设施菜田土壤pH 和初始 对反硝化产物比的影响
曹文超,郭景恒,宋 贺,刘 骕,陈吉吉,王敬国*
(1 中国农业大学资源与环境学院,北京 100193;2 安徽农业大学农学院,安徽合肥 230036)
曹文超1,郭景恒1,宋 贺2,刘 骕1,陈吉吉1,王敬国1*
(1 中国农业大学资源与环境学院,北京 100193;2 安徽农业大学农学院,安徽合肥 230036)
【目的】设施菜田土壤反硝化作用是N2O排放和氮素损失的重要途径。本研究通过室内厌氧培养试验,在不同pH和初始条件下,比较设施菜田土壤反硝化氮素气体排放及产物比的变化特征。【方法】以设施菜田土壤为研究对象,通过添加一定量低浓度的酸碱溶液调节土壤pH分别为酸性、中性和碱性条件,调节后的实测pH分别为5.63、6.65和7.83;同时以谷氨酸钠作为有效性碳,除未添加有效性碳作为对照处理 (CK)外,其他有效性碳与硝酸盐的比值分别调节为5∶1、15∶1和30∶1,三种pH条件下均设置 4 个水平,每个水平3次重复。利用自动连续在线培养系统 (Robot系统),在厌氧条件下监测不同处理土壤产生的 N2O、NO、N2和CO2浓度的动态变化,通过计算N2O/(N2O + NO + N2)指数估算反硝化过程N2O的产物比。【结果】增加土壤的pH能显著减少设施菜田土壤N2O和NO的产生量,酸性 (pH 5.63) 土壤的N2O、NO产生量峰值在不同初始比下均显著高于中性 (pH 6.65) 和碱性 (pH 7.83) 土壤 (P< 0.05)。中性和碱性土壤在高下有利于减少反硝化过程N2O的产生,而酸性土壤条件下差异并不显著。中性土壤条件下增加有机碳含量会降低NO产生量,而在酸性和碱性土壤上有机碳的添加对NO产生量没有显著影响。土壤pH和初始比对土壤N2O的产生有极显著的交互效应 (P< 0.001)。酸性和中性土壤上添加有机碳能够显著增加土壤N2的产生速率 (P< 0.05),且与对照相比,不同pH的土壤添加有机碳后均显著促进反硝化过程中N2O向N2的转化。在不同初始下碱性土壤的CO2产生量显著高于酸性和中性土壤,同时与对照相比,添加有机碳显著增加了土壤的CO2产生量 (P< 0.05)。酸性土壤的N2O产物比在不同初始下均极显著高于碱性土壤 (P< 0.01),且不同初始下的土壤N2O产物比随pH的增加显著下降,二者呈极显著线性负相关关系(P< 0.01)。【结论】土壤pH降低是设施菜田土壤N2O和NO排放量较高的重要原因。而且,增加初始土壤有效碳含量促进了土壤的反硝化损失,并在中性和碱性土壤中N2O的产生量减少。土壤pH升高和初始增加均降低了产物比,但增加了土壤反硝化作用速率。在利用N2O排放通量和产物比估算土壤反硝化氮素损失时,土壤pH和有效碳含量是必须考虑的两个重要因素。
反硝化;pH;;N2O;日光温室
反硝化过程是土壤氮损失的主要途径之一,而且该过程产生的N2O和NO会给环境带来危害。其中,N2O是重要的温室气体,与全球气候变暖和臭氧层破坏密切相关[1–2];而NO是一种具有高度活性且有毒的化合物,会损耗臭氧[3]和污染空气[4],同时,NO经光化学氧化和水合作用生成HNO2和HNO3,对土壤和水体系统酸化产生影响[5]。农业活动占全球人为活动排放N2O和NO总量比例分别约为60%和10%[6]。以我国农田土壤为例,其N2O直接排放量为4.74 × 105t,占全国N2O排放的55.8%[7]。大量研究表明N2O和NO的排放与氮肥的施用密切相关,施用氮肥后增加土壤N2O和NO的排放量[8–11]。
我国设施蔬菜种植系统中,农户为了追求高产,过量施用化学氮肥。大部分地区设施菜田每季化肥氮素投入量通常会超过1200 kg/hm2[9],约为小麦、玉米田的2~7倍[10–12]。除了大量施用化学氮肥外,有机肥料的投入量也很大。以寿光设施菜田为例,有机肥年均施用量高达177 t/hm2[13]。此外,该地区设施菜田全年灌溉水总量在748~1957 mm之间,平均灌溉量高达1307 mm[14]。这些都为土壤反硝化微生物提供了大量的底物和适宜的环境条件,进而促进反硝化作用的发生和N2O的大量排放。据估计,我国设施菜田反硝化年损失量可达N 45.8 kg/hm2[15],反硝化过程对N2O排放总量的贡献率可达22.5%~57.7%[16]。由于方法原因,土壤反硝化氮损失的直接定量测定比较困难。已有研究表明,利用反硝化产物比和大量的N2O田间观测数据,可以估算土壤反硝化损失[17–18]。
