黄瓜花叶病毒致病性研究进展
邱艳红王超楠,2朱水芳
(1. 中国检验检疫科学研究院植物检疫研究所,北京 100176;2. 中国农业大学植物保护学院,北京 100193)
黄瓜花叶病毒致病性研究进展
邱艳红1王超楠1,2朱水芳1
(1. 中国检验检疫科学研究院植物检疫研究所,北京 100176;2. 中国农业大学植物保护学院,北京 100193)
黄瓜花叶病毒(Cucumber mosaic virus,CMV)属于正义单链RNA病毒,寄主广泛,危害严重,是当前最具影响力的植物病毒之一,也是世界公认的重要植物病害。CMV自1916年报道以来,国内外学者从病毒基因、基因编码产物以及与寄主相互作用等多个方面展开了大量研究。随着高通量测序技术与蛋白质组学技术的发展,病毒相关致病机制也取得突破性进展。介绍了CMV编码蛋白,CMV相关的卫星RNA以及寄主因子在CMV侵染植物后的症状发展过程中的作用,并对今后的CMV致病性研究进行了讨论,旨为CMV的相关研究提供参考。
黄瓜花叶病毒;致病机制;小干扰RNA;蛋白互作
黄瓜花叶病毒(Cucumber mosaic virus,CMV)是雀麦花叶病毒科(Bromoviridae)黄瓜花叶病毒属(Cucumovirus)的典型成员。对CMV的描述最早可以追溯到1916年,当时Doolittle和Jagger[1,2]同时报道了CMV可引起黄瓜和其它葫芦科植物上的病害。目前,CMV分布在世界各地,特别是在温带和热带地区。CMV株系繁多,寄主广泛,能够侵染1 200多种植物,是禾谷类作物、牧草、木本和草本观赏植物、蔬菜及果树上发生最广、危害最大的病害[3]。
CMV的基因组含有3条正义单链RNA(RNA1、RNA2和RNA3),共编码5种蛋白(1a、2a、2b、MP和CP)。有些CMV除基因组RNA以外还携带非编码的卫星RNA(Satellite RNA,satRNA)[4,5]。CMV侵染后可引起植株矮化(Stunt)、叶片畸形(Leaf malformation)以及花叶(Mosaic)等症状,研究表明这些症状的产生与CMV基因以及基因编码产物均有相关性[4]。因而本文就黄瓜花叶病毒的相关致病因子以及参与病毒致病过程的寄主蛋白进行综合阐述,旨在为黄瓜花叶病毒进一步研究提供参考意义。
1 CMV致病相关的病毒编码蛋白
1.1 1a蛋白
1a蛋白由RNA1编码,是病毒重要的复制酶组分,具备RNA依赖RNA聚合酶(RdRp)的活性,也是影响寄主症状的重要因子[5]。通过比较CMV的Fny株系(CMV-Fny)和CMV的Sny株系(CMVSny)在西葫芦上的系统发病速度发现,RNA1不仅影响病毒的复制,而且影响病毒在细胞间移动及长距离运输[6]。CMV-Ns株系能在多种烟草上引起系统性坏死反应,研究发现这与la蛋白的第461位半胱氨酸特性直接相关[7]。1a蛋白在第443位和第472位氨基酸之间有两个两亲性的α螺旋结构,将第461位半胱氨酸突变为精氨酸或赖氨酸时影响了螺旋结构的完整性和两亲性,导致坏死反应的消失。而第461位半胱氨酸突变为丙氨酸或丝氨酸时并不改变1a蛋白的高级结构,因而不影响病毒在寄主上的坏死症状[8]。此外,1a蛋白的第865、896、957和980位氨基酸共同影响了病毒的胞间移动与复制,导致CMV-P1株系可以在含有抗性基因Cmr1的辣椒上进行系统侵染[9,10]。
1.2 2a蛋白
2a蛋白由RNA2编码与1a蛋白共同完成病毒的复制,同时影响寄主症状的发展。Du等[11]发现将2a蛋白的C端缺失会降低病毒在寄主体内含量,减轻在烟草上的致病症状。Choi等[12]利用重组的方法证明2a蛋白和MP共同影响病毒在菠菜叶中的细胞间移动速率,进而影响症状发展。另外,2a蛋白还是引起细胞坏死反应的重要因子,该蛋白第631位氨基酸突变可导致在豇豆上的坏死反应消失[13,14]。
