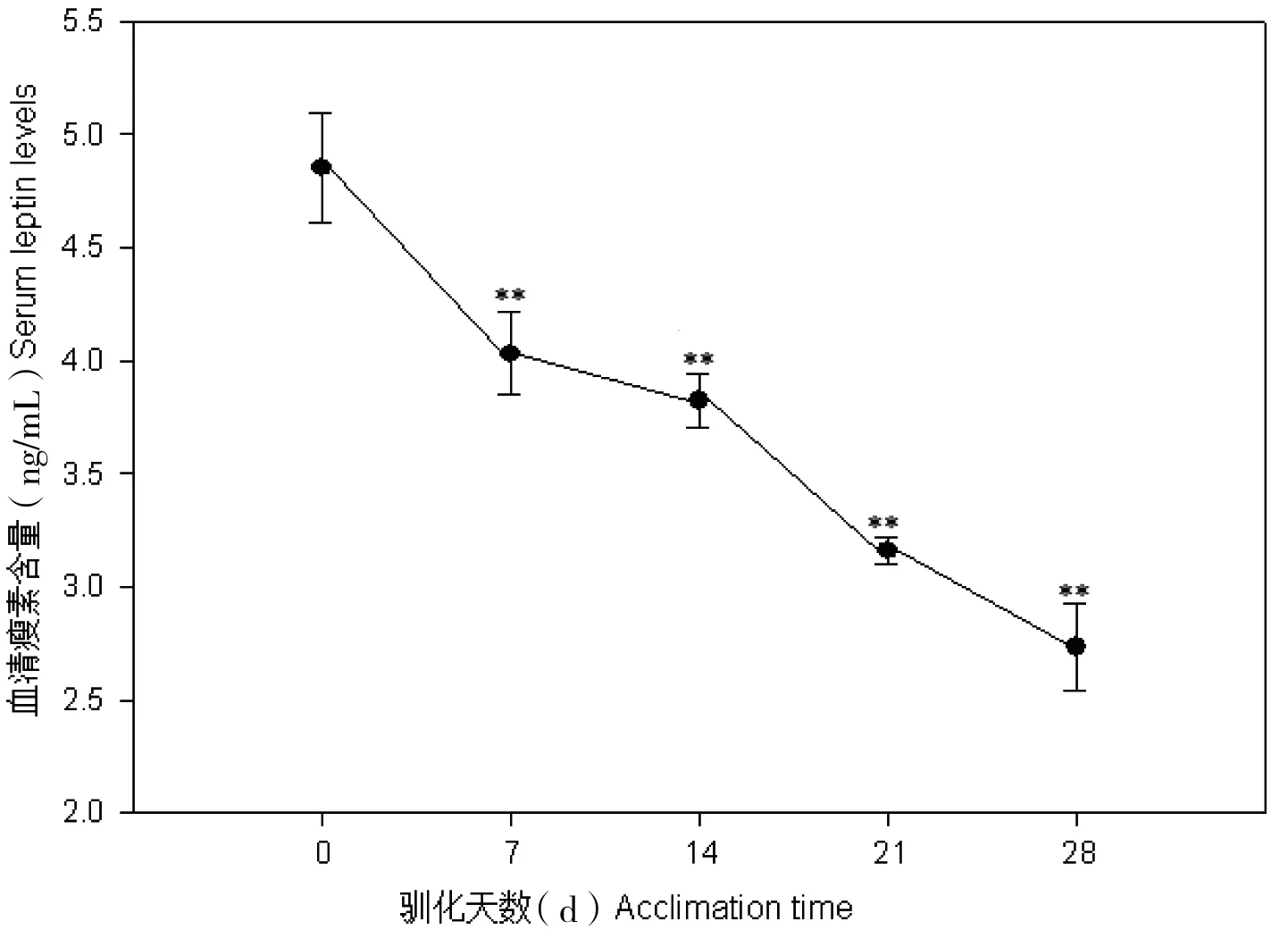
图1 模拟冬季环境中中缅树鼩血清瘦素含量的变化
*P<0.05;**P<0.01(与0天比较)
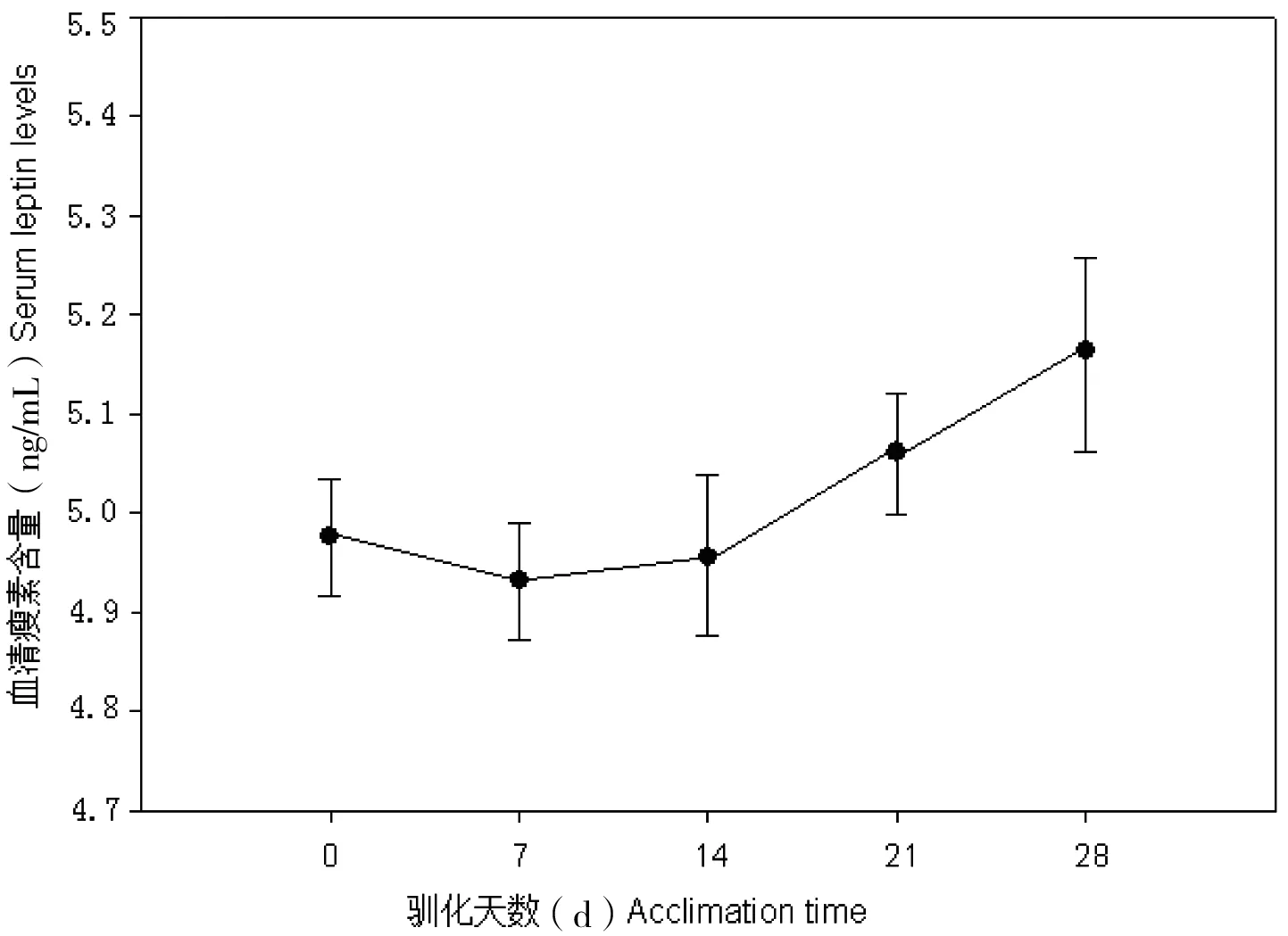
图2 模拟夏季环境中中缅树鼩血清瘦素含量的变化
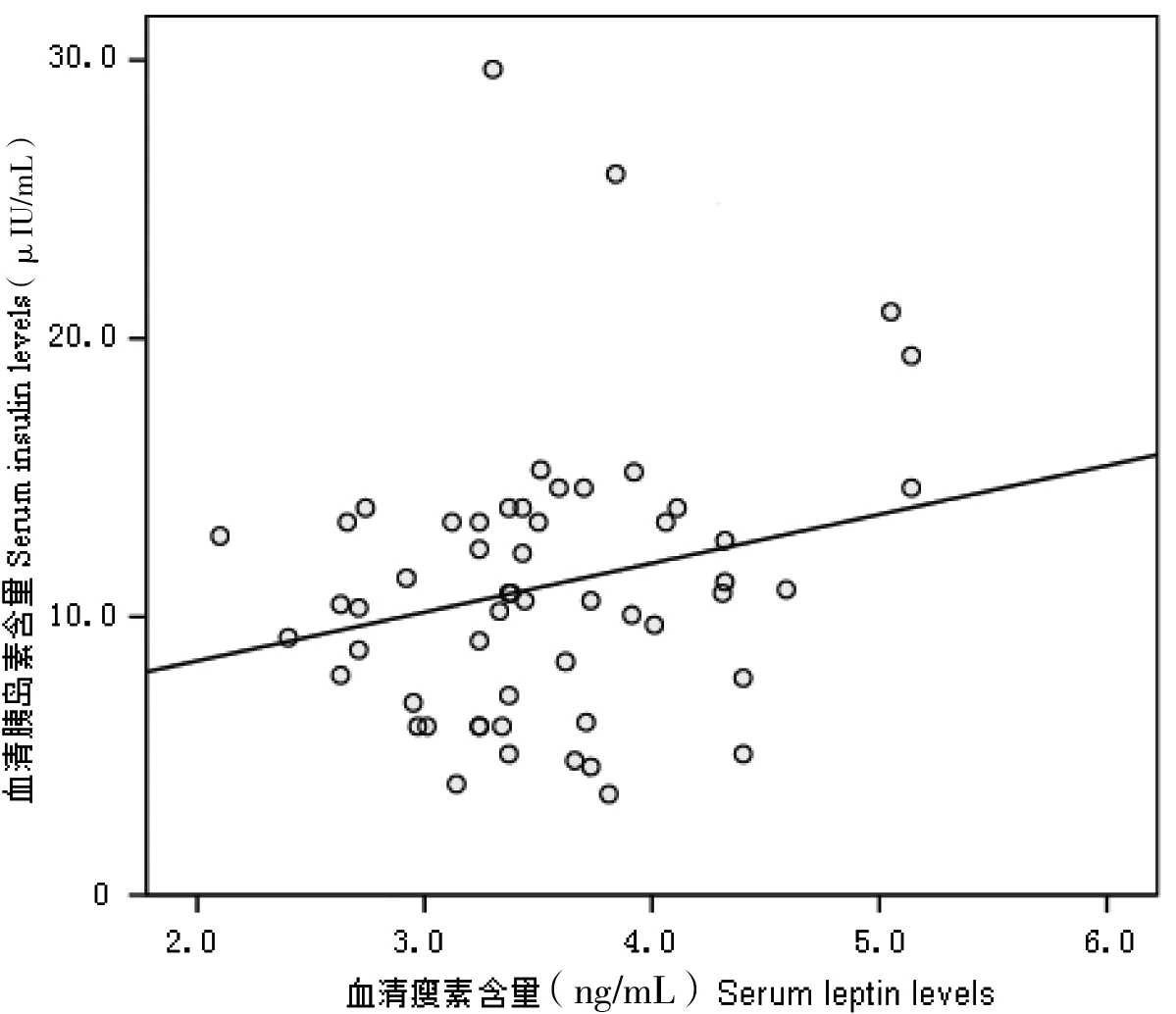
图3 中缅树鼩血清瘦素浓度与胰岛素的相关性
2.2 甲状腺素含量
在模拟冬季环境中,血清T3浓度显著增加,T4浓度显著降低,T3/T4的比率显著增加(图4);血清促甲状腺素含量较0天增加了44.13%。在模拟夏季环境中,血清T3和T4浓度变化差异不显著(P>0.05),T3/ T4比率显著降低(F=2.12,P<0.05, 图5);血清促甲状腺素含量较0天减少了27.92%。
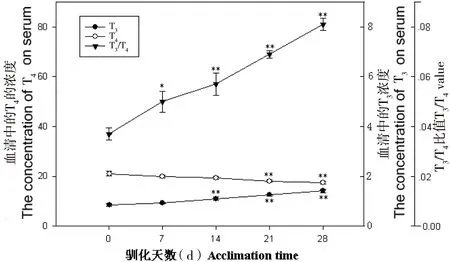
图4 模拟冬季环境中中缅树鼩血清甲状腺激素含量的变化