众所周知,土壤中每1 mol的NH3-N氧化为NO3–-N即可释放1 mol质子,在没有生物利用的情况下,成为导致土壤酸化的重要原因之一。同时,在土壤根际微域环境中,根系对阳离子的吸收超过阴离子时产生的酸性分泌物也会造成土壤局部酸化。已有研究表明,设施菜田每年仅由施肥产生的土壤酸化潜势就超过H+220 kmol/hm2[19],相同区域设施菜田土壤pH值比粮田土壤低0.5个单位[20]。土壤pH值的降低会显著影响反硝化的损失量[21–22]和气态产物比的变化[23]。而且,设施菜田表层土壤的可溶性有机碳含量显著高于农田土壤[24],这有利于其形成土壤微域厌氧环境。设施菜田通常底施大量的有机肥和大水漫灌,在生长季初期 (1到2周内) 也会产生较大范围的厌氧环境。有证据表明,施用有机肥后提高土壤水分含量,反硝化作用对N2O的贡献可在64%~77%之间[25]。
综上所述,土壤pH和有机碳是影响反硝化过程与N2O排放的重要环境因子,它们之间存在着交互作用,但目前相关的研究较少、亟待深入。本研究选择pH下降明显、有机肥施用量很高的设施蔬菜集中种植区代表性土壤,通过在线连续监测的自动培养系统,研究外源碳加入和pH对反硝化产物比的影响,从而为探讨通过降低产物比以实现N2O减排的措施提供理论依据。对土壤反硝化产物比及其影响因素的深入了解,又可为利用N2O的田间观测数据准确估算土壤反硝化氮损失提供参考。
1 材料与方法
1.1 采样点概况及土壤样品采集
供试土壤采自中国农业大学山东省寿光市罗家村 (36°55′N,118°35′E) 设施菜田长期定位试验点。该地区年平均温度和降雨分别为12.4℃和558 mm。试验始于2004年2月,每年种植两季番茄,即每年2~6月为冬春季,8月至次年1月为秋冬季,7月份休闲。试验初始0—10 cm土层土壤理化性质为全氮1.37 g/kg,有机质18.3 g/kg,pH 5.7 (0.05 mol/L CaCl2),有效磷和速效钾含量均为299 mg/kg,有关试验点的更多信息可参见He等[26]的研究。
在冬春季和秋冬季番茄移栽前,分别基施风干鸡粪 8 t/hm2和 10 t/hm2(约为 N 146 kg/hm2和 211 kg/hm2),尿素作为追肥随灌溉水施用,每次用量为N 120 kg/hm2,冬春季为6次,秋冬季共5次。本试验以农户习惯施肥土壤作为供试样品,为避免鸡粪施用的影响,于2015年6月番茄最后收获期采集0—20 cm土壤样品,充分混合后用冰盒运回实验室,在4℃下保存备用。
1.2 试验设计与方法
分别称取相当于10.0 g烘干土重的新鲜土样于120 mL血清瓶中,根据已知的土壤硝酸盐含量和谷氨酸钠添加量配置一定浓度的溶液,将溶液用注射器加入至土壤中后,调节土壤质量含水量为25%。用橡胶塞和铝盖密封,并利用抽真空–充氦气系统(北京帅恩科技有限公司) 反复用He (99.999%) 气冲洗血清瓶3次,最后充上He气。将所有血清瓶置于20℃恒温水浴槽中培养,平衡血清瓶内气压后,利用Robot自动培养系统每间隔8 h在线连续监测血清瓶内N2O、NO、N2和CO2的浓度变化。每个处理均重复3次。
Robot自动培养系统包括自动进样和气体分析模块。其中自动进样模块包括双向旋转的蠕动泵(Gilson Model 222,Gilson,法国) 和顶空自动采样器(CTC GC-Pal)。气体分析模块由气相色谱 (Agilent 7890A) 和氮氧化物分析仪 (Model 200E,美国) 组成。而气相色谱中包含热导检测器 (TCD)、电子捕获检测器 (ECD) 和火焰离子化检测器 (FID) 3个检测器,能够监测N2O (ECD、TCD),N2(TCD),CO2(TCD) 和O2(TCD) 气体浓度的变化。氮氧化物分析仪用来定量监测NO气体浓度。有关Robot自动培养系统的运行模块细节可参见Molstad等[28]和McMillan 等[29]的研究。
