云南绿春县天然次生林和4种人工林树冠层蚂蚁群落多样性
赵婧文,卢志兴,陈又清
(中国林业科学研究院资源昆虫研究所,云南 昆明 650224)
云南绿春县天然次生林和4种人工林树冠层蚂蚁群落多样性
赵婧文,卢志兴,陈又清*
(中国林业科学研究院资源昆虫研究所,云南 昆明 650224)

天然次生林;人工林;树种选择;树冠层蚂蚁;群落多样性
Abstract:[Objective]To explore the effect of secondary natural forest and plantations on the diversity of canopy foraging ant communities and the influence of tree selections in plantations on the diversity of canopy foraging ant communities.[Method]Investigations of ant communities in secondary natural forest and four plantations were conducted by trap at Lüchun County, Yunnan Province. Meanwhile, the plant diversity and foliage density were investigated. [Result](1) 17998 ant individuals were collected, which belonging to 68 species, 29 genera, and 6 subfamilies of Formicidae. (2) Ant abundance ranked as lac insect-corn agroforests > lac insect plantations > eucalyptus plantations > rubber plantations > secondary natural forests. (3) Ant richness ranked as lac insect-corn agroforests > lac insect plantations > secondary natural forests > eucalyptus plantations > rubber plantations. (4) ACE index ranked as lac insect-corn agroforests > lac insect plantations > secondary natural forests > eucalyptus plantations >rubber plantations. (5) Significantly negative relationship was found between canopy foraging ant abundance, species richness and litter coverage, foliage density of the zone above 300 cm, and the tree crown density, but positive relationship was found between the ant abundance, species richness and herbage coverage. Meanwhile, there were significantly negative relations between ACE index and litter