多环芳烃典型电子性质与其大型蚤光致毒性的构效关系研究
谷成刚,朱梦荣, 2,刘畅, 2,提清清, 2,何欢,孙成, #,蒋新, *
1. 中国科学院南京土壤研究所,土壤环境与污染修复重点实验室,南京 2100082. 中国科学院大学,北京 1000493. 南京大学环境学院,污染控制与资源化研究国家重点实验室,南京 210023
多环芳烃典型电子性质与其大型蚤光致毒性的构效关系研究
谷成刚1,朱梦荣1, 2,刘畅1, 2,提清清1, 2,何欢3,孙成3, #,蒋新1, *
1. 中国科学院南京土壤研究所,土壤环境与污染修复重点实验室,南京 2100082. 中国科学院大学,北京 1000493. 南京大学环境学院,污染控制与资源化研究国家重点实验室,南京 210023
多环芳烃(PAHs)是环境中广泛分布的持久性有毒有机污染物,备受研究者关注。基于密度泛函理论(DFT)先期计算PAHs前线分子轨道能隙可能与其光致毒性诱发所需吸收光照辐射能有一致性,本研究选取非取代PAHs对大型蚤(Daphnia magna)光致毒性实验数据,通过DFT计算典型电子性质,由偏最小二乘(PLS)分析方法优化发展了定量构效关系模型,经与前人结果比较和验证其拟合优度、稳定性和内外部预测性能均有显著提升,可在应用域(AD)范围内准确预测PAHs光致毒性而满足风险评估需求。构效关系分析结果表明,PAHs光致毒性与分子前线轨道能隙紧密相关,除苯并[k]荧蒽和屈可能具有不同的光致毒性作用机制之外,多数PAHs若具有较低的前线轨道能隙、较小分子稳定性和较大分子变形性,均将有利于促进其光致毒性作用的发生;结合PAHs光致毒性与分子前线轨道能隙间的相关关系,可推测DFT计算前线轨道能隙宽度在2.740~4.208 eV之间和对应光照辐射波段约为295 nm~450 nm时,PAHs污染暴露将可能诱发较高的光致毒性效应。这为太阳光照射下PAHs光致毒性作用机制阐释和风险评价提供了数据支持与理论依据。
多环芳烃;大型蚤;光致毒性;密度泛函理论方法;电子性质;构效关系
Received3 January 2017accepted8 March 2017
Abstract: Polycyclic aromatic hydrocarbons (PAHs) pertain to the category list of persistent toxic substances which are ubiquitously detected in the environment and thereby arouse much concerns of scientific community. Based on the possible coherence of solar irradiation with the energy gap of molecular frontier orbitals by preliminary computation of density functional theory (DFT), in this study the experimental phototoxicity of PAHs to Daphnia magna was selected in priori, and the corresponding quantitative structure-activity relationship (QSAR) between the typically DFT-calculated electronic properties and phototoxicity was carefully developed by partial least square (PLS) analyses. After critical validation and comparison with previous studies, the goodness of fitting, robustness and internal or external predictability was clearly enhanced to a certain degree. Thus, within the decided applicability domain (AD) the QSAR was suggested to serve as precise prediction tool to meet the demand of risk assessment. QSAR analysis indicated the tight correlation relationship of phototoxicity with energy gap of