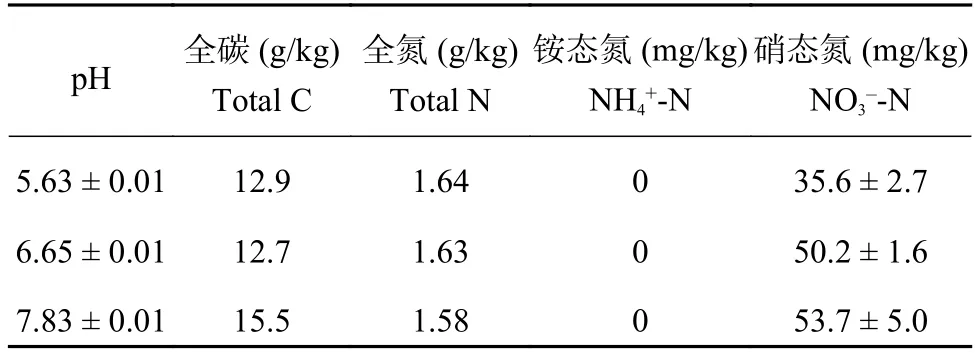
表1 不同土壤pH下初始理化性质Table 1 Initial soil physical and chemical properties with different soil pH
1.3 土壤基础参数测定
1.4 数据处理
气体数据计算根据在线监测的血清瓶中气体数值和已知标准气体浓度,计算相应气体每克干土的产生量。研究厌氧培养下反硝化产物比N2O/(N2O +NO + N2) 的变化,以计算N2O的产生量指数IN2O,计算公式如下:

其中:N2O (t)、N2(t)、NO (t) 均为培养时间t下的累积产生量。时间t的选择依据是以N2O产生速率为正值或N2O产生量达最大值时作为终止时间基准,即利用从初始培养至达到N2O最大累积产生量时的反硝化产物比均值进行比较。
试验数据采用SigmaPlot 12.5作图,利用SPSS 20.0分析软件进行单、双因素方差分析。
2 结果与分析
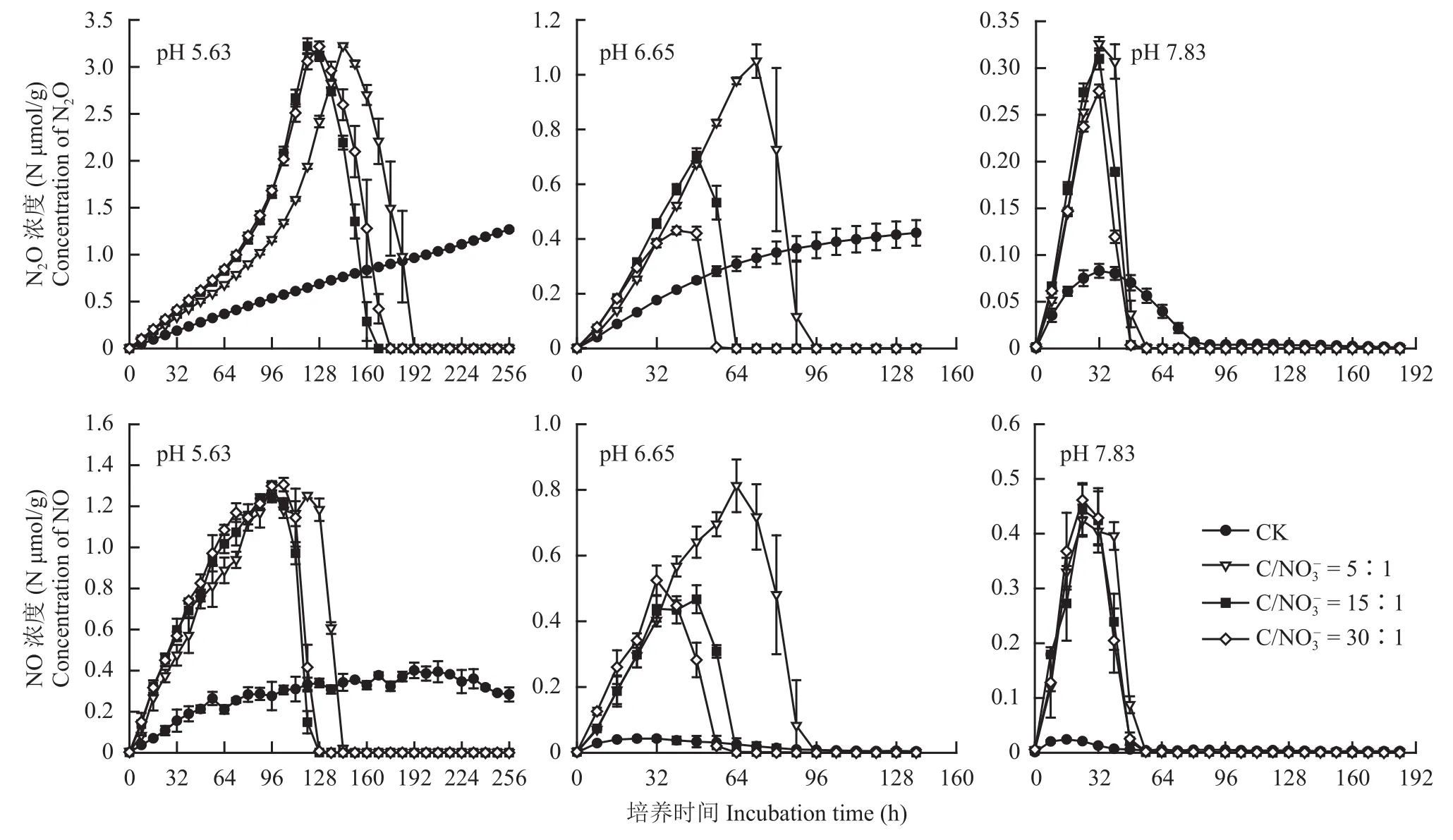
图1 不同pH和初始 下土壤N2O和NO产生量的动态变化Fig. 1 Dynamics of N2O and NO production during the anaerobic incubation from soil under different pH(acidic, neutral and alkaline) and levels
由图1整体来看,连续培养过程中土壤反硝化N2O产生量变化明显,且随土壤pH的增加N2O产生量显著减少。除未添加谷氨酸钠的对照处理 (CK)外,其它3个不同初始C/NO3–处理中N2O产生量的动态变化过程相类似,均呈现先增加达到峰值后再减少的趋势。在pH 5.63条件下,初始C/NO3–比为5∶1、15∶1和30∶1的处理中,N2O产生量分别在144、120和128 h时达到峰值,且三者均差异不显著 (图1)。在中性条件下 (pH 6.65),初始比为5∶1、15∶1和30∶1的处理中,N2O产生量分别在72、48和40 h时达到峰值,分别为N 1.05、0.71 和 0.43 μmol/g,三者差异极显著 (P< 0.01)。单从峰值来看,在土壤pH为7.83时,添加谷氨酸钠的3个处理均在连续培养32 h时达到峰值,且初始C/NO3–为30∶1的处理显著降低了N2O的排放 (P<0.05)。可见,中性和碱性土壤中添加有效性碳有利于减少反硝化过程N2O的产生,而酸性条件下添加有效性碳并不能降低N2O产生量。
与N2O类似,土壤反硝化NO的产生量也随土壤pH的增加而减少。在酸性条件下 (pH 5.63),添加谷氨酸钠的处理均在培养96 h后达到峰值,且差异不显著 (图1)。未添加谷氨酸酸钠的处理 (CK),NO的产生量较少且下降缓慢,导致其累积排放时间较长,这可能与土壤中可利用性碳不足导致缺乏电子供体有关。在pH为6.65的中性土壤条件下,初始为5∶1的处理在培养64 h时NO产生量达到峰值,为N 0.81 μmol/g,分别是初始为15∶1和30∶1处理峰值的1.74和1.55倍 (图1)。而在pH为7.83的碱性土壤中,NO的产生量均在培养24 h时达到峰值,且初始为 5∶1、15∶1 和30∶1的处理间NO产生量差异不显著。可见,酸性和碱性土壤中有机碳的添加对NO产生量不会产生显著影响,而在中性条件下有机碳含量会降低NO产生量。
由图2可知,随着培养时间的延长不同土壤pH下各处理的N2产生量均呈上升趋势,且添加谷氨酸钠后N2产生量均达到稳定状态。由其平衡的时间来看,随着土壤pH的增加N2O还原为N2的速率显著加快。除pH值为7.83的碱性土壤外,N2产生速率在pH为5.63的酸性土壤和pH为6.65的中性土壤中均随初始土壤C/NO3–的增加而增大。可见,在一定程度上增加土壤pH值并同时向土壤中添加可利用性碳可促进反硝化过程中N2O向N2的转化,有利于减少温室气体N2O的排放。