*P<0.05; **P<0.01; compared with 0 day
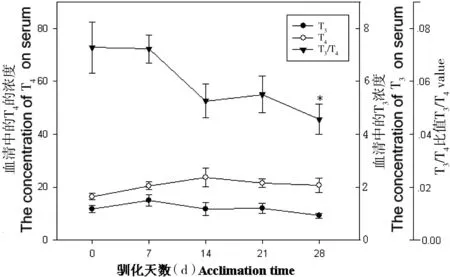
图5 模拟夏季环境中中缅树鼩血清甲状腺激素含量的变化
*P<0.05; compared with 0 day
3 讨论
瘦素主要由脂肪组织分泌,其在动物体重调节和能量代谢中起重要的作用[12-14]。本研究结果表明,模拟冬季环境显著降低中缅树鼩的血清瘦素水平,而模拟夏季环境对其血清瘦素含量没有影响。在模拟冬季环境中,中缅树鼩的瘦素含量下降,有利于其增加食物摄入,弥补冷胁迫条件下产热消耗的增加。胰岛素是影响瘦素合成与分泌的最重要的因素[15-16]。胰岛素能增强大鼠瘦素含量及分泌[17],脂肪组织瘦素含量在禁食时减少而喂养后增加,这与胰岛素浓度的变化是相似的,说明胰岛素可以调整瘦素的合成与分泌[18]。本研究结果表明中缅树鼩的瘦素与胰岛素呈显著的正相关,与上述的研究结果一致,说明胰岛素能正向的调节瘦素的合成与分泌。