molecular frontier orbitals. And with the exception of benzo[k]fluoranthene and chrysene for different photoinduced toxicological mechanism, it is suitable for the great majority of PAHs that the lower energy gap of molecular frontier orbitals, the less molecular stability and higher deformability shall favor the occurrence of phototoxicity. In virtue of the correlation between energy gap of frontier orbitals and phototoxicity, the span of energy gap 2.740-4.208 eV by DFT and the predicted corresponding wave spectrum of solar irradiation within about 295 nm-450 nm was proposed as the necessity of PAHs exposure to induce the higher phototoxicity. The study could be anticipated for providing data framework and theoretical guideline for phototoxicological mechanism illumination and risk assessment of PAHs under solar irradiation.Keywords: PAHs; Daphnia magna; phototoxicity; density functional theory; electronic properties; QSARs
多环芳烃(PAHs)是由2个及2个以上的苯环以稠环方式相连接的化合物,主要来自于石油、煤炭等人类工业活动所需材料的不完全燃烧和火山喷发、森林火灾以及高等植物的合成释放等天然因素,在生物与非生物环境介质中均有分布[1-4]。由于PAHs具有典型的致癌、致畸、致突变“三致”效应、生殖毒性和环境持久性[5-8],已成为威胁生态系统安全和人体健康的重要有机污染物,其早在20世纪80年代就被美国环境保护署(EPA)同等列为优先控制污染物中的一类[9],一直以来备受各国研究者的普遍关注。
PAHs因其稠环电子结构形成共轭大Π键,能够在光照作用下吸收可见或紫外光波段而引发一系列的光化学反应,如通过光修饰作用转化为毒性更大的产物,或通过生成自由基、单线态氧而造成对各种生物大分子(如核酸、脂质或蛋白质等)的损伤,整体表现出光致毒性。于20世纪中期,就有研究指出PAHs对哺乳动物、水生生物等都能表现出光致毒性效应[10],而后续的研究也逐渐深入广泛,开展了以大型蚤(Daphnia magna)[11-14]、发光菌(Vibrio fischeri)[15-16]和绿藻(Scenedesmus vacuolatus)[17]等为指示生物的实验研究,提出了相应的光致毒性作用机制,为PAHs的生物效应和风险评估提供了科学依据。研究表明,在120 μW·cm-2的UV-A(320 nm~400 nm)和25 μW·cm-2的UV-B(290 nm~320 nm)紫外辐射作用下,PAHs,如蒽能够与DNA大分子共价结合而诱发对典型水生生物-大型蚤(Daphnia magna)的光致毒性效应[11, 18]。为进一步揭示光致毒性作用机制,前人已从PAHs电子结构和分子光谱特征等参数化表征出发,发展了与其光致毒性之间的定量构效关系(QSARs),并在光敏化和光修饰因子的基础上提出了双向作用机制[19-22]。Newsted等[11]基于杂原子取代和非取代PAHs对大型蚤的光致急性毒性,以最低单重激发态和三重激发态能量发展了非线性构效关系。兼顾PAHs对光的吸收效率、水溶液中稳定性、辐射源能量与光照密度等因素间的竞争关系,Mekenyan等[23]在半经验分子轨道计算方法AM1和PM3结构优化的基础上,探讨了分子前线轨道能隙(EGAP)与大型蚤光致毒性间的相关关系,继而修正了非线性构效关系,并预测PAHs分子前线轨道能隙在7.2±0.4 eV时,可诱发对大型蚤的光致毒性。