coverage, herbage coverage, and tree crown density, but a positive correlation between ACE index and foliage density of the zone from 175 cm to 199.9 cm. [Conclusion]Plantation has a certain positive role to the ant diversity conversation, especially has a significantly effect by choosing native tree. Reasonable management of plantation is conducive to the conservation of biological diversity.
Keywords: secondary natural forest; plantation; tree species selection; canopy foraging ant; community diversity
人工林由于其经济价值日益成为全球森林的重要组分[1]。中国人工林面积居世界首位,面积达6 933万hm2,云南省绿春县人工林主要包括桉树林、橡胶林和紫胶林等。人工林的大面积种植使人们开始关注其对生物多样性的保护是否有利。有研究表明,人工林会降低生物多样性[2-3]。人工林中的物种丰富度和多度均减少,甚至当地种、特有种和被保护的稀有物种缺失,同时常见种的多度增加,使少量常见、分布广泛的物种占据优势地位[4-5]。除此之外,人工林的树种选择是影响生物多样性的因素之一,选择非乡土树种使本地物种无法适应这些外来树种及其所营造的环境[6-7],但目前国内针对人工林树种选择对生物多样性影响的研究,多是针对林内植物多样性,而对节肢动物的研究很少。
蚂蚁是地球上分布最广泛的昆虫,以其良好的分类知识库和对环境变化的敏感性经常被用来作为环境的指示生物[8]。研究发现,树冠层节肢动物群落中90%以上的个体为蚂蚁[9],而且树冠层蚂蚁的捕食压力,能够显著影响树冠节肢动物群落的多样性、群落结构和动态[10-11],进而对生态系统服务产生影响。树冠层蚂蚁多样性受到植被结构[12]、植物多样性[13]、栖息地干扰[14]和食物资源分布[15]等多种因素的影响。树冠层蚂蚁多数来自地表,但目前对于环境因子变化对树冠层蚂蚁群落的影响研究很少[16],特别是相关研究还未探讨地表环境特征与人工林垂直结构特征与树栖蚂蚁群落多样性之间的关系。
本研究对云南省绿春县桉树林、紫胶玉米混农林、天然次生林、橡胶林和紫胶林5种类型样地中树冠层蚂蚁群落多样性、植物多样性及地表栖境特征进行调查,通过比较分析人工林与天然林的蚂蚁群落多样性和植物群落多样性的差异及二者的相关性,进而分析天然林和人工林对蚂蚁群落多样性的影响及人工林树种的差异对蚂蚁群落多样性保护作用,以期为人工林的经营管理和生物多样性的保护提供依据。
1 材料与方法
1.1研究地概况


表1 不同样地概况
1.2调查方法
1.2.1树冠层蚂蚁群落调查 于2012年10月份(雨季)和2013年4月份(旱季),调查5种类型样地内的树冠层蚂蚁群落,具体方法为:每个样地设置3条100m长的样带,样带间距大于50m,在每条样带上选择5株乔木,至少间距10m,保证样本独立性,共计15株乔木,所选择的乔木胸径在10cm以上,对所选乔木进行编号。将树栖蚂蚁诱集陷阱固定在树干上距离地面1.5m处,以浓度为50%的乙二醇作为陷阱溶液。在陷阱中使用支架设置诱饵提高诱集效果,支架下端浸没在陷阱溶液中,蚂蚁不能接触到诱饵,诱饵为金枪鱼和蜂蜜的混合物。设置48h后收集陷阱中的所有蚂蚁,置于含有70%酒精溶液的离心管中,带回实验室进行鉴定并统计数量,无法鉴定到种的以形态种对待[17],根据种类鉴定结果整理出物种组成名录。