图2 不同pH和初始下土壤N2和CO2产生量的动态变化Fig. 2 Dynamics of N2 and CO2 production from three pH soils (acidic, neutral and alkaline) during the anaerobic incubation with different levels
碳源为反硝化微生物提供反应所需的电子供体和能源,CO2的产生量可间接反映微生物的活性。由图2可知,各pH土壤在添加有效性碳后,CO2产生量均显著高于对照处理 (CK),且在相同培养时间和初始下,CO2产生量随土壤pH的增加而显著增大 (P< 0.01)。酸性土壤中,连续培养256 h后对照处理CO2产生量为2.14 μmol/g,初始为5∶1、15∶1和30∶1处理的CO2产生量分别是对照处理的3.55、3.93和3.92倍 (图2)。在土壤pH为6.65条件下,连续培养136 h后,初始为5∶1处理的CO2产生量为5.69 μmol/g (图2),且与初始为15∶1和30∶1的处理均差异极显著 (P< 0.01)。碱性土壤中,在土壤连续培养112 h后,CO2产生量在添加有效性碳的不同处理间差异显著 (P< 0.05)。此外,随着pH增加,土壤的平均呼吸速率也明显增加。
由表3可知,当N2O达最大累积产生量时,土壤pH和分别对N2O产生量有极显著作用(P< 0.001),且二者交互效应极显著 (P< 0.001)。同时,整体来看,在不同初始下各土壤 pH 对N2O累积产生量影响显著 (P< 0.05)。而对于不同初始C/NO3–来说,与对照处理 (CK) 相比,添加有效性碳后,显著增加了土壤N2O的产生,但高比值 (30∶1) 的N2O最大累积量显著低于其他两个水平处理 (P< 0.05)。
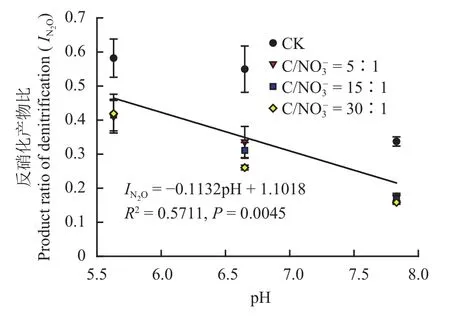
图3 不同初始下反硝化产物比和土壤pH的关系Fig. 3 Relationship between the product ratio of denitrification and soil pH with different initial levels
表2 不同pH和初始下厌氧培养土壤反硝化产物比 比较分析Table 2 Compared analysis for the product ratio of denitrification in soils under the anaerobic incubation with different pH and initial levels
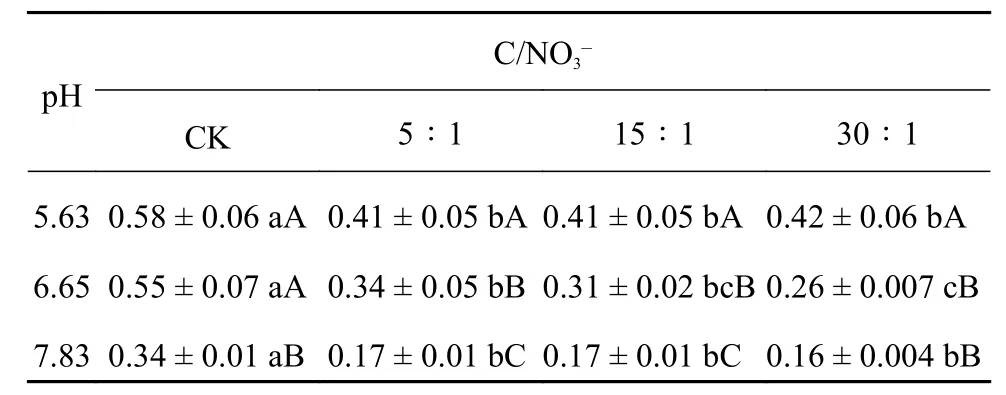
表2 不同pH和初始下厌氧培养土壤反硝化产物比 比较分析Table 2 Compared analysis for the product ratio of denitrification in soils under the anaerobic incubation with different pH and initial levels
注(Note):同行数据后不同小写字母表示 间差异显著 (P < 0.05),同列数据后不同大写字母表示 pH 间差异极显著 (P <0.01); 表中数值为平均值 ± 标准差 (n = 3)。 Values followed by different lowercase letters in a row indicate significant differences (P <0.05) among different ratios and capital letters in a column indicate significant differences (P < 0.01) among different pH; Values are mean ± SD (n = 3).