由于PAHs分子前线轨道能隙宽时,能量足够大的光子才有可能激发前线分子轨道的电子发生跃迁、引发光化学反应而表现出光致毒性,然而(模拟)太阳光照的紫外短波段可能无法接触环境界面,实际引发PAHs的光致毒性并不显著;相比之下,PAHs分子前线轨道能隙窄时,分子稳定性差、易降解,实际引发PAHs的光致毒性也较小。为此,Ferreira[24]建立了PAHs分子前线轨道能隙与其光致毒性的Gaussian型函数关系,并在后来将分子前线轨道能隙宽度由7.2±0.4 eV扩大到7.2±0.7 eV用于预测大型蚤光致毒性[25]。分子前线轨道能隙是外层电子发生跃迁和转移的屏障,是分子稳定性和反应性的电子结构基础。然而,比较不同PAHs光致毒性效应所对应的光照辐射波长(小于5.0 eV)和半经验分子轨道计算方法AM1计算的前线轨道能隙,可发现AM1计算前线轨道能隙接近光照辐射能量的2倍,如表1,这可能源于电子相关处理的缺失。相对而言,密度泛函理论方法(DFT)能够以泛函形式考量电子相关问题,先期计算结果表明,DFT计算PAHs分子前线轨道能隙和光致毒性效应所对应的光照辐射能量是相近的(见表1)。因此,基于DFT对PAHs电子结构和能量计算表征,减少半经验分子轨道计算方法带来的误差,可能有助于提高PAHs光致毒性构效关系及其预测准确性。
本文利用DFT三参数混合泛函理论方法对PAHs电子结构优化计算,并在基态电子结构与能级参数Gaussian型函数关系转化的基础上,由偏最小二乘分析(PLS)方法[26-27]优化发展PAHs对大型蚤(Daphnia magna)[23]光致毒性效应的定量构效关系,以期在相关模型内外部预测性能得以显著提升的前提下,在相似结构应用域(AD)内用于准确预测PAHs光致毒性效应并阐释相关分子作用机制。相关工作开展,将为PAHs水生生态光致毒性效应评价提供数据基础和科学依据。
1 材料与方法(Materials and methods)
1.1 PAHs电子结构优化计算


(1)

表1 PAHs光吸收能量与不同前线轨道能隙的比较Table 1 Comparison between the absorbed solar energy and the calculated energy gap of frontier orbitals of PAHs by AM1 and DFT respectively
注:PAHs对大型蚤光致毒性的实测吸收波长和AM1计算PAHs的EGAP分别来自文献[11, 25];所采纳DFT计算方法为B3LYP/6-311G**。
Note: Data of wave length absorbed for PAHs phototoxicity to Daphnia magna, and EGAPcomputed by AM1 referenced from [11, 25]; DFT method was employed as B3LYP/6-311G**.
η=(ELUMO-EHOMO)/2=EGAP/2
(2)
绝对硬度(η)表示分子体系的稳定性和反应进行的难易程度:η越大,分子越“硬”,电子从最高占有轨道向最低未占轨道的跃迁和电子转移越不容易发生;反之,分子体系越“软”,电子跃迁越容易发生[35]。有研究表明,绝对硬度与分子极化率具有一定相关性,反映了分子在外加电场下外层电子云的变形性[37-38]。PAHs在光照辐射或与生物受体分子作用时,电子转移的潜势可由电子转移量予以表征,因此分子体系能量下降最大化情况下的电子转移量,即最大电子转移量(ΔNmax)可由式(3)计算求得。
ΔNmax=(ELUMO+EHOMO)/(EHOMO-ELUMO)
(3)
考虑PAHs电子性质参数在后续构效关系中的统计学显著性,主要将电子轨道能级和绝对硬度及其相关参数列于表2。
1.2 数据来源与模型建立方法
为对比研究PAHs对大型蚤光致毒性的分子前线轨道能隙预测宽度,本文选取17种非取代PAHs对水生生物-大型蚤(Daphnia magna)光致毒性实验数据(Exp.)[23],以调整半数致死时间ALT50(S)为毒理学指标,表示为对数形式-logALT50(pALT50)。pALT50越大,PAHs对大型蚤的光致毒性效应越显著。相关数据见表2。


表2 PAHs电子性质参数及其光致毒性实测、预测值和相关残差Table 2 PAHs electronic properties, and the experimental, predicted and residuals of corresponding phototoxicity
注:*标示为PAHs光致毒性构效关系的测试集;PAHs电子性质参数单位:eV。
Note: Test set labeled by asterisks for QSAR of PAHs phototoxicity; Electronic properties of PAHs in eV.