甲状腺激素在动物适应性产热中起着重要的作用[19-21]。中缅树鼩在模拟冬季环境中,血清T3浓度显著增加,T4浓度显著下降,T3/T4比率显著增加,动物经过冷刺激后,一方面血循环中T4利用率加速,另一方面外周组织中的T4分布增加,刺激T4在外周组织中脱碘,产生活性更强的T3,这也成为血清T3浓度增加的主要原因,模拟冬季环境中T3/T4的比率的增加导致产热能力的增加。在模拟夏季环境中,中缅树鼩血清T3和T4差异不显著,T3/T4的比率下降,在28 d后达到显著水平,说明在模拟夏季环境中其甲状腺激素刺激产热的能力有所降低。促甲状腺激素(thyroid-stimulating hormone, TSH)是由垂体前叶分泌的一种糖蛋白激素,对甲状腺组织的生长、分化等起着非常重要的作用[22-28]。急性冷暴露能够引起大鼠血清TSH浓度的快速升高[29]。大鼠处于3℃~4℃冷环境中,血清中的TSH在30 min内能够提高1.5倍[30]。中缅树鼩在模拟冬季环境中促甲状腺激素水平先上升后下降,可能是因为低温短光照刺激了促甲状腺激素的分泌,进而提高血清中甲状腺激素水平,当甲状腺水平达到一定浓度时,高水平的甲状腺激素则通过下丘脑-垂体-甲状腺轴的反馈作用,缓慢地调节了促甲状腺激素的分泌,从而维持机体内分泌激素的平衡。
综上所述,中缅树鼩的血清瘦素水平与体重变化趋势不同,表现出了一定的特异性,胰岛素水平与瘦素水平呈显著的正相关,说明在中缅树鼩中,胰岛素能够正向的调节和刺激瘦素的分泌。模拟冬季环境显著刺激了甲状腺素T3含量的增加,从而增强了产热能力;模拟冬季环境在前期刺激了促甲状腺激素的分泌,并通过下丘脑-垂体-甲状腺轴调节了血清甲状腺激素的水平,在驯化中期,当甲状腺激素达到一定浓度后,又通过下丘脑-垂体-甲状腺轴反馈抑制了促甲状腺激素的分泌。内分泌激素在中缅树鼩季节性变化过程中起着非常重要的作用。
[1]EBLING F J P. Hypothalamic control of seasonal changes in food intake and body weight[J]. Frontiers in Neuroendocrinology, 2015, 37: 97-107.