1.3 2b蛋白
2b蛋白由CMV的来源于RNA2的亚基因组RNA4A编码。一方面2b蛋白作为基因沉默抑制因子(Viral suppressor of RNA silencing,VSR),保护病毒基因组不被降解,从而影响病毒的积累量,间接影响病毒的致病性[15]。另一方面,2b蛋白在抑制基因沉默介导的抗病毒通路的同时影响了寄主微小RNA(microRNA,miRNA)的代谢途径,导致相关致病症状的产生[16,17]。转CMV-Fny 2b蛋白的拟南芥表现出明显的植株矮小等症状。进一步研究发现2b蛋白可以干扰寄主拟南芥中miR159的代谢途径,影响miR159靶基因的转录,导致寄主症状的产生[17,18]。
此外,将CMV-Q的2b蛋白进行突变以后会导致病毒在烟草上的侵染症状延迟以及减弱,甚至导致该病毒不能在黄瓜上系统侵染,因而推测2b蛋白是通过影响病毒的系统移动而间接影响症状的发展[19]。Diaz-Pendon等[20]也发现CMV-Q的2b蛋白虽然影响了病毒在拟南芥中的系统侵染,然而却不是症状发展所必须的。
1.4 MP蛋白
MP由病毒的RNA3编码,负责病毒在细胞间的移动以及长距离运输[5]。CMV-Fny侵染烟草以后会在系统叶上交替出现花叶和症状不明显现象,而将MP的第51位的Asn和第240位Ile分别替换为Lys和Phe时系统叶将会一直保持花叶症状,进一步研究发现两个氨基酸的替换会引起寄主体内MP的增加,因而推断症状的交替出现跟病毒的运输有很大关系[21]。然而,带有同样两个氨基酸突变的病毒RNA侵染菠菜会影响病毒的系统移动[22]。说明MP的氨基酸突变对症状的影响具有寄主特异性。
1.5 CP蛋白
CP由病毒来源于RNA3的亚基因组RNA4编码,在病毒的包裹、复制、细胞间移动以及长距离运输中都起到非常关键的作用,也是病毒最重要的症状决定因子[23,24]。
CMV侵染后通常引起花叶症状,如CMV-M侵染烟草后可引起严重的黄白化症状,CMV-Fny侵染烟草后引起绿斑驳症状。研究证明花叶症状的产生与CMV的CP有直接相关性。Rao和Francki[25]利用假重组技术证实寄主出现的花叶症状由CMV的RNA3决定。Shintaku等[26]进一步将CMV-Fny和CMVM 的RNA3不同区域进行重组后发现,花叶症状由CP上第129位氨基酸(CP129)所决定。将CMV的pepo株系CP129突变为酸性氨基酸类、碱性氨基酸类、以及非极性氨基酸类等,然而并没有发现氨基酸的极性与症状之间的关系[27],说明CP129对CP的结构并没有起决定性的作用。此外,从CP的三级结构上也可以看出,CP129并未裸露在CP的表面,只是αE-βEF环的第一个氨基酸,因而CP129的改变很有可能只是影响了该αE-βEF环的灵活性[28]。
花叶症状的产生通常伴随着叶绿体相关代谢受损[29]。我们利用高通量测序技术分析CMV-M侵染烟草后不同阶段的基因表达谱发现,大量与叶绿体相关的基因表达受抑制[30]。将CMV-pepo的CP129突变为不同氨基酸后可引发不同花叶症状,进一步研究发现不同花叶症状的叶片中其叶绿体相关基因的表达量也不同[31]。CMV侵染烟草后还可以引起6个蛋白不同程度的降解,其中光合系统II-放氧复合物降解最为明显[32]。Di Carli[33]研究小组通过差异双向凝胶电泳与质谱分析技术发现CMV侵染的野生型番茄与抗CMV的转基因番茄蛋白表达模式不同,其中很多与光合作用相关的蛋白存在差异表达。