1.2.2植物群落调查 乔灌木群落调查:每块样地选择3个10m×10m的大样方,调查并记录乔木和灌木的植物种类、数量、胸径、树高和郁闭度。
草本植物群落调查:在放置树栖陷阱的乔木附近设置1个1m×1m的小样方,调查草本植物的种类、数量和盖度,同时估计小样方内的枯落物盖度并用5点法测量枯落物厚度并计算其平均值作为该样方的枯落物厚度值。
植物垂直密度变化:使用利维杆(长3m,直径1cm的铁杆)方法。以地表陷阱位置为参考,使用时将利维杆立于距陷阱水平距离1m的位置,垂直于地面,以每25cm作为一个区段,共12个区段,统计该位置每个区段内植物与铁杆接触点的数量(不考虑植物与铁杆的接触面积),对于超过300cm高度的植物密度,只统计有无,有植物覆盖记为1,无则记为0[18]。
1.3分析方法
1.3.1抽样充分性判断 使用R语言中的iNEXT软件包进行基于个体数的物种稀疏和预测曲线的绘制,根据曲线特征进行抽样充分性的判断[19]。
1.3.2多样性比较 在比较树冠层蚂蚁群落多样性时,以设置的样带为重复,即按编号以5个陷阱为一组,每块样地有3个重复。统计蚂蚁物种丰富度和多度,并使用软件Estimate S计算ACE估计值。数据分析时,满足单因素方差分析条件时使用单因素方差分析(One-way ANOVA)中LSD 多重比较方法,不满足时,进行Krushkal-wallis H 检验,比较样地间树冠层蚂蚁群落多样性的差异。
1.3.3群落结构相似性 对不同物种的多度数据进行有/无转换,重点关注不同类型植被树冠层蚂蚁群落在物种组成上的差异,使用PRIMER v7中的非度量多维尺度分析(Non-metric Multi-Dimensional Scaling,nMDS)分析不同类型样地树冠层蚂蚁群落结构差异;使用群落结构相似性比较不同类型样地间群落结构差异的显著性[20];对不同类型样地蚂蚁群落进行层次聚类分析(Hierarchical Cluster analysis),选取40%相似性水平将结果以圆圈的方式叠加到nMDS结果图中。
1.3.4指示物种分析 使用R语言中的indicspecies软件包对不同类型样地进行指示物种分析,分析时使用的分组基于nMDS群落结构分析结果,即相似蚂蚁群落结构的样地为同一类型,计算不同物种的特异度和保真度,同时计算IndVal值,以IndVal指示值大于0.7并与样地显著相关(P<0.05)的物种作为指示物种[21]。
1.3.5相关性分析 使用SPSS18.0软件中的相关性分析对树冠层蚂蚁多样性和植物多样性进行分析,其中,树冠层蚂蚁多样性包括蚂蚁多度和物种丰富度;植物多样性包括草本植物的多度、物种丰富度和盖度,乔灌木植物的多度、物种丰富度和盖度,枯落物的盖度和厚度以及植物垂直密度。
2 结果分析
2.1物种组成及抽样充分性
5种类型样地共采集蚂蚁17998头,隶属于6亚科29属68种,其中,桉树林共采集到蚂蚁3606头,隶属于4亚科13属23种;紫胶林-玉米混农林共采集到蚂蚁5421头,隶属于5亚科17属37种;天然次生林共采集到蚂蚁1850头,隶属于5亚科19属36种;橡胶林共采集到蚂蚁2472头,隶属于5亚科15属24种;紫胶林共采集到蚂蚁4649头,隶属于5亚科20属37种。5种类型样地的树冠层蚂蚁群落基于个体数的物种稀疏和预测曲线(图1)表明:图中实线部分为实际曲线,虚线部分为预测曲线,5条曲线预测部分都趋于平缓,说明这5种类型的样地物种数随个体数增加基本不再变化,表明抽样比较充分。
2.2树冠层蚂蚁群落多样性
