Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
Injury to central nervous system (CNS) tissues in adult mammals oen leads to neuronal loss, scarring, and permanently lost neurologic functions, and this default healing response is increasingly linked to a pro-inflammatory innate immune response. Extracellular matrix (ECM) technology can reduce inflammation, while increasing functional tissue remodeling in various tissues and organs, including the CNS. However,ECM bioscaffold delivery and the rapid ECM degradation rate in vivo have complicated advancing ECM technology in the CNS.e recent discovery of bioactive matrix bound vesicles (MBV) within ECM bioscaffolds represents a potentially significant vertical advance in our understanding of how ECM bioscaffolds modulate the default healing response in various tissues and organs. MBV enable new approaches to delivering ECM derived bioactive components in a minimally invasive manner, and open new areas of investigation into how the ECM derived MBV regulate tissue and organ organization during development, normal function, aging, and aer injury or disease.is perspective provides an overview of MBV, initial studies on macrophages, neuroblastoma cells,and primary neurons, and discusses the potential diagnostic and therapeutic potentials of MBV in CNS applications.
ECM bioscaffolds are widely used pre-clinically and clinically to positively modulate the innate immune response to reduce scarring and promote functional tissue remodeling(Dziki et al., 2017). ECM is the protein and glycosaminoglycan matrix in tissues and organs that provides mechanical and biochemical support, facilitates cellular communication,and directs cellular phenotype and function. ECM bioscaffolds derived by decellularizing healthy xenogeneic tissues can promote constructive tissue remodeling and functional recovery, over scarring, in diverse tissues, including esophagus,lower urinary tract, and musculotendinous tissues, among others. Recent pre-clinical studies suggest ECM bioscaffolds can also promote positive tissue remodeling in the nervous system (Ren et al., 2015), in part, by positively regulating the innate immune response (Tukmachev et al., 2016). However,the bioactive factors within ECM bioscaffolds are not well understood.us, the recent discovery of bioactive MBV in virtually all ECM bioscaffolds analyzed and the ability of purified MBV to regulate diverse cellular phenotypes suggests that MBV are critical bioactive factors within ECM bioscaffolds (Huleihel et al., 2016).is holds particular promise for CNS applications in which ECM delivery is complicated by the necessity for minimally invasive approaches (Massensini et al., 2015).
MBV appear to represent a distinct class of extracellular vesicles (EV) localized to the collagen fibrillary network within the ECM. MBV were originally discovered due to the presence of non-degradable, non-coding nucleic acids, specifically miRNAs, within various ECM bioscaffolds (Huleihel et al., 2016). Huleihel et al. (2016) showed MBV are a protective source of miRNAs in all laboratory and commercially produced ECM bioscaffolds tested, including ECM bioscaffolds from muscle, intestine, urinary bladder, and even CNS tissues, suggesting MBV are ubiquitous ECM components positioned to play a role in ECM organization and function. MBV readily bind to collagen fibrils and evidence suggests the localization of MBV to tightly packed collagen fibrils protect MBV from detergent-mediated degradation during ECM decellularization. In agreement, MBV are small, nanoscale vesicles ranging in size from about 10 to 300 nm.is size places MBV in the range of exosomes, which are secreted from virtually all cell types and found in all bodily fluids. However,MBV differ from exosomes not only in their localization but also in their general lack of typical extra-vesicular exosome markers, like the tetraspanins CD9, CD63, and CD81 (Huleihel et al., 2016), suggesting MBV originate from a unique,ECM-specific, cellular origin.
MBV can recapitulate effects of the ECM bioscaffolds from which they were derived and these effects appear to depend on MBV miRNA cargoes (Huleihel et al., 2017a). Like other EV, MBV carry unique and complex cargoes, including lipids,proteins, cytokines, carbohydrates, and other small molecules(Figure 1), capable of activating a variety of extra- and intracellular signaling pathways. As with other EVs, initial studies indicate the cellular responses to MBV depend on both extra-vesicular proteins and the intra-vesicular miRNA cargoes(Faust et al., 2017). Interestingly, miRNA cargoes in MBV,derived from different tissues, are enriched in highly conserved miRNAs involved in cell cycle regulation and differentiation (Faust et al., 2017; Huleihel et al., 2017b), suggesting MBV and their cargoes may play a fundamental role in ECM mediated tissue and organ development, organization, and function, possibly by regulating cellular phenotypes in a site appropriate manner. Moreover, several miRNAs highly enriched in MBVs are known to regulate neuronal signaling pathways underlying neuronal differentiation and neurite growth, including miRNAs-30b, -125b, and -133b (van der Merwe and Steketee, 2017).
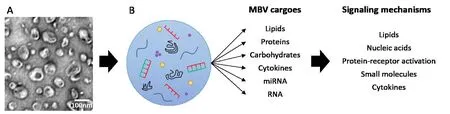
Figure 1 MBV carry diverse cargoes that can signal via multiple mechanisms.