[2]ZHU W L, WANG Z K. Seasonal changes in body mass, serum leptin levels and hypothalamic neuropeptide gene expression in maleEothenomysolitor[J]. Comparative Biochemistry and Physiology, 2015, 184: 83-89.
[3]XING X, YANG M, WANG D H. The expression of leptin, hypothalamic neuropeptides and UCP1 before, during and after fattening in the Daurian ground squirrel (Spermophilusdauricus)[J]. Comparative Biochemistry and Physiology, 2015, 184(3): 105-112.
[4]SCHERBARTH F, DIEDRICH V, DUMBELL R A, et al. Somatostatin receptor activation is involved in the control of daily torpor in a seasonal mammal[J]. American Journal of Physiology-Regulatory, 2015, 309(6): 668-674.
[5]ABELENDA M, LEDESMA A, RIAL E. Leptin administration to cold-acclimated rats reduces both food intake and brown adipose tissue thermogenesis[J]. Journal of Thermal Biology, 2003, 28(6-7): 525-530.
[6]BING C, FRANKISH H M, PICKAVANCE L,et al. Hyperphagia in cold-exposed rats is accompanied by decreased plasma leptin but unchanged hypothalamic NPY[J]. Am J Physiol, 1998, 274(2): 62-68.
[7]KLUG B J, BRIGHAM R M. Changes to metabolism and cell physiology that enable mammalian hibernation[J]. Springer Science Reviews, 2015, 3(1): 39-56.
[8]SAITO M, YONESHIRO T, MATSUSHITA M. Roles of brown adipose tissue in seasonal variations of thermogenesis in men[J]. The FASEB Journal, 2015, 29(1): 993-1015.
[9]MIAO Y, WU W, DAI Y, et al. Liver X receptor β controls thyroid hormone feedback in the brain and regulates browning of subcutaneous white adipose tissue[J]. Proceedings of the National Academy of Sciences, 2015, 112(45): 14006-14011.
[10]ZHAO Z J, WANG D H. Effects of photoperiod on energy budgets and thermogenesis in Mongolian gerbils (Merionesunguiculatus)[J]. Journal of Thermal Biology, 2006, 31(4): 323-331.
[11]LI Y G, YAN Z C, WANG D H. Physiological and biochemical basis of basal metabolic rates in Brandt′s voles (Lasiopodomysbrandtii) and Mongolian gerbils (Merionesunguiculatus)[J]. Comparative Biochemistry and Physiology, 2010, 157(3): 204-211.
[12]SCHILLING J, HOSPES R, KAYA G, et al. Serum leptin in neonatal lambs is associated with temperature, plasma lipids and metabolites[J]. Experimental and Clinical Endocrinology & Diabetes, 2015, 123(7): 398-404.
[13]POLYZOS S A, ARONIS K N, KOUNTOURAS J, et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis[J]. Diabetologia, 2016, 59(1): 30-43.
[14]LI H, ZHANG T, LENG R, et al. Plasma/serum leptin levels in patients with systemic lupus erythematosus: a meta-analysis[J]. Archives of Medical Research, 2015, 46(7): 551-556.
[15]NABAVI S, RAFRAF M, SOMI M H, et al. Probiotic yogurt improves body mass index and fasting insulin levels without affecting serum leptin and adiponectin levels in non-alcoholic fatty liver disease (NAFLD)[J]. Journal of Functional Foods, 2015, 18: 684-691.
[16]SI Z, HU K, WANG C, et al. The effects of neuromuscular facilitation techniques on osteoporosis of hemiplegia limbs and serum leptin level in patients or rats with cerebral infarction[J]. Brain Injury, 2016, 30(4): 474-479.
[17]SALADIN R, DE VOS P, GUERRE-MILLO M. Transient increase in obese gene expression after food intake or insulin administration[J]. Nature, 1995, 377(6549): 527-529.
[18]CUSIN I, SAINSBURY A, DOYLE P. The ob gene and insulin: a relationship leading to clues to the understanding of obesity[J]. Diabetes, 1995, 44(12): 1467-1470.
[19]MORTAZAVI S M J, HABIB A, GANJ-KARIMI A H, et al. Alterations in TSH and thyroid hormones following mobile phone use[J]. Iranian Journal of Medical Sciences, 2009, 24(4): 274-278.