表2表明:5种类型样地树冠层蚂蚁群落物种多度之间差异显著(χ2=13.178,P<0.05),紫胶林-玉米混农林>紫胶林>桉树林>橡胶林>天然次生林,紫胶林-玉米混农林显著高于天然次生林和橡胶林,紫胶林显著高于天然次生林,其他样地间差异不显著;5种类型样地树冠层蚂蚁群落物种丰富度差异极显著(F4,25=18.129,P<0.001),紫胶林-玉米混农林>紫胶林>天然次生林>橡胶林>桉树林,紫胶林-玉米混农林显著高于其他4种类型样地,天然次生林和紫胶林显著高于桉树林和橡胶林,其他样地间差异不显著;5种类型样地树冠层蚂蚁ACE估计值差异极显著(χ2=17.702,P<0.01),紫胶林-玉米混农林>紫胶林>天然次生林>桉树林>橡胶林,紫胶林-玉米混农林显著高于其他4种类型样地,紫胶林显著高于桉树林和橡胶林,天然次生林显著高于橡胶林,其他样地间差异不显著。
2.3树冠层蚂蚁群落结构比较
5种类型样地的树冠层蚂蚁群落结构差异极显著(ANOSIM GlobalR=0.577,P<0.01),其中,紫胶林的树冠层蚂蚁群落结构与紫胶林-玉米混农林相似;天然次生林的2个重复样地的树冠层蚂蚁群落结构不相似;桉树林的树冠层蚂蚁群落结构与其余类型样地较不相似;橡胶林的树冠层蚂蚁群落结构与其余样地明显不相似(图2)。
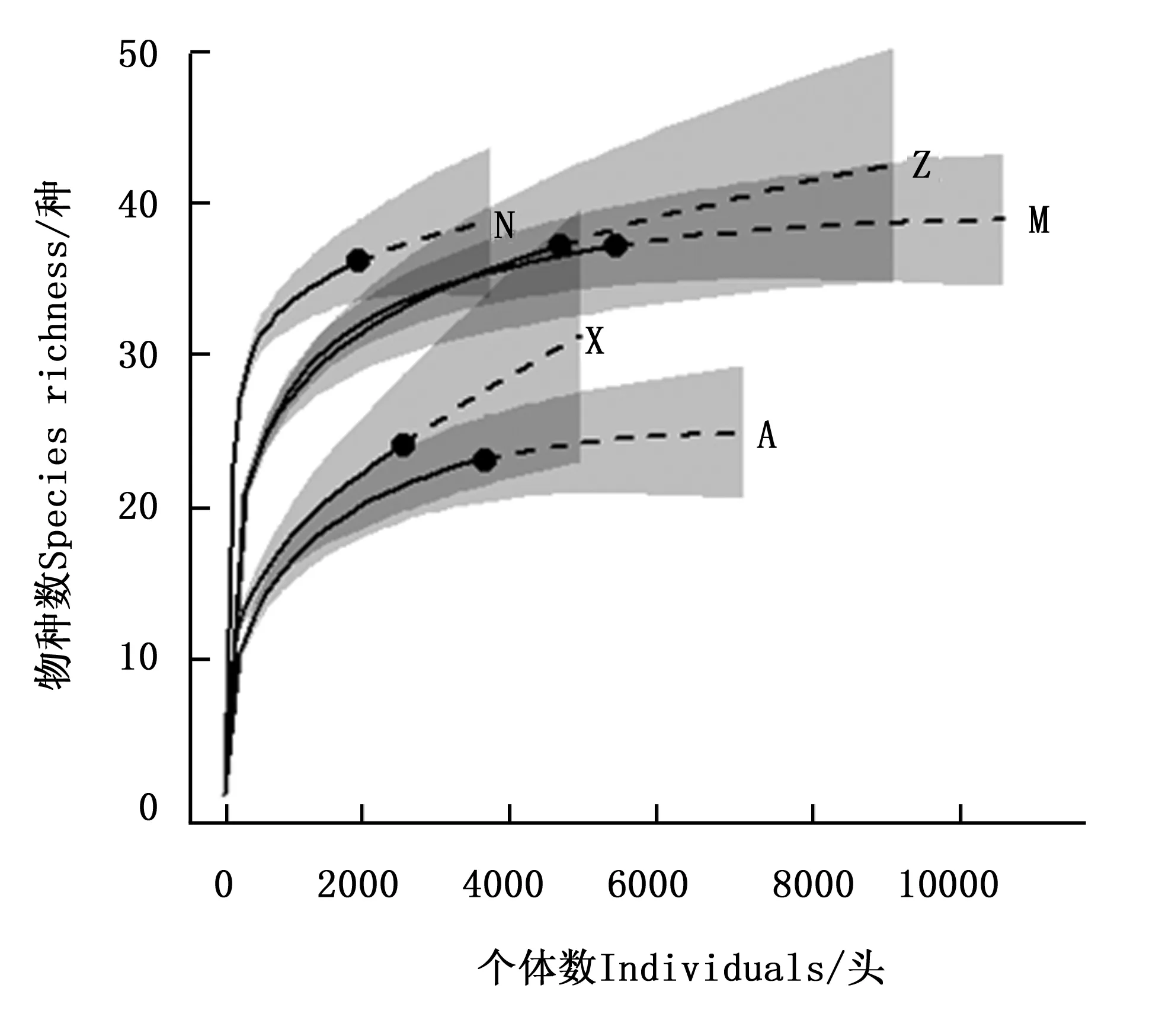
A、M、N、X和Z分别代表桉树林、紫胶林-玉米混农民、天然次生林、橡胶林和紫胶林。A, M, N, X and Z represented Eucalyptus plantations, Lac insect-corn agroforests, Seondary natural forests, Rubber plantations and Lac insect plantations respectively.图1 不同样地树冠层蚂蚁群落基于个体数的物种稀疏和预测曲线Fig.1 Rare species number and extrapolation curves of canopy foraging ant communities in different sites based on individuals
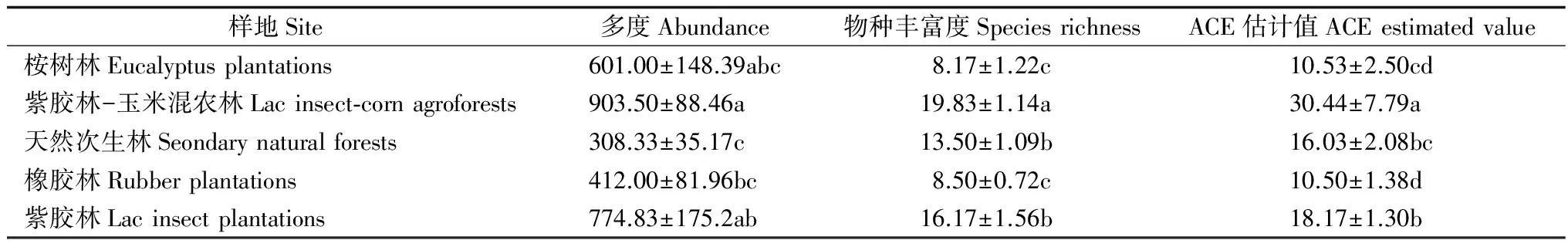
样地Site多度Abundance物种丰富度SpeciesrichnessACE估计值ACEestimatedvalue桉树林Eucalyptusplantations601.00±148.39abc8.17±1.22c10.53±2.50cd紫胶林-玉米混农林Lacinsect⁃cornagroforests903.50±88.46a19.83±1.14a30.44±7.79a天然次生林Seondarynaturalforests308.33±35.17c13.50±1.09b16.03±2.08bc橡胶林Rubberplantations412.00±81.96bc8.50±0.72c10.50±1.38d紫胶林Lacinsectplantations774.83±175.2ab16.17±1.56b18.17±1.30b
注:表中数值为均值±标准误。同列不同字母表示在0.05水平的差异显著。ACE估计值是对样地中实际物种数的估计。
Note: Data in the table as mean ±standard error. The different letters within the same column mean significant difference (P<0.05). ACE index was an estimate of the actual number of species in the sample plot.