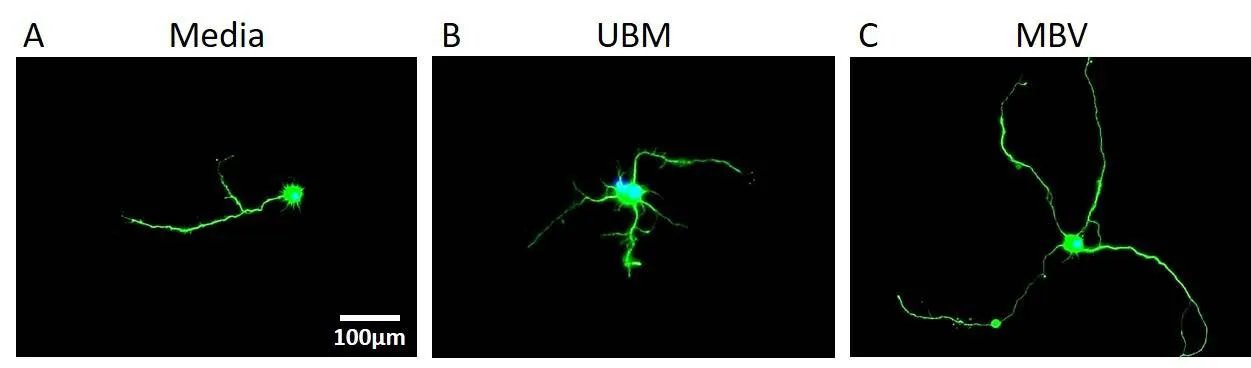
Figure 2 MBV derived from UBM induce significantly longer neurite extensions in CNS neurons compared to media and UBM conditions (Faust et al.,2017).
MBV derived from UBM can also regulate differentiation and axon growth in neuroblastoma and primary CNS neurons. In neuroblastoma cells, UBM MBV increased neuronal differentiation and neurite extension at a faster rate than the UBM ECM (Huleihel et al., 2016). In primary CNS neuron cultures, MBV increased axon growth specifically, without altering survival or dendrite growth (Faust et al., 2017). MBV also increased retinal ganglion cell axon growth compared to the UBM ECM from which they were derived and media controls (Figure 2). Moreover, similar to macrophages (Huleihel et al., 2017b), increased axon growth appears to depend on MBV miRNAs. UBM derived MBV are internalized by virtually all neurons within minutes and delivered labeled miRNA cargoes to the cytoplasm (Faust et al., 2017), and in our preliminary experiments increased axon growth is differentially regulated by pre-transfecting MBV with sequence specific antagomirs against miRNAs-30b, -125b, and 133b.
Can cell or tissue specific MBV be used to prevent scarring and promote functional tissue remodeling in the CNS (van der Merwe and Steketee, 2016)? ECM has been shown to prevent scarring in numerous tissues throughout the body, but it is unknown whether MBV recapitulate these ECM effects in vivo as they do in vitro. Injury to the CNS results in permanently lost neurologic function, partly due to the innate immune response after injury, which results in a persistent inflammatory response and astrogliosis, eventually leading to scar tissue deposition and cessation of neurologic function.Thus, multifunctional biologics that can both modulate the inflammatory innate immune response and increase CNS axon growth are attractive approaches to treat CNS injury and promote functional recovery. MBV enable the combination of CNS growth promotion and macrophage polarization,indicating MBV may be used in vivo to alter the default healing response and promote functional remodeling aer CNS injury. MBV may offer a superior alternative to whole ECM since MBV are easy to introduce in vivo, thereby offering a minimally invasive therapy.
MBV can be used as biomarkers to assess tissue health and disease state. Exosomal EV cargoes change with trauma and disease and have successfully been used as biomarkers for numerous diseases. However, the effects of injury and disease on MBV cargoes remains unknown. ECM bioactivity changes with regard to source tissue injury and disease state.erefore, MBV cargoes and surface markers are hypothesized to also change aer injury and potentially act to potentiate injuries and disease. For instance, ECM isolated from different aged tissues have different effects on cellular responses in vivo and in vitro (Sicari et al., 2012), logically suggesting MBV and their cargoes may differ based on source tissue age, and future studies will focus on determining how MBV composition changes throughout cellular development and aer injury.
MBV are remarkably resistant to degradation and even lyophilization, therefore MBV may provide a new metric for analyzing cadaver tissues and bones.ough further work is necessary to confirm the localization and function of MBV in vivo, the presence of MBV within the ECM supports the notion that MBV are optimally positioned to play a role in regulating tissue and organ architecture during normal function, aging, and aer injury.us, analysis of MBV cargoes could provide minimally invasive diagnostic approaches for analyzing injury severity or disease progression.