病毒侵染后对叶绿体相关基因和蛋白的影响会进一步破坏叶绿体的结构。我们通过扫描电子显微镜观察叶片的亚细胞结构时发现,CMV侵染所引起的黄白化叶片中叶绿体数量减少,而且叶绿体含有较少的类囊体膜[34]。此外,CMV侵染的烟草叶片中过氧化氢含量明显升高[35]。Song等[36]的研究也发现CMV侵染干扰了叶绿体和线粒体的电子传递活性并影响其抗氧化系统,细胞器中过氧化氢的积累,导致细胞器的氧化胁迫。在植物体内,非原生质体、微体、线粒体和叶绿体均可以产生过氧化氢,其中叶绿体是过氧化氢的主要产生位点。而高浓度的过氧化氢对细胞有毒害作用。然而,过氧化氢的产生是否与叶绿体的电子传递干扰、叶绿体结构变化以及病毒侵染后植物的症状发展有关仍需进一步的研究论证。
2 CMV致病相关的卫星RNA
satRNA是一类非编码的RNA分子,通常需要辅助病毒提供蛋白来完成复制、包裹、运动及传播等过程[5]。satRNA对辅助病毒的致病性影响可分为3类:增强型、减弱或致弱型及不改变型。由于在复制过程中satRNA与辅助病毒的基因组RNAs形成竞争关系,因而会导致辅助病毒的基因组RNAs在寄主中的积累量降低,从而减轻病毒在寄主上的症状表现,但依然有少数satRNA能在烟草或者番茄上引起坏死[37]、花叶等症状[38,39]。然而,CMV-satRNA与番茄不孕病毒(Tomato aspermy virus,TAV)混合接种寄主植物时,TAV在寄主上的症状减轻,但TAV的基因组含量并没有降低[40,41]。此外,CMV-satRNA与小西葫芦黄化花叶病毒(Zucchini yellow mosaic virus,ZYMV)共同侵染时,ZYMV的基因组含量反而增加[42],因而satRNA的致病性是satRNA、辅助病毒和侵染寄主三者之间相互作用的结果[43]。
1982年,Gonsalves等[38]就发现WL2 satRNA可以引起西红柿叶片的白化症状,并且叶片中叶绿素含量和胡萝卜素含量下降非常明显。在B5 satRNA侵染的番茄叶片中也发现了叶绿素含量降低现象[39]。直到2011年,科研人员发现CMV在侵染烟草过程中,其satRNA产生的小干扰RNA(Small interfering RNA,siRNA)是引发叶片黄化症状的关键因子。病毒siRNA是植物DCL蛋白切割病毒双链RNA而产生的一类约21-24 nt的RNA,是植物抗病毒免疫反应的重要中间产物[44]。然而,由于病毒siRNA与叶绿素合成基因(Chlorophyll biosynthetic gene,CHLI)的mRNA存在22 nt互补区域,因而随着satRNA的siRNA产生,CHLI的mRNA会被降解,影响了叶绿素合成并导致烟草叶片的黄化症状[45,46]。此外,我们利用CMV-M侵染烟草的体系,获得了大量病毒siRNA数据。通过与烟草基因组比对发现,CMV基因组的siRNA也可靶向烟草基因[47]。而病毒siRNA在CMV-M致病过程中的具体功能需要做进一步的研究。
3 CMV致病相关的寄主蛋白
由于病毒自身编码蛋白有限,病毒进入植物细胞后会利用各种寄主资源完成其侵染过程。表1是目前已鉴定出的与CMV编码蛋白互作的寄主蛋白。在CMV的复制过程中,1a蛋白首先锚定在液泡膜上或者液泡膜蛋白上。2a蛋白可通过N端126位氨基酸与1a蛋白进行互作,并定位在液泡膜上,利用寄主体内的其他蛋白协同完成病毒的复制。而当病毒完成复制以后,2a蛋白被磷酸化,停止与1a蛋白的互作,并游离在细胞质中,参与病毒的其它侵染过程[5]。