C/NO3–CK 5∶1 15∶1 30∶1 5.630.58 ± 0.06 aA0.41 ± 0.05 bA0.41 ± 0.05 bA0.42 ± 0.06 bA 6.650.55 ± 0.07 aA0.34 ± 0.05 bB0.31 ± 0.02 bcB0.26 ± 0.007 cB 7.830.34 ± 0.01 aB 0.17 ± 0.01 bC0.17 ± 0.01 bC 0.16 ± 0.004 bB pH
表3 土壤pH和初始对N2O产生量的因子分析Table 3 Multivariate analysis of variance for the effects of pH and initial on the N2O production
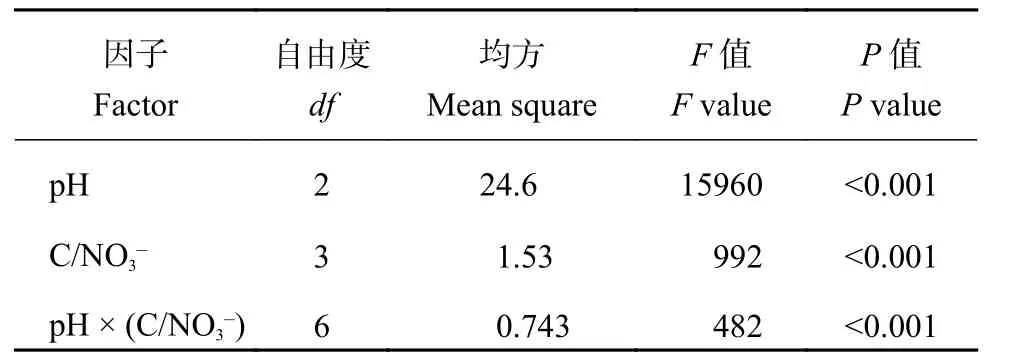
表3 土壤pH和初始对N2O产生量的因子分析Table 3 Multivariate analysis of variance for the effects of pH and initial on the N2O production
P 值P value pH 2 24.6 15960 <0.001 C/NO3– 3 1.53 992 <0.001 pH × (C/NO3–) 6 0.743 482 <0.001因子Factor自由度df均方Mean square F 值F value
3 讨论
土壤pH值是影响反硝化作用特征的重要因素。在低pH值条件下 (pH 5.63),对照处理 (CK) 的氮素气态产物以N2O和NO为主,这是由于低pH阻碍了N2O还原酶的形成[30]。同时,基于在Paracoccus denitrificans上研究的结果,低pH也会干扰周质空间下的蛋白组装[31]。本研究发现,不同初始下土壤N2O产生量指数随pH的增加显著下降,且二者呈极显著线性负相关关系 (图3),这与Liu等[32]和Qu等[23]的研究结果类似。同时,在碱性土壤和中性土壤中添加有效碳后,反硝化作用N2O产物比值 (0.16~0.34) 均低于Schlesinger等[21]汇总的农田土壤反硝化产物比结果 (0.37),而在酸性土壤中有无添加有效性碳N2O产物比均较高 (0.41~0.58)。由于土壤pH对反硝化N2O产物比的影响不可忽视,因而,对不同pH的土壤仅用单一的产物比并结合N2O排放通量估算土壤反硝化的总损失量存在较大的误差。
NO是反硝化作用过程的中间产物,同时也是编码亚硝酸盐还原酶 (NIR) 和一氧化氮还原酶 (NOR)转录的诱导因子[33]。Bergaust等[33]发现,在模式菌株P. denitrificans上NO还可以诱导nosZ基因的转录。本研究结果表明,NO的最大累积量在低pH土壤 (pH 5.63) 中显著高于其他pH处理 (图1),且在土壤溶液中NO浓度的最大值为6.2 μmol/L (数据未显示)。这是由于NO在低pH条件下的产生速率大于其消耗速率,还可能与低pH下的微生物会优先将硝酸盐完全还原为亚硝酸盐,而不是还原亚硝酸盐有关。此外,在低pH土壤条件下,尤其在土壤亚硝态氮出现累积时,化学分解反应等非生物因素对NO的排放贡献也不容忽视[34],还需进一步探究。
与对照相比 (CK),各pH土壤在添加谷氨酸钠后均显著增加了反硝化速率,且土壤反硝化速率的最大值也随有效性碳含量的增加而增大,这与Jahangir等[35]的研究结果相一致。原因可能是有效性碳增加了土壤nir和nosZ的基因拷贝浓度[36],促进了反硝化微生物酶的活性[37]。同时,可利用性碳源的添加也会增加细胞的呼吸作用,显著促进CO2的排放,且随土壤pH值的增加呈上升趋势 (图2)。在同一初始比土壤下,土壤pH的调节对N2O和CO2两种温室气体的排放存在某种程度上的“此消彼长”效应 (trade-off effect)。增加土壤pH后,N2O产生量明显减少的同时CO2的产生量也会显著增加,这可能与提高土壤pH后促进了土壤碳、氮的矿化有关[38]。
虽然在低pH条件下,有效碳的添加对反硝化N2O的产物比没有影响,但在中性和石灰性土壤上,碳的有效性显著影响该产物比。因此,在利用N2O排放通量来估算土壤反硝化损失时,活性有机碳含量是应该予以重视的一个重要因素。
表4 培养结束后不同pH和初始下铵态氮及硝态氮含量及气态氮素损失总量Table 4 Concentrations of ammonium and nitrate and the total N gaseous loss in the end of the incubation
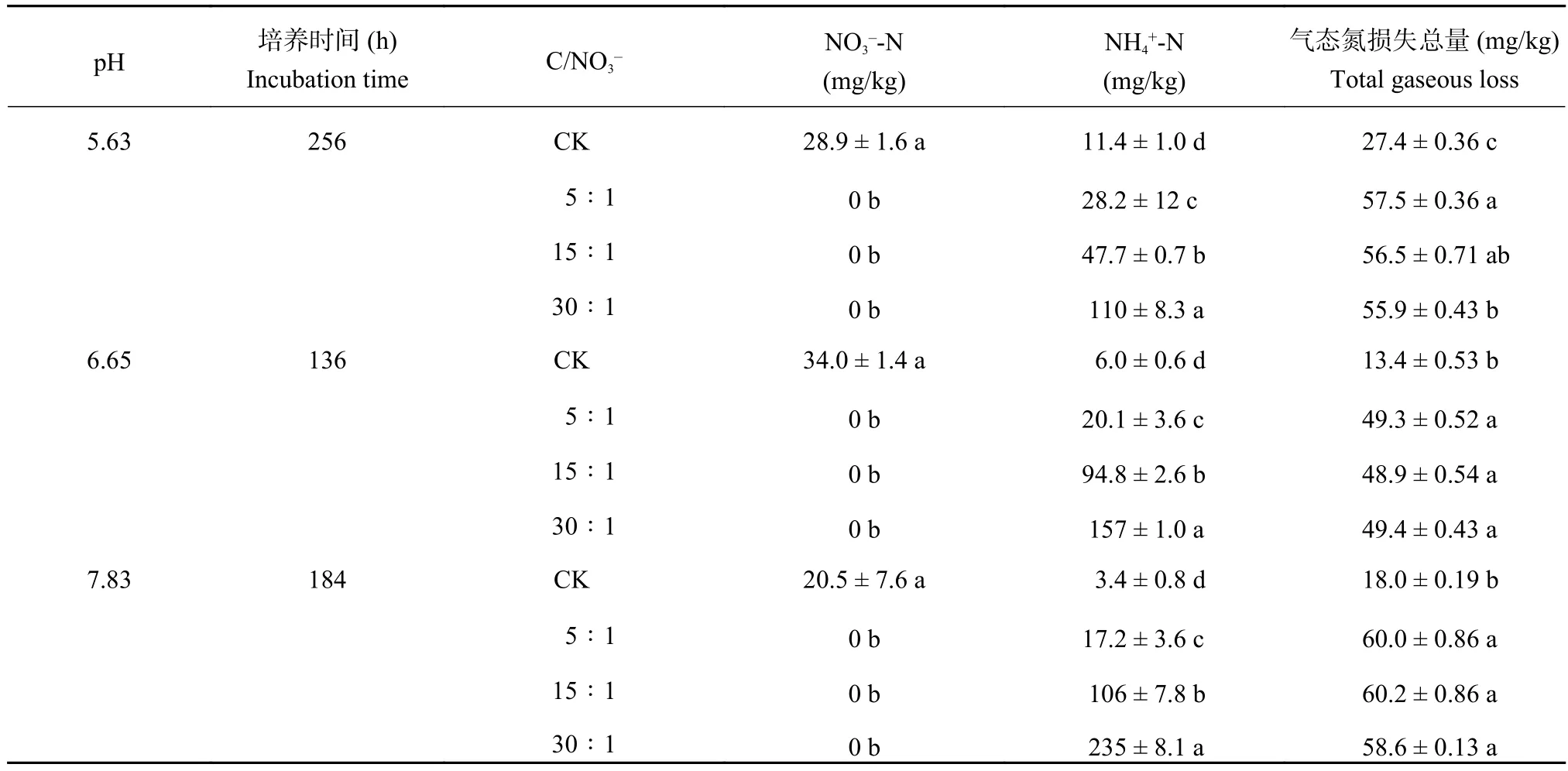
表4 培养结束后不同pH和初始下铵态氮及硝态氮含量及气态氮素损失总量Table 4 Concentrations of ammonium and nitrate and the total N gaseous loss in the end of the incubation