2.4指示物种
紫胶林和紫胶林-玉米混农林的指示物种为平和弓背蚁、网纹刺结蚁、红头弓背蚁和罗氏心结蚁;橡胶林的指示物种为黑细长蚁和黄猄蚁(表3);其他样地无指示物种。
2.5蚂蚁群落与环境因子相关性分析

3 讨论
有研究表明,乡土树种人工林对蚂蚁多样性有一定的保护作用[22-23],外来树种人工林使蚂蚁物种丰富度降低[24],或者虽然能够保持和天然次生林相近的蚂蚁丰富度,但组成明显有差异[25],甚至使少数常见的物种多度增加,占据优势地位[5]。本研究中,在天然林和4种人工林的比较中,外来人工桉树林和橡胶林与天然次生林相比,树冠层蚂蚁物种丰富度和ACE估计值降低,但少数常见种多度增加,并且与天然次生林群落结构差异显著,与前人研究一致[4-5],说明2种外来人工林能够维持一定的蚂蚁群落,但保护作用不及天然次生林;而乡土树种人工紫胶林和紫胶林一玉米混农林树冠层蚂蚁多度、物种丰富度和ACE估计值均增加,并且2种人工林群落结构相似,对蚂蚁群落的保护作用显著优于外来人工林。

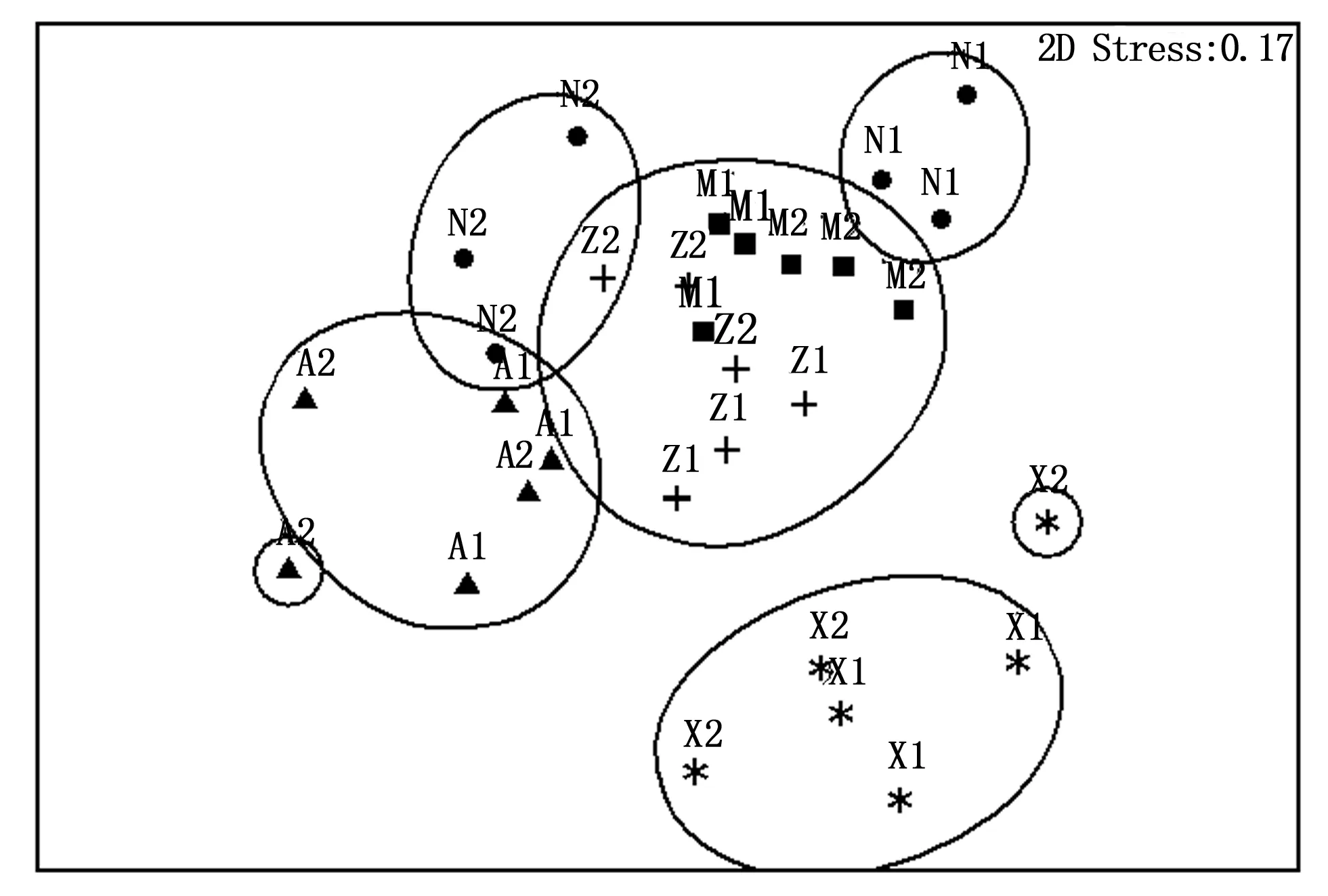
图中黑圈表示40%相似性水平。A、M、N、X和Z分别代表桉树林、紫胶林-玉米混农民、天然次生林、橡胶林和紫胶林。The dark circles represented similarity level of forty percent.A, M, N, X and Z represented Eucalyptus plantations, Lac insect-corn agroforests, Seondary natural forests Rubber plantations and Lac insect plantations respectively.图2 不同类型样地树冠层蚂蚁群落结构相似性Fig.2 The similarity of structures of canopy foraging ant communities in different sites