利用酵母双杂交技术已筛选到烟草的Tcoi1蛋白可以与1a蛋白的甲基转移酶结构域进行互作,大量表达Tcoi1有利于CMV侵染,而降低Tcoi1的表达会抑制CMV侵染活性,推测该Tcoi1蛋白可以将病毒1a蛋白进行甲基化,从而帮助病毒完成复制与运输过程[48]。烟草中的Tcoi2蛋白也可以与1a蛋白的甲基转移酶结构域进行互作,而功能是将1a蛋白的甲基转移酶结构域进行磷酸化,推测1a蛋白的磷酸化可能影响1a蛋白与2a蛋白或者其它寄主因子的互作[49]。此外,烟草BRP1(Bromodomain containing RNA binding Protein)蛋白与1a蛋白也存在互作,然而沉默BRP1后并不能完全抑制病毒的复制,推测该蛋白很可能是通过影响病毒复制复合物的稳定性而间接影响病毒复制[50]。从辣椒(Capsicum annuum ‘Bukang’)中还分离鉴定出与1a蛋白解旋酶结构域互作的甲酸脱氢酶(Formate dehydrogenase,FDH)和钙网蛋白前体(Calreticulin-3 precursor,CRT3),在沉默FDH的烟草中病毒不能系统移动,而在沉默CRT3的烟草中病毒不能复制和系统侵染[51]。烟草所编码的Tsip1蛋白与1a和2a蛋白均可以互作。在超表达Tsip1的烟草中病毒RNA的含量降低,而沉默Tsip1的植株中病毒含量持续增加,推测Tsip1蛋白可以通过与1a、2a蛋白形成复合物来直接影响病毒的复制[52]。烟草所编码的calcineurin B-like互作蛋白激酶(NtCIPK12)可以与2a蛋白互作,并对2a蛋白进行磷酸化[53],影响2a蛋白与1a蛋白的互作[54]。
Argonaute蛋白(AGO)是植物小RNA代谢通路中的重要因子。2b蛋白可以通过与拟南芥的AGO1和AGO4互作来影响其剪切活性,不仅干扰植物基因沉默抗病毒通路,同时干扰寄主体内miRNA代谢途径[55-57]。除了发挥VSR功能以外,2b蛋白还是宿主吸引昆虫媒介的诱导因子(A viral inducer of host attractiveness to insect vectors,VIA)。JAZ蛋白(Jasmonate ZIM-domain proteins)是茉莉酸信号通路中的重要抑制因子,2b蛋白通过与JAZ蛋白互作抑制该蛋白的降解,从而抑制茉莉酸信号通路并增强寄主植物对蚜虫的吸引力[58]。此外,2b蛋白可以与拟南芥的过氧化氢酶(CAT3)互作并抑制其酶活性。由于CAT是过氧化氢的重要水解酶类,CAT活性受抑将导致过氧化氢含量积累以及细胞的坏死[59]。
CsAO4是黄瓜所编码的,定位在细胞壁上的抗坏血酸氧化酶(Ascorbate oxidase)。病毒侵染后可诱发该蛋白在烟草体内的表达。将编码该蛋白的基因敲除后可降低CMV在系统叶片上的积累,而将该蛋白进行过量表达后并没有明显增加CMV在系统叶上的积累,推测是该蛋白在病毒侵染早期,与MP一同协助病毒运输至邻近细胞[60]。
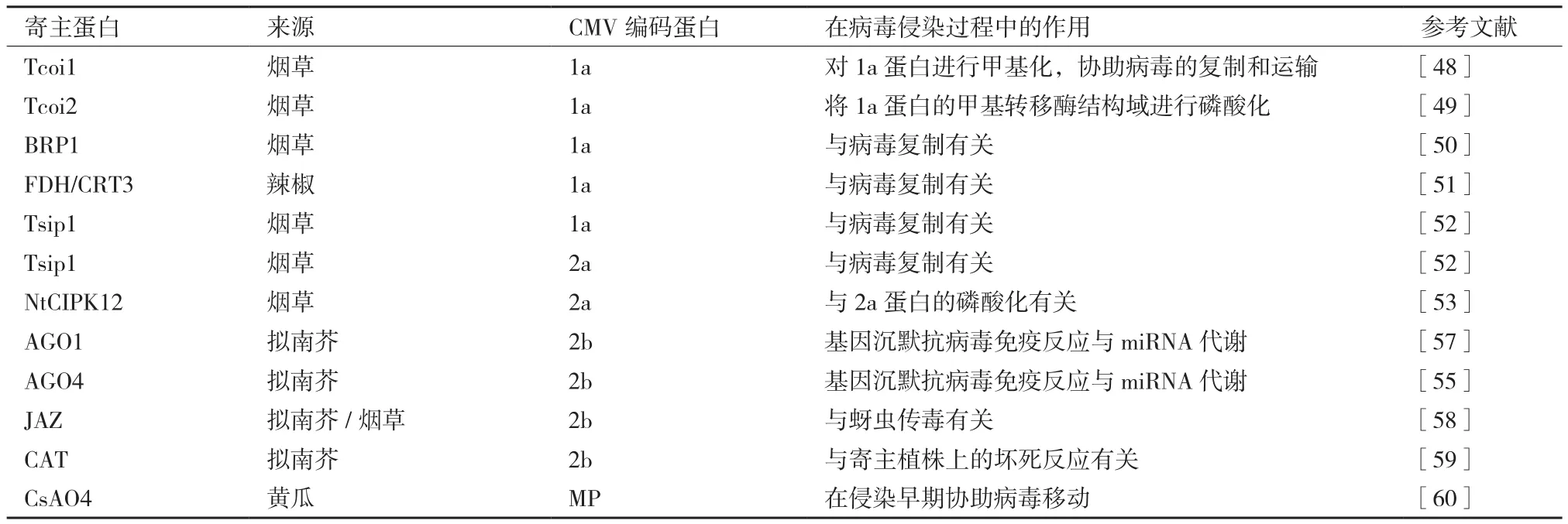
表1 与CMV编码蛋白互作的主要寄主蛋白
4 结语
植物病毒致病的分子机制一直是植物病毒领域的研究热点。利用酵母双杂交(Yeast two hybrid,YTH)、双分子荧光(Bimolecular fluorescence comp-lement,BiFC)及免疫共沉淀(Coimmunoprecipitation,COIP)等技术已鉴定出多个参与CMV侵染的寄主蛋白。此外,利用转录组测序技术以及蛋白质组学研究发现大量参与病毒致病过程的寄主基因和蛋白,为进一步揭示CMV致病相关的分子机制奠定基础。