注(Note):同列数据后不同小写字母表示相同 pH 值不同间差异显著 (P < 0.05) Values followed by different lowercase letters in a column indicate significant differences (P < 0.05) among different ratios; 表中数值为平均值 ± 标准差 (n = 3) Values are mean ± SD (n = 3).
气态氮损失总量 (mg/kg)Total gaseous loss 5.63 256 CK 28.9 ± 1.6 a 11.4 ± 1.0 d 27.4 ± 0.36 c 5∶1 0 b 28.2 ± 12 c 57.5 ± 0.36 a 15∶1 0 b 47.7 ± 0.7 b 56.5 ± 0.71 ab 30∶1 0 b 110 ± 8.3 a 55.9 ± 0.43 b 6.65 136 CK 34.0 ± 1.4 a 6.0 ± 0.6 d 13.4 ± 0.53 b 5∶1 0 b 20.1 ± 3.6 c 49.3 ± 0.52 a 15∶1 0 b 94.8 ± 2.6 b 48.9 ± 0.54 a 30∶1 0 b 157 ± 1.0 a 49.4 ± 0.43 a 7.83 184 CK 20.5 ± 7.6 a 3.4 ± 0.8 d 18.0 ± 0.19 b 5∶1 0 b 17.2 ± 3.6 c 60.0 ± 0.86 a 15∶1 0 b 106 ± 7.8 b 60.2 ± 0.86 a 30∶1 0 b 235 ± 8.1 a 58.6 ± 0.13 a pH 培养时间 (h)Incubation time C/NO3– NO3–-N(mg/kg)NH4+-N(mg/kg)
[ 1 ]Morley N, Baggs E M, Dörsch P,et al. Production of NO, N2O and N2by extracted soil bacteria, regulation by NO2–and O2concentrations[J]. Federation of European Microbiological Societies Microbiology Ecology, 2008, 65: 102–112.
[ 2 ]Wan S, Ward T L, Altosaar I. Strategy and tactics of disarming GHG at the source: N2O reductase crops[J]. Trends in Biotechnology, 2012,30(8): 410–415.
[ 3 ]Thomson A J, Giannopoulos G, Pretty J,et al. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions[J].Philosphical Transactions of the Royal Society Biological Sciences,2012, 367: 1157–1168.
[ 4 ]Kartal B, Tan N C G, Van de Biezen E,et al. Effect of nitric oxide on anammox bacteria[J]. Applied and Environmental Microbiology,2010, 76(18): 6304–6306.
[ 5 ]Bergaust L, Bakken L R, Frostegård Å. Denitrification regulatory phenotype, a new term for the characterization of denitrifying bacteria[J]. Biochemical Society Transactions, 2011, 39: 207–212.
[ 6 ]Zhang Y, Lin F, Jin Y,et al. Response of nitric and nitrous oxide fluxes to N fertilizer application in greenhouse vegetable cropping systems in southeast China[J]. Scientific Reports, 2016, 6: 20700.
[ 7 ]董红敏, 李玉娥, 陶秀萍,等. 中国农业源温室气体排放与减排技术对策[J]. 农业工程学报, 2008, 24(10): 269–273.Dong H M, Li Y E, Tao X P,et al. China greenhouse gas emissions from agricultural activities and its mitigation strategy[J]. Transactions of the CSAE, 2008, 24(10): 269–273.