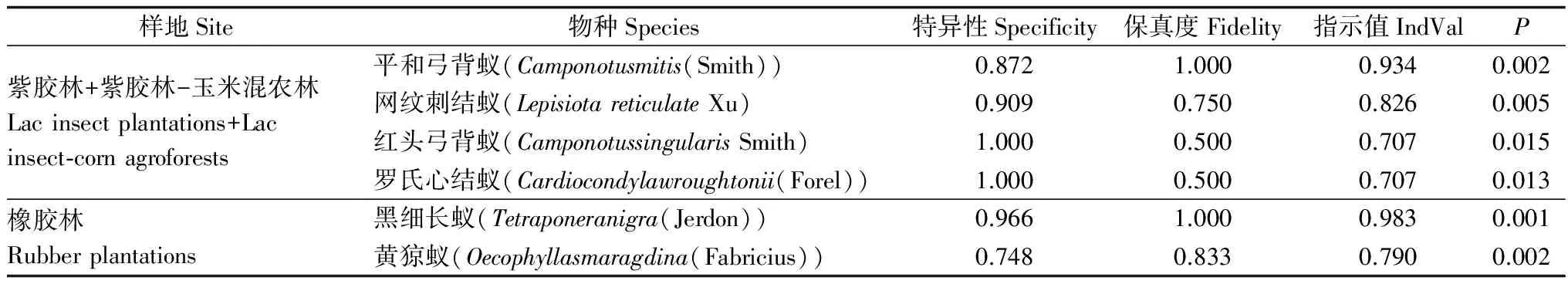
样地Site物种Species特异性Specificity保真度Fidelity指示值IndValP紫胶林+紫胶林-玉米混农林Lacinsectplantations+Lacinsect⁃cornagroforests橡胶林Rubberplantations平和弓背蚁(Camponotusmitis(Smith))0.8721.0000.9340.002网纹刺结蚁(LepisiotareticulateXu)0.9090.7500.8260.005红头弓背蚁(CamponotussingularisSmith)1.0000.5000.7070.015罗氏心结蚁(Cardiocondylawroughtonii(Forel))1.0000.5000.7070.013黑细长蚁(Tetraponeranigra(Jerdon))0.9661.0000.9830.001黄猄蚁(Oecophyllasmaragdina(Fabricius))0.7480.8330.7900.002
注:特异性是该物种在各样地中出现的概率,保真度为该物种在分组中出现的概率。
Note: Specificity mean the proportion of the species appeared in different sites. Fidelity mean the proportion of the species appeared in groups.