随着基因沉默介导的植物抗病毒免疫反应机制的研究,尤其CMV-satRNA的siRNA沉默寄主植物基因引发黄白化症状的发现,将植物病毒的siRNA参与病毒致病过程的研究推向热点,也为植物病毒致病性研究开辟了新的研究领域。
虽然CMV的致病机制研究取得了很大进展,但关于CP及第129位氨基酸如何影响寄主症状的产生以及如何影响叶绿体结构和功能都有待进一步研究。此外,病毒基因组产生的siRNA如何靶标于寄主基因以及在致病过程中的作用仍需探究。
[1] Doolittle SP. A new infectious mosaic disease of cucumber[J]. Phytopathology, 1916, 6:145-147.
[2] Jagger IC. Experiments with the cucumber mosaic disease[J]. Phytopathology, 1916, 6:148-151.
[3] Palukaitis P, Roossinck MJ, Dietzgen R G, et al. cucumber mosaic virus[J]. Advances in Virus Research, 1992, 41:281-348.
[4] Mochizuki T, Ohki ST. Cucumber mosaic virus:viral genes as virulence determinants[J]. Molecular Plant Pathology, 2012, 13(3):217-225.
[5] Palukaitis P, Garcia-Arenal F, Cucumoviruses[J]. Advances in Virus Research, 2003, 62:241-323.
[6] Galon A, Kaplan I, Roossinck MJ, et al. The kinetics of infection of zucchini squash by cucumber mosaic virus indicate a function for RNA1 in virus movement[J]. Virology, 1994, 205(1):280-289.
[7] Diveki Z, Salanki K, Balazs E. The necrotic pathotype of the cucumber mosaic virus(CMV)Ns strain is solely determined by amino acid 461 of the 1a protein[J]. Molecular Plant-Microbe Interactions, 2004, 17(8):837-845.
[8] Salanki K, Gellert A, Naray-Szabo G, et al. Modeling-based characterization of the elicitor function of amino acid 461 of cucumber mosaic virus 1a protein in the hypersensitive response[J]. Virology, 2007, 358:109-118.
[9] Kang WH, Seo JK, Chung BN, et al. Helicase domain encoded by cucumber mosaic virus RNA1 determines systemic infection of Cmr1 in pepper[J]. PLoS One, 2012, 7(8):e43136.