(4)


(5)
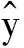

2 结果(Results)
2.1 PAHs前线轨道能级/能隙与光致毒性的相关性


图1 PAHs大型蚤光致毒性与前线轨道能级/能隙的相关性Fig. 1 Correlation between energy levels/energy gap of frontier molecular orbitals and phototoxicity of PAHs to Daphnia magna
pALT50= -3.291 +0.336η' +0.299E'LUMO+0.283E'HOMO
(6)


图2 PAHs大型蚤光致毒性预测值与实测值的比较关系Fig. 2 Comparisons between the predicted and experimental phototoxicity of PAHs to Daphnia magna
构效关系的应用域AD表征如图3所示。由图可以看出,PAHs电子结构信息的杠杆值h均小于临界h*(1.0),说明构效关系模型在具有相似稠环结构的应用域内能够准确预测PAHs的光致毒性,且荧蒽(14#)相较而言具有较高的杠杆值,属于高杠杆样本点,表明其四元环分子结构信息对构效关系发展具有显著影响。就预测残差而言,95%置信区间内所有PAHs光致毒性的预测残差绝对值均低于2.5δ,说明构效关系能够给予相对准确的PAHs光致毒性预测值。若究其严格的±2δ标准化残差区间,屈(3#)和苯并[k]荧蒽(15#)将分别界定于残差区间之外,分别属于被低估和高估的奇异值。然而,就PAHs光致毒性相关构效关系的预测性能而言,在±2.5δ标准化残差区间范围内其准确性在总体上仍可以满足风险评估的具体需求。

图3 PAHs光致毒性Williams图Fig. 3 Williams plot sketched for phototoxicity of PAHs
2.2 PAHs光致毒性构效关系的性能比较

3 讨论(Discussion)
为定量评估PAHs电子性质参数对其光致毒性变异解释的贡献和相对大小,计算了电子性质参数在PLS提取主成分上的相对权重(W*)及其VIPs值。本研究构效关系引入并保留了3个性质参数,其在第一主成分上的相对权重W*和VIPs参见柱状图4。与分子绝对硬度有关的η'具有最大权重(0.603)和最高VIP(1.04),结合光致毒性与η'之间的正相关关系,即较高的η'能够诱发较高的光致毒性,表明PAHs基态分子的前线轨道能隙和分子稳定性对PAHs光致毒性具有重要影响,只是影响关系并非线性;尽管有较高前线轨道能隙和分子稳定性的PAHs能够诱发光致毒性效应,但对于大多数PAHs化合物而言,在一定范围内较小的前线轨道能隙和较低的分子稳定性,才能促使光致毒性发生,这可能是由于PAHs较小的前线轨道能隙有利于光照能量的吸收而发生光敏化反应。由于绝对硬度与分子的极化率、分子体积具有一定相关性[37-38],绝对硬度越小、极化率和分子体积越大,核外电子在外加或邻近分子电场作用下的变形性越大,使得分子正、负电荷重心不断发生瞬间相对位移并产生瞬间偶极,从而愈发有利于非极性或极性分子之间的瞬间偶极-瞬间偶极相互吸引作用,即色散作用。这与前人分析结果具有较好的一致性[46]。相比之下,E'LUMO和E'HOMO的权重(W*,0.568和0.560)和VIP(0.98,0.97)略小;由于ELUMO和EHOMO分别与前线分子轨道得失电子有关,其包涵的电子结构能级排布特征信息,在一定程度上可与前线轨道能隙紧密相关。

图4 PAHs电子性质参数的相对权重与VIPs比较Fig. 4 Comparisons of relative weights and VIPs among electronic properties of PAHs
前人分别根据PAHs光致毒性的预测值与实测值,将PAHs划分为高等、中等和低等光致毒性暴露污染物,如菲、芴和三亚苯具有相对较低的光致毒性,其调整半数致死时间pALT50远小于-2.95[23, 25]。因此,pALT50等于-2.95成为定量评估PAHs光致毒性大小的“分水岭”,大于该值则表明PAHs能够诱发较高或中等光致毒性。以本研究所取得构效关系对非取代PAHs光致毒性进行预测(表2),且将预测值与转换绝对硬度η'进行线性拟合,得到拟合方程。由于茚并[1,2,3-cd]芘(20#)电子结构杠杆值h较大(4.557),表明其与其他PAHs化合物的结构信息质点距离较远,电子结构具有较大的差异。为此,在排除20#化合物的基础上,PAHs光致毒性预测值与η'的线性相关性由原来的R2为0.956提高到0.978,如图5所示。当pALT50大于-2.95时,可以计算出相对高毒性PAHs所对应的绝对硬度η区间,即1.370 eV<η<2.104 eV,对应前线轨道能隙值区间2.740 eV 图5 PAHs大型蚤光致毒性预测值与分子绝对硬度 间的相关关系Fig. 5 Correlation relationship between molecular hardness and predicted pALT50 of PAHs to Daphnia magna 综上所述,基于DFT理论方法发展的PAHs对大型蚤光致毒性的构效关系,其拟合优度、稳定性和内外部预测性能均有显著提升,可在应用域范围内准确预测PAHs光致毒性,从而满足风险评估需求。构效关系分析结果表明,除苯并[k]荧蒽和屈可能具有不同的光致毒性作用机制之外,对于多数PAHs而言,若具有较低的前线轨道能隙、较小的分子稳定性和较大的分子变形性,均将有利于促使光致毒性作用的发生;且DFT计算PAHs前线轨道能隙宽度在[2.740,4.208]eV之间和对应光照辐射波段约为295 nm~450 nm时,PAHs污染暴露将可能诱发较高的光致毒性效应。这为PAHs对大型蚤光致毒性作用机制阐释和风险评价提供了数据支持与理论依据。 [1] Gewurtz S B, Lazar R, Haffiner G D. Comparison of polycyclic aromatic hydrocarbon and polychlorinated biphenyl dynamics in benthic invertebrates of Lake Erie, USA [J]. Environmental Toxicology and Chemistry, 2000, 19: 2943-2950 [2] White J C, Triplett T. Polycyclic aromatic hydrocarbons (PAHs) in the sediments and fish of the Mill River, New Haven, Connecticut, USA[J]. Bulletin of Environmental Contamination and Toxicology, 2002, 68: 104-110 [3] Liang Y, Tse M F, Young