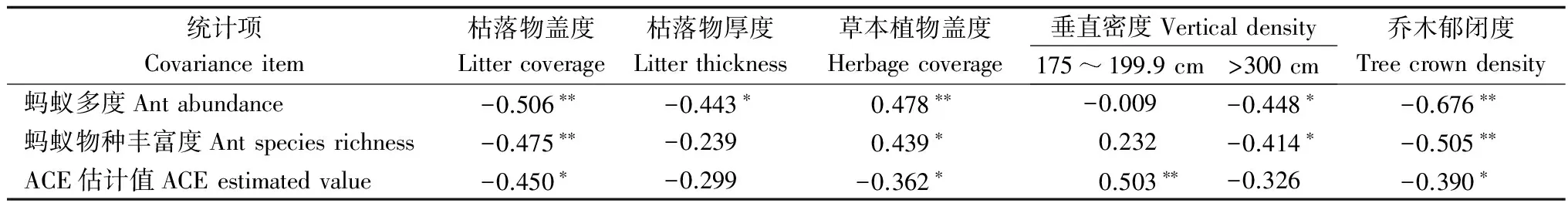
表4树冠层蚂蚁群落多样性与环境因子相关性分析
注:*代表在0.05水平上的显著相关,**代表0.01水平的显著相关。
Note:One asterisk mean significant correlation (P<0.05). Two asterisks mean significant correlation (P< 0.01)
蚂蚁作为指示生物能较好指示环境变化[31]。平和弓背蚁和红头弓背蚁同属于弓背蚁属,广泛分布于各种栖境中,能够利用的食物资源非常广泛[32];罗氏心结蚁个体较小,喜欢潮湿地区或者沼泽[33],这3种蚂蚁为紫胶林和紫胶林-玉米混农林的指示物种,说明这2种乡土树种人工林能够提供一个栖境异质性较高的环境,使得多种蚂蚁共存。黄猄蚁多发生在有干扰的地区[34],该蚂蚁的出现,证明橡胶林受到了干扰。
4 结论
本研究结果显示,人工林对蚂蚁多样性有一定的保护作用,特别是乡土树种人工林较外来人工林对蚂蚁多样性有更好的保护作用,但如何对人工林进行合理的管护,才能既满足经济发展又保护生物多样性,值得进一步研究。
[1] FAO,State of the world’s forests[J]. Food and Agriculture Organization of the United Nations, 2011, 54(2):165-166.
[2] Cicuzza D, Kessler M, Clough Y,etal. Conservation value of cacao agroforestry systems for terrestrial herbaceous species in central Sulawesi, Indonesia[J]. Biotropica, 2011, 43(6): 755-762.
[3] Gibson L, Lee T M, Gibson L,etal. Primary forests are irreplaceable for sustaining tropical biodiversity[J]. Nature, 2011, 478(7369): 378-381.
[4] Beukema H, Danielsen F, Vincent G,etal. Plant and bird diversity in rubber agroforests in the lowlands of Sumatra, Indonesia[J]. Agroforestry Systems, 2007, 70(3): 217- 242.
[5] Steffan-Dewenter I, Kessler M, Barkmann J,etal. Tradeoffs between income, biodiversity, and ecosystem functioning during tropical rainforest conversion and agroforestry intensification[J]. Proceedings of the National Academy of Sciences, 2007, 104(12): 4973-4978.
[6] 任 海, 王 俊. 试论人工林下乡土树种定居限制问题[J]. 应用生态学报, 2007, 18(8): 1855-1860.
[7] Djègo J, Sinsin B. Effect of introduced exotic trees on the species diversity of the plant communities of their undergrowth[J]. Systematics & Geography of Plants, 2006, 76(2): 191-209.
[8] Dejean A, Corbara B, Orivel J,etal. Rainforest canopy ants: the implications of territoriality and predatory behavior[J]. Functional Ecosystems and Communities, 2007, 1(2): 105-120.
[9] Davidson D W, Cook S C, Snelling R R,etal. Explaining the abundance of ants in lowland tropical rainforest canopies[J]. Science, 2003, 300(5621): 969-972.
[10] Floren A, Biun A, Linsenmair E K. Arboreal ants as key predators in tropical lowland rainforest trees[J]. Oecologia, 2002, 131(1): 137-144.
[11] Philpott S M, Perfecto I, Vandermeer J. Effects of Management Intensity and Season on Arboreal Ant Diversity and Abundance in Coffee Agroecosystems[J]. Biodiversity & Conservation, 2006, 15(1): 139-155.
[12] Klimes P, Idigel C, Rimandai M,etal. Why are there more arboreal ant species in primary than in secondary tropical forests?[J]. Journal of Animal Ecology, 2012, 81(5): 1103-1112.
[13] Basset Y, Cizek L, Cuénoud P,etal. Arthropod diversity in a tropical forest[J]. Science, 2012, 338(6113): 1481-1484.
[14] Schonberg L A, Longino J T, Nadkarni N M,etal. Arboreal ant species richness in primary forest, secondary forest, and pasture habitats of a tropical montane landscape[J]. Biotropica, 2004, 36(3): 402-409.
[15] Blüthgen N, Stork N E. Ant mosaics in a tropical rainforest in Australia and elsewhere: a critical review[J]. Austral Ecology, 2007, 32(1): 93-104.
[16] Yusah K M, Foster W A. Tree size and habitat complexity affect ant communities (Hymenoptera: Formicidae) in the high canopy of Bornean rain forest[J]. Myrmecological News, 2016, 23: 15-23.
[17] 徐正会. 西双版纳自然保护区蚁科昆虫生物多样性研究[M]. 昆明:云南科技出版社, 2002.