[10] Kang WH, Hoang NH, Yang HB, et al. Molecular mapping and characterization of a single dominant gene controlling CMV resistance in peppers(Capsicum annuum L. )[J]. Theoretical and Applied Genetics, 2010, 120:1587-1596.
[11] Du ZY, Chen FF, Zhao ZJ, et al. The 2b protein and the C-terminus of the 2a protein of Cucumber mosaic virus subgroup I strains both play a role in viral RNA accumulation and induction of symptoms[J]. Virology, 2008, 380(2):363-370.
[12] Choi SK, Palukaitis P, Min BE, et al. Cucumber mosaic virus 2a polymerase and 3a movement proteins independently affect both virus movement and the timing of symptom development in zucchini squash[J]. Journal of General Virology, 2005, 86:1213-1222.
[13] Hu ZZ, Zhang TQ, Yao M, et al. The 2a protein of cucumber mosaic virus induces a hypersensitive response in cowpea independently of its replicase activity[J]. Virus Research, 2012, 170:169-173.
[14] Karasawa A, Okada I, Akashi K, et al. One amino acid change in cucumber mosaic virus RNA polymerase determines virulent/ avirulent phenotypes on cowpea[J]. Phytopathology, 1999, 89:1186-1192.
[15] Csorba T, Kontra L, and Burgyan J. Viral silencing suppressors:Tools forged to fine-tune host-pathogen coexistence[J]. Virology, 2015, 479:85-103.
[16] Feng JL, Liu SS, Wang MN, et al. Identification of microRNAs and their targets in tomato infected with cucumber mosaic virus based on deep sequencing[J]. Planta, 2014, 240(6):1335-1352.
[17] Du ZY, Chen AZ, Chen WH, et al. Using a viral vector to reveal the role of microRNA159 in disease symptom induction by a severe strain of cucumber mosaic virus[J]. Plant Physiology, 2014, 164(3):1378-1388.
[18] Lewsey M, Robertson FC, Canto T, et al. Selective targeting of miRNA-regulated plant development by a viral counter-silencing protein[J]. Plant Journal, 2007, 50(2):240-252.
[19] Ding SW, Li WX, Symons RH. A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement[J]. The EMBO Journal, 1995, 14(23):5762-5772.
[20] Diaz-Pendon JA, Li F, Li WX, et al. Suppression of antiviralsilencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs[J]. Plant Cell, 2007, 19(6):2053-2063.
[21] Galon A, Kaplan IB, Palukaitis P. Characterization of cucumber mosaic virus. II. Identification of movement protein sequences that influence its accumulation and systemic infection in tobacco[J]. Virology, 1996, 226(2):354-361.
[22] Kaplan IB, Galon A, Palukaitis P. Characterization of cucumber mosaic virus. III. Localization of sequences in the movement protein controlling systemic infection in cucurbits[J]. Virology, 1997, 230(2):343-349.
[23] Callaway A, Giesman CD, Gillock E, et al. The multifunctional capsid proteins of plant RNA viruses[J]. Annual Review of Phytopathology, 2001, 39(1):419-460.
[24] Salanki K, Kiss L, Gellert A, et al. Identification a coat protein region of cucumber mosaic virus(CMV)essential for longdistance movement in cucumber[J]. Archives of Virology, 2011, 156(12):2279-2283.
[25] Rao A, Francki R. Distribution of determinants for symptom production and host range on the three RNA components of cucumber mosaic virus[J]. Journal of General Virology, 1982, 61(2):197-205.
[26] Shintaku MH, Lee Z, Palukaitis P. A single amino acid substitution in the coat protein of cucumber mosaic virus induces chlorosis in tobacco[J]. Plant Cell, 1992, 4(7):751-757.
[27] Mochizuki T, Ohki ST. Single amino acid substitutions at residue 129 in the coat protein of cucumber mosaic virus affect symptom expression and thylakoid structure[J]. Archives of Virology, 2011, 156(5):881-886.
[28] Gellert A, Salanki K, Naray-Szabo G, et al. Homology modelling and protein structure based functional analysis of five cucumovirus coat proteins[J]. Journal of Molecular Graphics & Modelling, 2006, 24(5):319-327.
[29] Zhao J, Zhang X, Hong Y, et al. Chloroplast in plant-virus interaction[J]. Frontiers in Microbiology, 2016, 7:1565.