[ 8 ]Bouwman A F, Boumans L J M, Batjes N H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data[J]. Global Biogeochemical Cycles, 2002, 16(4): 1–13.
[ 9 ]Zhu J H, Li X L, Christie P,et al. Environmental implications of low nitrogen use efficiency in excessively fertilized hot pepper (Capsicum frutescensL.) cropping systems[J]. Agriculture, Ecosystems and Environment, 2005, 111(1): 70–80.
[10]Cui F, Yan G X, Zhou Z X,et al. Annual emissions of nitrous oxide and nitric oxide from a wheat-maize cropping system on a silt loam calcareous soil in the North China Plain[J]. Soil Biology and Biochemistry, 2012, 48: 10–19.
[11]Ding W X, Cai Y, Cai Z C,et al. Nitrous oxide emissions from an intensively cultivated maize-wheat rotation soil in the North China Plain[J]. Science of the Total Environment, 2007, 373(2): 501–511.
[12]Pang X B, Mu Y J, Lee X,et al. Nitric oxides and nitrous oxide fluxes from typical vegetables cropland in China: Effects of canopy,soil properties and field management[J]. Atmospheric Environment,2009, 43(16): 2571–2578.
[13]余海英, 李廷轩, 张锡洲. 温室栽培系统的养分平衡及土壤养分变化特征[J]. 中国农业科学, 2010, 43(3): 514–522.Yu H Y, Li T X, Zhang X Z. Nutrient budget and soil nutrient status in greenhouse system[J]. Scientia Agricultura Sinica, 2010, 43(3):514–522.
[14]宋效宗. 保护地生产中硝酸盐的淋洗及其对地下水的影响[D]. 北京: 中国农业大学博士学位论文, 2007.Song X Z. Nitrate leaching and its effects on groundwater in intensive vegetable cropping systems in Northern China [D]. Beijing: PhD Dissertation of China Agricultural University, 2007.
[15]Cao B, He F Y, Xu Q M,et al. Denitrification losses and N2O emissions from nitrogen fertilizer applied to a vegetable field[J].Pedosphere, 2006, 16(3): 390–397.
[16]Zhu T, Zhang J, Cai Z. The contribution of nitrogen transformation processes to total N2O emissions from soils used for intensive vegetable cultivation[J]. Plant and Soil, 2011, 343(1–2): 313–327.
[17]Cui S H, Shi Y L, Groffman P M,et al. Centennial-scale analysis of the creation and fate of reactive nitrogen in China (1910—2010)[J].Proceedings of the National Academy of Sciences, 2013, 110:2052–2057.
[18]Schlesinger W H. On the fate of anthropogenic nitrogen[J].Proceedings of the National Academy of Sciences, 2009, 106:203–206.
[19]Guo J H, Liu X J, Zhang Y,et al. Significant acidification in major Chinese croplands[J]. Science, 2010, 327(5968): 1008–1010.
[20]王敬国. 设施菜田退化土壤修复与资源高效利用[C]. 北京: 中国农业大学出版社, 2011.Wang J G. Management of degraded vegetable soils in greenhouses[C]. Beijng: China Agricultural University Press, 2011.
[21]Čuhel J, Šimek M. Proximal and distal control by pH of denitrification rate in a pasture soil[J]. Agriculture, Ecosystems and Environment, 2011, 141(1): 230–233.
[22]Šimek M, Cooper J E. The influence of soil pH on denitrification:progress towards the understanding of this interaction over the last 50 years[J]. European Journal of Soil Science, 2002, 53(3): 345–354.
[23]Qu Z, Wang J, Almøy T,et al. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils[J]. Global Change Biology, 2014, 20(5): 1685–1698.
[24]田静, 郭景恒, 陈海清, 等. 土地利用方式对土壤溶解性有机碳组成的影响[J]. 土壤学报, 2011, 48(2): 338–346.Tian J, Guo J H, Chen H Q,et al. Effect of land use on composition of soil dissolved organic carbon[J]. Acta Pedologica Sinica, 2011,48(2): 338–346.
[25]Baral K R, Arthur E, Olesen J E,et al. Predicting nitrous oxide emissions from manure properties and soil moisture: An incubation experiment[J]. Soil Biology and Biochemistry, 2016, 97: 112–120.
[26]He F F, Jiang R F, Chen Q,et al. Nitrous oxide emissions from an intensively managed greenhouse vegetable cropping system in Northern China[J]. Environmental Pollution, 2009, 157(5):1666–1672.
[27]张瑜. 中国农田土壤酸化现状、原因与敏感性的初步研究[D]. 北京: 中国农业大学硕士学位论文, 2009.Zhang Y. Preliminary study on acidification in Chinese agricultural soils: current status, potential reason and sensitivity [D]. Beijing: MS Thesis of China Agricultural University, 2009.
[28]Molstad L, Dörsch P, Bakken L R. Robotized incubation system for monitoring gases (O2, NO, N2O, N2) in denitrifying cultures[J].Journal of Microbiological Methods, 2007, 71(3): 202–211.
[29]McMillan A, Phillips R, Berben P,et al. Automated N2O/N2analysis—a new tool for studying denitrification dynamics and testing mitigation strategies[A]. Flrc Worshop. Nutrient Management for the Farm, Catchment and Community[C]. 2014: 92.
[30]Liu B, Frostegård Å, Bakken L R. Impaired reduction of N2O to N2in acid soils is due to a posttranscriptional interference with the expression ofnosZ[J]. Mbio, 2014, 5(3): e01383–14.
[31]Bergaust L, Mao Y, Bakken L R,et al. Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrogen oxide reductase inParacoccus denitrificans[J]. Applied and Environmental Microbiology, 2010, 76(19): 6387–6396.
[32]Liu B, Mørkved P T, Frostegård Å,et al. Denitrification gene pools,transcription and kinetics of NO, N2O and N2production as affected by soil pH[J]. Fems Microbiology Ecology, 2010, 72(3): 407–417.