Conclusion:Regenerative medicine therapies are increasingly investigating in vivo tissue reconstruction strategies to promote functional recovery aer injury.ese strategies utilize the intrinsic ability of cells to organize and differentiate into functional tissues aer injury by promoting site appropriate remodeling in vivo instead of ex vivo tissue engineering and re-implantation.e concept that the secreted EVs may provide a safer, more tunable, and easily deliverable platform for modulating the default healing response in the CNS has recently been discussed (van der Merwe and Steketee, 2016).MBV are a natural component of the ECM in healthy tissues,have been shown to be non-cytotoxic, can promote CNS neurite growth, promote an anti-inflammatory macrophage phenotype, and can easily be administered in vivo, therefore making MBV an attractive option to promote neuroprotection and constructive remodeling in the CNS aer injury.
Finally, compared to other extracellular vesicles derived from undifferentiated clonal cell lines in vitro, MBV are likely more relevant to positive tissue remodeling since MBV are derived from healthy, pro-regenerative adult tissues.
Yolandi van der Merwe, Anne E. Faust, Michael B. Steketee*
Department of Bioengineering, Swanson School of Engineering,
University of Pittsburgh, Pittsburgh, PA, USA (van der Merwe Y)Department of Ophthalmology, School of Medicine, University of
Pittsburgh, Pittsburgh, PA, USA (van der Merwe Y, Faust AE,Steketee MB)
McGowan Institute for Regenerative Medicine, University of Pittsburgh, Pittsburgh, PA, USA (van der Merwe Y, Faust AE,Steketee MB)
Center for Neuroscience University of Pittsburgh, University of Pittsburgh, Pittsburgh, PA, USA (Steketee MB)
*Correspondence to: Michael B. Steketee, Ph.D.,
SteketeeM@UPMC.edu.
orcid: 0000-0001-7179-8898 (Michael B. Steketee)
Accepted:2017-10-10
How to cite this article:van der Merwe Y, Faust AE, Steketee MB (2017)Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds. Neural Regen Res 12(10):1597-1599.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Open peer reviewer:Stefan Wiese, Ruhr University Bochum, Germany.
Dziki JL, Huleihel L, Scarritt ME, Badylak SF (2017) Extracellular matrix bioscaffolds as immunomodulatory biomaterials. Tissue Eng Part A doi:10.1089/ten.TEA.2016.0538.
Faust A, Kandakatla A, van der Merwe Y, Ren T, Huleihel L, Hussey G,Naranjo JD, Johnson S, Badylak S, Steketee M (2017) Urinary bladder extracellular matrix hydrogels and matrix-bound vesicles differentially regulate central nervous system neuron viability and axon growth and branching. J Biomater Appl 31:1277-1295.
Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, Stolz DB,Badylak SF (2016) Matrix-bound nanovesicles within ECM bioscaffolds.Science advances 2:e1600502.
Huleihel L, Bartolacci JG, Dziki JL, Vorobyov T, Arnold B, Scarritt ME, Pineda Molina C, LoPresti ST, Brown BN, Naranjo JD, Badylak SF (2017a) Matrix-bound nanovesicles recapitulate extracellular matrix effects on macrophage phenotype. Tissue Eng Part A doi: 10.1089/ten.TEA.2017.0102.
Huleihel L, Dziki JL, Bartolacci JG, Rausch T, Scarritt ME, Cramer MC, Vorobyov T, LoPresti ST, Swineheart IT, White LJ, Brown BN, Badylak SF(2017b) Macrophage phenotype in response to ECM bioscaffolds. Semin Immunol 29:2-13.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L,Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A,Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481-487.
Massensini AR, Ghuman H, Saldin LT, Medberry CJ, Keane TJ, Nicholls FJ,Velankar SS, Badylak SF, Modo M (2015) Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater 27:116-130.
Ren T, van der Merwe Y, Steketee MB (2015) Developing extracellular matrix technology to treat retinal or optic nerve injury(1,2,3). eNeuro 2.
Sicari BM, Johnson SA, Siu BF, Crapo PM, Daly KA, Jiang H, Medberry CJ,Tottey S, Turner NJ, Badylak SF (2012) The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 33:5524-5533.
Tukmachev D, Forostyak S, Koci Z, Zaviskova K, Vackova I, Vyborny K,Sandvig I, Sandvig A, Medberry CJ, Badylak SF, Sykova E, Kubinova S(2016) Injectable extracellular matrix hydrogels as scaffolds for spinal cord injury repair. Tissue Eng Part A 22:306-317.
van der Merwe Y, Steketee MB (2016) Immunomodulatory approaches to CNS injury: extracellular matrix and exosomes from extracellular matrix conditioned macrophages. Neural Regen Res 11:554-556.
van der Merwe Y, Steketee MB (2017) Extracellular vesicles: biomarkers,therapeutics, and vehicles in the visual system. Curr Ophthalmol Rep doi:10.1007/s40135-017-0153-0.
10.4103/1673-5374.217324
- 中国神经再生研究(英文版)的其它文章
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration
- Effect of glial cells on remyelination after spinal cord injury
- In vitro neuroprotective effects of ciliary neurotrophic factor on dorsal root ganglion neurons with glutamate-induced neurotoxicity
- miR-30c promotes Schwann cell remyelination following peripheral nerve injury
- End-to-side neurorrhaphy repairs peripheral nerve injury: sensory nerve induces motor nerve regeneration