[30] Lu J, Du ZX, Kong J, et al. Transcriptome analysis of Nicotiana tabacum infected by cucumber mosaic virus during systemic symptom development[J]. PLoS One, 2012, 7(8):e43447.
[31] Mochizuki T, Ogata Y, Hirata Y, et al. Quantitative transcriptional changes associated with chlorosis severity in mosaic leaves of tobacco plants infected with cucumber mosaic virus[J]. Molecular Plant Pathology, 2014, 15(3):242-254.
[32] Takahashi H, Ehara Y, Hirano H. A protein in the oxygen-evolving complex in the chloroplast is associated with symptom expression on tobacco leaves infected with cucumber mosaic virus strain Y[J]. Plant Molecular Biology, 1991, 16(4):689-698.
[33] Di Carli M, Villani ME, Bianco L, et al. Proteomic analysis of the plant-virus interaction in cucumber mosaic virus(CMV)resistant transgenic tomato[J]. Journal of Proteome Research, 2010, 9(11):5684-5697.
[34] Lei R, Jiang H, Hu F, et al. Chlorophyll fluorescence lifetime imaging provides new insight into the chlorosis induced by plant virus infection[J]. Plant Cell Reports, 2017, 36(2):327-341.
[35] Lei R, Du ZX, Qiu YH, et al. The detection of hydrogen peroxide involved in plant virus infection by fluorescence spectroscopy[J]. Luminescence, 2016, 31(5):1158-1165.
[36] Song XS, Wang YJ, Mao WH, et al. Effects of cucumber mosaic virus infection on electron transport and antioxidant system in chloroplasts and mitochondria of cucumber and tomato leaves[J]. Physiologia Plantarum, 2009, 135(3):246-257.
[37] Xu P, Roossinck MJ. cucumber mosaic virus D satellite RNA-induced programmed cell death in tomato[J]. Plant Cell, 2000, 12(7):1079-1092.
[38] Gonsalves D, Provvidenti R, Edwards MC. Tomato white leaf:the relation of an apparent satellite RNA and cucumber mosaic virus[J]. Phytopathology, 1982, 72:1533-1538.
[39] Garcia-Arenal F, Zaitlin M, Palukaitis P. Nucleotide sequence analysis of six satellite RNAs of cucumber mosaic virus:primary sequence and secondary structure alterations do not correlate with differences in pathogenicity[J]. Virology, 1987, 158:339-347.
[40] Moriones E, Diaz I, Rodriguez-Cerezo E, et al. Differential interactions among strains of Tomato aspermy virus and satellite RNAs of cucumber mosaic virus[J]. Virology, 1992, 186(2):475-480.
[41] Moriones E, Fraile A, Garcia-Arenal F. Host-associated selection of sequence variants from a satellite RNA of cucumber mosaic virus[J]. Virology, 1991, 184(1):465-468.
[42] Wang Y, Gaba V, Yang J, et al. Characterization of synergy between cucumber mosaic virus and potyviruses in cucurbit hosts[J].Phytopathology, 2002, 92(1):51-58.
[43] Gal-On A, Kaplan I, Palukaitis P. Differential effects of satellite RNA on the accumulation of cucumber mosaic virus RNAs and their encoded proteins in tobacco vs zucchini squash with two strains of CMV helper virus[J]. Virology, 1995, 208(1):58-66.
[44] Ding SW. RNA-based antiviral immunity[J]. Nature Reviews Immunology, 2010, 10(9):632-644.
[45] Smith NA, Eamens AL, Wang MB. Viral small interfering RNAs target host genes to mediate disease symptoms in plants[J]. PLoS Pathogens, 2011, 7(5):e1002022.
[46] Shimura H, Pantaleo V, Ishihara T, et al. A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery[J]. PLoS Pathogens, 2011, 7(5):e1002021.
[47] Qiu YH, Zhang YJ, Hu F, et al. Characterization of siRNAs derived from cucumber mosaic virus in infected tobacco plants[J]. Archives of Virology, 2017, 162(7):2077-2082.
[48] Kim MJ, Huh SU, Ham BK, et al. A novel methyltransferase methylates cucumber mosaic virus 1a protein and promotes systemic spread[J]. Journal of Virology, 2008, 82(10):4823-4833.