[33]Bergaust L, van Spanning R R J M, Frostegård Å,et al. Expression of nitrous oxide reductase inParacoccus denitrificansis regulated by oxygen and nitric oxide through FnrP and NNR[J]. Microbiology,2012, 158: 826–834.
[34]Van Cleemput O, Samater A H. Nitrite in soils: accumulation and role in the formation of gaseous N compounds[J]. Fertilizer Research,1995, 45(1): 81–89.
[35]Jahangir M M R, Khalil M I, Johnston P,et al. Denitrification potential in subsoils: a mechanism to reduce nitrate leaching to groundwater[J]. Agriculture, Ecosystems and Environment, 2012,147: 13–23.
[36]Barrett M, Khalil M I, Jahangir M M R,et al. Carbon amendment and soil depth affect the distribution and abundance of denitrifiers in agricultural soils[J]. Environmental Science and Pollution Research,2016, 23(8): 7899–7910.
[37]Shannon K E M, Saleh–Lakha S, Burton D L,et al. Effect of nitrate and glucose addition on denitrification and nitric oxide reductase(cnorB) gene abundance and mRNA levels inPseudomonas mandeliiinoculated into anoxic soil[J]. Antonie Van Leeuwenhoek, 2011,100(2): 183–195.
[38]Baggs E M, Smales C L, Bateman E J. Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil[J]. Biology and Fertility of Soils, 2010, 46(8): 793–805.
[39]宋贺, 王成雨, 陈清, 等. 长期秸秆还田对设施菜田土壤反硝化特征和 N2O 排放的影响[J]. 中国农业气象, 2014, 35(6): 628–634.Song H, Wang C Y, Chen Q,et al. Effects of long-term amendment of residue on denitrification characteristics and N2O emissions in greenhouse soil[J]. Chinese Journal of Agrometeorology, 2014,35(6): 628–634.
[40]Saggar S, Jha N, Deslippe J,et al. Denitrification and N2O:N2production in temperate grasslands: processes, measurements,modelling and mitigating negative impacts[J]. Science of the Total Environment, 2013, 465: 173–195.
[41]Obia A, Cornelissen G, Mulder J,et al. Effect of soil pH increase by biochar on NO, N2O and N2production during denitrification in acid soils[J]. PloS One, 2015, 10(9): e0138781.
Effects of pH and initial labileratio on denitrification in a solar greenhouse vegetable soil
CAO Wen-chao1, GUO Jing-heng1, SONG He2, LIU Su1, CHEN Ji-ji1, WANG Jing-guo1*
(1 College of Resource and Environment, China Agricultural University, Beijing 100193, China;2 College of Agronomy, Anhui Agricultural University, Hefei, Anhui 230036, China)
【Objectives】Denitrification is one of predominant process for N2O emission from solar greenhouse vegetable soils. An anoxic incubation experiment was conducted to explore effects of soil pH and initial labileratio on nitrogen gaseous emissions (i.e. N2O, NO and N2) and the N2O product during the denitrification in a solar greenhouse vegetable soil.【Methods】A typical greenhouse vegetable soil in Shouguang City was selected for the study, and soil pH was adjusted to acidic, neutral and alkaline by adding a certain amount of lowconcentration of acid (0.1 mol/L HCl) or alkaline (0.1 mol/L NaOH) solution. The final soil pH values were 5.63,6.65 and 7.83 (adjusted), respectively. Sodium glutamate (C5H8NO4Na) was added as a labile organic carbon source, and the ratios of initial labile C towere adjusted to 5∶1, 15∶1 and 30∶1 by adding different amounts of sodium glutamate and no sodium glutamate addition in the control (CK). Four levels of initial labile C/NO3–ratios were set up in the three soils with different pH, and there were three replicates in each level. A robotized incubation system was used to monitor the kinetics of gaseous products (N2O, NO, N2, and CO2) of denitrification under anoxic conditions and to estimate the N2O product ratio of denitrification by calculating Index N2O/(N2O + NO + N2).【Results】The increase in soil pH significantly reduced N2O and NO production in the soil, and the peak values of N2O and NO production in the acidic soil were significantly higher than those of the alkaline and neutral soils with different initial C/NO3–ratios (P< 0.05). With the increase of initial labile C, the production of N2O was reduced in the neutral and alkaline soils, but kept unchanged in the acidic soil. The addition of sodium glutamate reduced NO emission in the neutral soil. However, there were no differences in NO production in other pH levels. A significant interaction on N2O production was observed between soil pH and initial labileratio (P< 0.001). The N2production rate significantly increased with sodium glutamate addition in the acidic and neutral soils (P< 0.05). The addition of sodium glutamate could promote the reduction of N2O to N2in denitrification process at different soil pH. The CO2production was significantly higher in the alkaline soil than those in the acidic and neutral soils. As compared with CK, the CO2production increased significantly with the sodium glutamate addition at different soil pH (P< 0.05). The N2O product ratio in the acidic soil was significantly higher than that in the alkaline soil under different initial labile(P< 0.01), the N2O product ratio was significantly decreased with the increase of soil pH, and a significant linear negative correlation relationship was observed between soil pH and the Index N2O (P< 0.01).【Conclusions】High N2O and NO emissions were usually found in greenhouse vegetable soils, primarily due to the decline of soil pH.Furthermore, increase in labile C availability promoted the N loss of denitrification, and decreased N2O production in neutral and alkaline soils. With the increase of both soil pH and labile, the N2O product ratio reduced,but the denitrification activity increased. Both soil pH and carbon availability were crucial factors for estimation of N loss of denitrification by multiplying N2O flux and N2O product ratio.
denitrification; pH;nitrous oxide; solar greenhouse
2017–02–27 接受日期:2017–04–04
国家自然科学基金项目(41230856,41301258)资助。
曹文超(1986—),男,山东郓城人,博士研究生,主要从事土
壤氮素转化及其环境效应方面的研究。E-mail:caochaoqun66@163.com。 * 通信作者 E-mail:wangjg@cau.edu.cn