[20]VISSENBERG R, MANDERS V D, MASTENBROEK S, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction[J]. Human Reproduction Update, 2015, 21(3): 378-387.
[21]DONZELLI R, COLLIGIANI D, KUSMIC C, et al. Effect of hypothyroidism and hyperthyroidism on tissue thyroid hormone concentrations in rat[J]. European Thyroid Journal, 2016, 5(1): 27-34.
[22]BURGER D, GAGNON A M, LOCHNAN H A, et al. Thyroid‐stimulating hormone acutely increases levels of circulating pro-coagulant microparticles[J]. Clinical Endocrinology, 2015, 83(2): 285-287.
[23]KARLSSON A C, FALLAHSHAHROUDI A, JOHNSEN H, et al. A domestication related mutation in the thyroid stimulating hormone receptor gene (TSHR) modulates photoperiodic response and reproduction in chickens[J]. General and Comparative Endocrinology, 2016, 228: 69-78.
[24]ISODA T, BABA S, MARUOKA Y, et al. Use of recombinant human thyroid-stimulating hormone (rhTSH) reduces the damage to salivary glands after radioiodine therapy for thyroid cancer[J]. Journal of Nuclear Medicine, 2015, 56(s3): 1638.
[25]YAU V M, LUTSKY M, YOSHIDA C K, et al. Prenatal and neonatal thyroid stimulating hormone levels and autism spectrum disorders[J]. Journal of Autism and Developmental Disorders, 2015, 45(3): 719-730.
[27]NISHIOKA E, HIRAYAMA S, UENO T, et al. Relationship between maternal thyroid-stimulating hormone (TSH) elevation during pregnancy and low birth weight: a longitudinal study of apparently healthy urban Japanese women at very low risk[J]. Early Human Development, 2015, 91(3): 181-185.
[28]KIM E Y, KIM S H, RHEE S J, et al. Relationship between thyroid-stimulating hormone levels and risk of depression among the general population with normal free T4levels[J]. Psychoneuroen Docrinology, 2015, 58: 114-119.
[29]DUCOMMUN P, SAKIZ E, GUILLEMIN R. Dissociation of the acute secretions of thyrotropin and adrenocorticotropin[J]. Am J Physiol, 1966, 210(6): 1257-1259.
[30]HERSHMAN J M, READ D G, BAILEY A L, et al. Effect of cold exposure on serum thyrotropin[J]. J Clin Endocrinol Metab, 1970, 30(4): 430-434.
StudyontheroleofendocrinehormoneregulationsinTupaiabelangeriundertheconditionofseasonalsimulationenvironment
ZHU Wan-long, XIE Jing, CAI Jin-hong, WANG Zheng-kun
(Key Laboratory of Ecological Adaptive Evolution and Conservation on Animals-Plants in Southwest Mountain Ecosystem of Yunnan Province Higher Institutes College, School of Life Sciences, Yunnan Normal University, Kunming 650500, China)
In order to investigate the roles of endocrine hormone in the regulation of body mass ofTupaiabelangeri, serum leptin levels, insulin levels, and the levels of thyroid hormone under simulating winter and summer environments were measured. The results showed that serum leptin levels inT.belangeriin simulating winter environment reduced significantly with the acclamation time; insulin levels were significantly correlated to leptin levels, indicating that insulin could positively modulaten and stimulate leptin secretion inT.belangeri; simulating winter environment increased thyroid hormone T3levels significantly, thereby enhancing the heat production capacity, and simulated summer environment had no obvious influence on the thyroid hormone T3levels, but showed a decreasing trend in a certain extent, weakened the heat production. All of these results suggested thatT.belangeriin seasonal change process will be through the adjustment of endocrine hormone levels to regulate the energy metabolism.
Tupaiabelangeri; endocrine hormone; body mass regulation
2016-05-27;
2016-06-06
国家自然科学基金项目(编号:31660121);云南省应用基础研究计划重点项目(编号:2016FA045);云南师范大学博士科研启动项目
朱万龙,副教授,研究方向为动物生理生态,E-mail: zwl_8307@163.com
10.3969/j.issn.2095-1736.2017.06.042
Q955
A
2095-1736(2017)06-0042-04