[18] Gunawardene N, Majer J, Edirisinghe J. Correlates of ant (Hymenoptera: Formicidae) and tree species diversity in Sri Lanka[J]. Myrmecological News, 2012, 17(17): 81-90.
[19] Chao A, Gotelli N J, Hsieh T C,etal. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies[J]. Ecological Monographs, 2014, 84(1): 45-67.
[20] Clarke K R, Gorley R N. PRIMER version6: user manual/tutorial[M]. Plymouth, UK:PRIMER-E,2006.
[21] Cáceres M D, Legendre P. Associations between species and groups of sites: indices and statistical inference[J]. Ecology, 2009, 90(12): 3566-3574.
[22] 卢志兴, 李可力, 张念念, 等. 巨桉林和天然次生林枯落物层蚂蚁多样性及指示种[J]. 林业科学研究, 2016, 29(4): 576-580.
[23] Delabie J H C, Jahyny B, Nascimento I C D,etal. Contribution of cocoa plantations to the conservation of native ants (Insecta: Hymenoptera: Formicidae) with a special emphasis on the Atlantic Forest fauna of southern Bahia, Brazil[J]. Biodiversity and Conservation, 2007, 16(8): 2359-2384.
[24] Hashim N R, Wan F A W J, Nasir M N S M. Ant diversity in a Peninsular Malaysian mangrove forest and oil palm plantation[J]. Asian Myrmecology, 2010, 3(1): 5-8.
[25] Denmead L H. Ant diversity, function and services across tropical land-use systems in Indonesia[D]. Göttingen:Georg-August Universität, 2016.
[26] Queiroz A C M, Ribas C R. Canopy cover negatively affects arboreal ant species richness in a tropical open habitat[J]. Brazilian Journal of Biology, 2016, 76(4): 864-870.
[27] Klimes P, Fibich P, Idigel C,etal.Disentangling the diversity of arboreal ant communities in tropical forest trees[J]. Plos One, 2015, 10(2): e0117853.
[28] Floren A, Wetzel W, Staab M. The contribution of canopy species to overall ant diversity (Hymenoptera: Formicidae) in temperate and tropical ecosystems[J]. Myrmecological News, 2014, 19(1): 65-74.
[29] 张念念, 陈又清, 卢志兴,等. 云南橡胶林和天然次生林枯落物层蚂蚁物种多样性、群落结构差异及指示种[J]. 昆虫学报, 2013, 56(11):1314-1323.
[30] 武子文, 毕兴丹, 卢志兴,等. 干热河谷地区不同恢复期火烧迹地地表蚂蚁群落比较[J]. 云南大学学报:自然科学版, 2017(1):130-136.
[31] Hoffmann B D, James C D. Using ants to manage sustainable grazing: Dynamics of ant faunas along sheep grazing gradients conform to four global patterns[J]. Austral Ecology, 2011, 36(6): 698-708.
[32] Garcia F H, Wiesel E, Fischer G. The Ants of Kenya (Hymenoptera: Formicidae)—Faunal Overview, First Species Checklist, Bibliography, Accounts for All Genera, and Discussion on Taxonomy and Zoogeography[J]. Journal of East African Natural History, 2007, 101(101): 127-222.
[33] Deyrup M, Davis L, Cover S. Exotic ants in Florida[J]. Transactions of the American Entomological Society, 2000, 126(3): 293-326.
[34] Torchote P, Sitthicharoenchai D, Chaisuekul C. Ant Species Diversity and Community Composition in Three Different Habitats: Mixed Deciduous Forest, Teak Plantation and Fruit Orchard[J]. Tropical Natural History,2010, 10(1): 37-51.

附表 不同样地蚂蚁名录及多度
续附表

不同样地蚂蚁名录及多度
(责任编辑:张 玲)
DiversityofCanopyForagingAntCommunitiesinSecondaryNaturalForestandPlantationsinLüchun,Yunnan
ZHAOJing-wen,LUZhi-xing,CHENYou-qing
(Research Institute of Resources Insects, Chinese Academy of Forestry, Kunming 650224, Yunnan, China)
S718.55
A
1001-1498(2017)05-0823-08
10.13275/j.cnki.lykxyj.2017.05.016
2017-04-15
国家自然科学基金项目(31470493和31270561)。
赵婧文(1991—),女,河北保定人,昆虫生态学硕士研究生.E-mail:zhaojingwen115@163.com.
* 通讯作者.