L, et al. Distribution patterns of polycyclic aromatic hydrocarbons (PAHs) in the sediments and fish at Mai Po Marshes Nature Reserve, Hong Kong [J]. Water Research, 2007, 41: 1303-1311 [4] Wang Z, Chen J, Qiao X, et al. Distribution and sources of polycyclic aromatic hydrocarbons from urban to rural soils: A case study in Dalian, China [J]. Chemosphere, 2007, 68: 965-971 [6] Lin C C, Chen S J, Huang K L, et al. PAHs, PAH-induced carcinogenic potency, and particle-extract-induced cytotoxicity of traffic-related nano/ultrafine particles [J]. Environmental Science and Technology, 2008, 42: 4229-4235 [7] Maertens R M, Gagne R W, Douglas G R, et al. Mutagenic and carcinogenic hazards of settled house dust II: Salmonella mutagenicity [J]. Environmental Science and Technology, 2008, 42: 1754-1760 [8] Papa E, Pilutti P, Gramatica P. Prediction of PAH mutagenicity in human cells by QSAR classification [J]. SAR and QSAR in Environmental Research, 2008, 19: 115-127 [9] Callahan M A, Slimak M W, Gabel N W, et al. Water-related environmental fate of 129 priority pollutants, EPA-440/4-79-029B [R]. Washington DC: U S Environmental Protection Agency, 1979 [10] Santamaria L, Prino G. The photodynamic substances and their mechanism of action [J]. Research Progress in Organic-Biological and Medicinal Chemistry, 1964, 1: 259-336 [11] Newsted J L, Giesy J P. Predictive models for photoinduced acute toxicity of polycyclic aromatic-hydrocarbons to Daphnia magna, Strauss (Cladocera, crustacea) [J]. Environmental Toxicology and Chemistry, 1987, 6: 445-461 [12] Wernersson A S, Dave G. Phototoxicity identification by solid phase extraction and photoinduced toxicity to Daphnia magna [J]. Archives of Environmental Contamination and Toxicology, 1997, 32: 268-273 [13] Huovinen P S, Soimasuo M R, Oikari A O J. Photoinduced toxicity of retene to Daphnia magna under enhanced UV-B radiation [J]. Chemosphere, 2001, 45: 683-691 [14] Lampi M A, Gurska J, McDonald K I C, et al. Photoinduced toxicity of polycyclic aromatic hydrocarbons to Daphnia magna: Ultraviolet-mediated effects and the toxicity of polycyclic aromatic hydrocarbon photoproducts [J]. Environmental Toxicology and Chemistry, 2005, 25: 1079-1087 [15] McConkey B J, Duxbury C L, Dixon D G, et al. Toxicity of a PAH photooxidatin product to the bacteria Photobacterium phosphoreum and the duckweed Lemna gibba: Effects of phenanthrene and its primary photoproduct, phenanthrenequinone [J]. Environmental Toxicology and Chemistry, 1997, 16: 892-899 [16] El-Alawi Y S, Dixon D G, Greenberg B M. Effects of a pre-incubation period on the photoinduced toxicity of polycyclic aromatic hydrocarbons to the luminescent bacterium Vibrio fischeri [J]. Environmental Toxicology, 2001, 16: 277-286 [17] Grote M, Schüürmann G, Altenburger R. Modelling photoinduced algal toxicity of polycyclic aromatic hydrocarbons [J]. Environmental Science and Technology, 2005, 39: 4141-4149 [18] Sina B K, Chignell C F. Binding of anthracene to cellular macromolecules in presence