[49] Kim MJ, Ham BK, Paek KH. Novel protein kinase interacts with the cucumber mosaic virus 1a methyltransferase domain[J]. Biochemical and Biophysical Research Communications, 2006, 340(1):228-235.
[50] Chaturvedi S, Seo JK, Rao ALN. Functionality of host proteins in cucumber mosaic virus replication:GAPDH is obligatory to promote interaction between replication-associated proteins[J]. Virology, 2016, 494:47-55.
[51] Choi Y, Kang MY, Lee JH, et al. Isolation and characterization of pepper genes interacting with the CMV-P1 helicase domain[J]. PLoS One, 2016, 11(1):e0146320.
[52] Huh SU, Kim MJ, Ham BK, et al. A zinc finger protein Tsip1 controls cucumber mosaic virus infection by interacting with the replication complex on vacuolar membranes of the tobacco plant[J]. New Phytologist, 2011, 191:746-762.
[53] Kang HK, Yang SH, LeeY P, et al. A tobacco CBL-interacting protein kinase homolog is involved in phosphorylation of the N-terminal domain of the cucumber mosaic virus polymerase 2a protein[J]. Biosci Biotechnol Biochem, 2012, 76(11):2101-2106.
[54] Kim SH, Palukaitis P, Park YI. Phosphorylation of cucumber mosaic virus RNA polymerase 2a protein inhibits formation of replicase complex[J]. The EMBO Journal, 2002, 21(9):2292-2300.
[55] Hamera S, Song X, Su L, et al. cucumber mosaic virus suppressor 2b binds to AGO4-related small RNAs and impairs AGO4 activities[J]. The Plant Journal, 2012, 69(1):104-115.
[56] Lewsey MG, Gonzalez I, Kalinina NO, et al. Symptom induction and RNA silencing suppression by the cucumber mosaic virus 2b protein[J]. Plant Signaling & Behavior, 2010, 5(6):705-708.
[57] Zhang X, Yuan YR, Pei Y, et al. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense[J]. Genes & Development, 2006, 20(23):3255-3268.
[58] Wu DW, Qi TC, Li WX, et al. Viral effector protein manipulates host hormone signaling to attract insect vectors[J]. Cell Research, 2017, 27(3):402-415.
[59] Inaba J, Kim BM, Shimura H, et al. Virus-induced necrosis is a consequence of direct protein-protein interaction between a viral RNA-silencing suppressor and a host catalase[J]. Plant Physiology, 2011, 156(4):2026-2036.
[60] Kumari R, Kumar S, Singh L, et al. Movement protein of cucumber mosaic virus associates with apoplastic ascorbate oxidase[J]. PLoS One, 2016, 11(9):e0163320.
(责任编辑 朱琳峰)
Research Advances on the Pathogenicity of Cucumber Mosaic Virus
QIU Yan-hong1WANG Chao-nan1,2ZHU Shui-fang1
(1. Institute of Plant Quarantine,Chinese Academy of Inspection and Quarantine,Beijing 100176;2. College of Plant Protection,China Agricultural University,Beijing 100193)
Cucumber mosaic virus(CMV)is the type member of single stranded RNA viruses. As it infects wide host species and induces serious symptoms,it has been considered as one of the most impact plant virus and also the severe plant disease in the world. Many domestic and overseas scholars have investigated extensively about the CMV genes,gene-encoded products and plant-virus interactions since it was reported in 1916. With the development of high-throughput sequencing technology and proteomic technology,the molecular mechanism of viral virulence has been made great progresses. In this review,we introduce the role of viral encoding proteins,its associated satellite RNA and host factors that are involved in the development of symptoms,and also discusse the further study on viral virulence of CMV,which will provide a useful reference for CMVrelated studies.
cucumber mosaic virus;pathogenic mechanism;small interfering RNA;protein-protein interaction
10.13560/j.cnki.biotech.bull.1985.2017-0278
2017-04-09
中国检验检疫科学研究院基本科研业务费项目(2017JK035)
邱艳红,女,助理研究员,研究方向:植物病毒学;E-mail:qiuyh@caiq.gov.cn
朱水芳,男,研究员,研究方向:植物病毒学;E-mail:zhusf@caiq.gov.cn