of light[J]. Photochemistry and Photobiology, 1983, 37: 33-37 [19] Huang X D, Krylov S N, Ren L S, et al. Mechanistic quantitative structure-activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons. II. An empirical model for the toxicity of 16 polycyclic aromatic hydrocarbons to the duckweed Lemna gibba L. G-3 [J]. Environmental Toxicology and Chemistry, 1997, 16: 2296-2303 [20] El-Alawi Y S, Huang X D, Dixon D G, et al. Quantitative structure-activity relationship for the photoinduced toxicity of polycyclic aromatic hydrocarbons to the luminescent bacteria Vibrio fischeri [J]. Environmental Toxicology and Chemistry, 2002, 21: 2225-2232 [21] Lampi M A, Gurska J, Huang X D, et al. A predictive quantitative structure-activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons to Daphnia magna with the use of factors for photosensitization and photomodification [J]. Environmental Toxicology and Chemistry, 2007, 26: 406-415 [22] Zhang Q. Predictive models on photolysis and photoinduced toxicity of persistent organic chemicals [J]. Frontiers of Environmental Science and Engineering, 2013, 7(6): 803-814 [23] Mekenyan O G, Ankley G T, Veith G D, et al. QSARs for photoinduced toxicity: I. Acute lethality of polycyclic aromatic hydrocarbons to Daphnia magna [J]. Chemosphere, 1994, 28: 567-582 [24] Ferreira M. Polycyclic aromatic hydrocarbons: A QSPR study [J]. Chemosphere, 2001, 44: 125-146 [25] Ribeiro F, Ferreira M. QSAR model of the phototoxicity of polycyclic aromatic hydrocarbons [J]. Journal of Molecular Structure: THEOCHEM, 2005, 719: 191-200 [26] Wold S, Albano C, Dunn W J, et al. Multivariate Data Analysis in Chemistry [M]// Kowalski B. (Ed.). Chemometrics: Mathematics and Statistics in Chemistry. Reidel, Dordrecht, 1984: 17-95 [27] Geladi P. Notes on the history and nature of partial least squares (PLS) modeling [J]. Journal of Chemometrics, 1988, 2: 231-246 [28] Lee C, Yang W, Parr R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density [J]. Physical Review B, 1988, 37: 785-789 [29] Becke A D. Density-functional thermochemistry.Ⅲ. The role of exact exchange [J]. Journal of Chemical Physics, 1993, 98: 5648-5652 [30] Peng C, Ayala P Y, Schlegel H B, et al. Using redundant internal coordinates to optimize geometries and transition states [J]. Journal of Computational Chemistry, 1996, 17: 49-54 [31] Peng C, Schlegel H B. Combining synchronous transit and Quasi-Newton methods for finding transition states [J]. Israel Journal of Chemistry, 1994, 33(4): 449-454 [32] Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 03, Revision B.03 [CP]. Pittsburgh PA: Gaussian Inc., 2003 [33] Mhin B J, Lee J E, Choi W. Understanding the congener-specific toxicity in polychlorinated dibenzo-p-dioxins: Chlorination pattern and molecular quadrupole moment [J]. Journal of the American Chemical Society, 2002, 124:144-148 [34] Cammarata A. An apparent correlation between the in vitro activity of chloramphenicol analogs and electronic polarizability [J]. Journal of Medicinal Chemistry, 1967, 10: 525-527 [35] Parr R G, Pearson R G. Absolute hardness: Companion parameters to absolute electronegativity [J]. Journal of the American Chemical Society, 1983, 105: 7512-7516 [36] Koopmans T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms [J]. Physica, 1934, 1: 104-113 [37] Politzer P A. A relationship between the charge capacity and the hardness of neutral atoms and groups [J]. Journal of Chemical Physics, 1987, 86: 1072-1073 [38] Ghanty T K, Ghosh S K. Correlation between hardness, polarizability, and size of atoms, molecules, and clusters [J]. Journal of Physical Chemistry, 1993, 97: 4951-4953 [39] Alexander G, Alexander T. Beware of Q2[J]. Journal of Molecular Graphics and Modelling, 2002, 20: 269-276 [40] Luco J M. Prediction of the brain-blood distribution of a large set of drugs from structurally derived descriptors using partial least-squares (PLS) modeling [J]. Journal of Chemical Information and Computer Science, 1999, 39: 396-404 [41] Eriksson L, Jaworska J, Worth A P, et al. Methods for reliability and uncertainty assessment and for applicability evaluation of classification- and regression-based QSARs [J]. Environmental Health Perspectives, 2003, 111(10):1361-1375 [42] Gramatica P. Principles of QSAR models validation: Internal and external [J]. QSAR & Combinatorial Science, 2007, 26(5): 694-701 [43] Roy K, Kar S, Ambure P. On a simple approach for determining applicability domain of QSAR models [J]. Chemometrics and Intelligent Laboratory Systems, 2015, 145: 22-29 [44] Klopman G, Kalos A N. Causality in structure-activity studies [J]. Journal of Computational Chemistry, 1985, 6: 492-506 [46] Wang Y, Chen J W, Li F, et al. Modelling photoinduced toxicity of PAHs based on DFT-calculated descriptors [J]. Chemosphere, 2009, 76(7): 999-1005 ◆ InvestigationofStructure-ActivityRelationshipbetweenTypicalElectronicPropertiesandPhototoxicityofPolycyclicAromaticHydrocarbonstoDaphniamagna Gu Chenggang1, Zhu Mengrong1,2, Liu Chang1,2, Ti Qingqing1,2, He Huan3, Sun Cheng3, #, Jiang Xin1, * 1. Key Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China2. University of Chinese Academy of Sciences, Beijing 100049, China3. State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023, China 10.7524/AJE.1673-5897.20170103003 2017-01-03录用日期2017-03-08 1673-5897(2017)3-516-10 X171.5 A 蒋新(1961—), 博士,研究员, 主要研究方向为典型持久性有机污染物的土壤环境化学行为与污染控制, 发表中英和德文学术论文190余篇。 国家自然科学基金项目(21377138;51578279);国家重点基础研究发展计划(2014CB441105);中科院“一三五”计划和领域前沿项目(ISSASIP1618)资助 谷成刚(1979-),男,博士,研究方向为土壤环境化学,E-mail: cggu@issas.ac.cn *通讯作者(Corresponding author), E-mail: jiangxin@issas.ac.cn #共同通讯作者(Co-corresponding author), E-mail: envidean@nju.edu.cn 谷成刚, 朱梦荣, 刘畅, 等. 多环芳烃典型电子性质与其大型蚤光致毒性的构效关系研究[J]. 生态毒理学报,2017, 12(3): 516-525 Gu C G, Zhu M R, Liu C, et al. Investigation of structure-activity relationship between typical electronic properties and phototoxicity of polycyclic aromatic hydrocarbons to Daphnia magna [J]. Asian Journal of Ecotoxicology, 2017, 12(3): 516-525 (in Chinese)

