联合黄连素治疗重性抑郁障碍患者躯体疼痛的随机、双盲、安慰剂、对照研究
杨程皓,马彦彦,安旭光,李 洁
(天津市安定医院,天津 300222*通信作者:李 洁,E-mail:tjlijie3827@163.com)
论著·临床
联合黄连素治疗重性抑郁障碍患者躯体疼痛的随机、双盲、安慰剂、对照研究
杨程皓,马彦彦,安旭光,李 洁*
(天津市安定医院,天津 300222
*通信作者:李 洁,E-mail:tjlijie3827@163.com)
目的 观测联合黄连素治疗对伴有躯体疼痛且既往三环类抗抑郁药(TCAs)和5-羟色胺和去甲肾上腺素再摄取抑制剂(SNRIs)治疗抵抗的重性抑郁障碍(MDD)的效果,探索抗抑郁治疗的新方向。方法 选取在天津市安定医院门诊就诊或住院治疗的伴有TCAs、SNRIs治疗抵抗的、符合《精神障碍诊断与统计手册(第4版)》(DSM-IV)重性抑郁障碍诊断标准的患者47例,采用随机数字表法分为研究组(n=24)和对照组(n=23)。研究组和对照组在现有治疗药物基础上分别联用黄连素和安慰剂,两组均治疗6周。于基线期和治疗6周末进行汉密尔顿抑郁量表17项版(HAMD-17)和简明疼痛量表(BPI)评定,于治疗6周末采用副反应量表(TESS)评定安全性。结果 治疗6周末,两组显著改善例数、改善例数比较差异均有统计学意义(χ2=4.286、3.901,P均<0.01),研究组HAMD-17和BPI各项评分均低于治疗前,且低于对照组(P均<0.01);研究组和对照组不良事件发生率比较差异无统计学意义(27.3% vs. 22.7%,χ2=0.063,P=0.342)。结论 联合黄连素治疗伴有躯体疼痛的SNRIs、TCAs治疗抵抗的MDD,可能有助于改善患者抑郁症状和躯体疼痛相关症状,且可能不显著增加不良反应发生率。
黄连素;治疗抵抗;抑郁症;躯体疼痛;简明疼痛问卷表
“抑郁”和“疼痛”是当今重要的公共健康问题。抑郁症是造成人体功能残疾的主要病因[1],在世界范围内这一人群总数超过3亿[2],极大程度地增加医疗资源消耗和经济负担。同时,有报告指出,约1亿美国人口遭受着慢性疼痛的侵袭,造成的经济损失高达6 350亿美元[3]。此外,抑郁症和疼痛经常同时存在,使问题更加复杂。数据显示,约30%~60%的疼痛患者存在抑郁情绪[4],约50%的抑郁症患者会出现躯体疼痛症状[5]。进一步研究显示,伴有抑郁情绪的疼痛患者,其疼痛症状更严重、预后更差、功能障碍更明显[6]。抑郁症患者的症状严重程度和疼痛明显相关[7]。疼痛程度越严重,疼痛部位越多,持续时间越久,抑郁症的治疗难度越大[8]。目前,针对伴有躯体疼痛的抑郁症患者,美国食品药品管理局(FDA)推荐的治疗药物主要包括三环类抗抑郁药(tricyclic antidepressants,TCAs)和5-羟色胺和去甲肾上腺素再摄取抑制剂(serotonin-norepinephrine reuptake inhibitors,SNRIs)[9]。但这些药物并不是总能取得理想的疗效,加之存在的药物不耐受、药物之间相互作用,急需探索新的治疗策略。
黄连素又称盐酸小檗碱,是黄连的有效成分之一,其生物学活性主要包括抗氧化应激、抗菌、抗病毒、抗炎[10-12]、激活阿片受体[13]等,使得黄连素能够发挥广泛而积极的神经生物学效用。黄连素不仅具有良好的抗抑郁效应[14-15],还能有效缓解疼痛[13,16]。鉴于抑郁症和疼痛的交互影响,故而考虑黄连素可能对抑郁症伴有躯体疼痛具有良好的效果。本研究针对既往经SNRIs和TCAs系统治疗疼痛症状改善不理想的重性抑郁障碍(major depressive disorder,MDD)患者,采用联合黄连素治疗,探讨其对抑郁症状、躯体疼痛的疗效及安全性。
1 对象与方法
1.1 对象

1.2 治疗方法
研究组在当前治疗药物的基础上联用黄连素300 mg tid(东北制药集团沈阳第一制药有限公司,2141008);对照组在当前治疗药物的基础上联用与黄连素外形、颜色等匹配的安慰剂。两组均治疗6周。研究期间两组抗抑郁药、增效治疗(抗精神病药物、情感稳定剂)剂量不能调整。
1.3 评定方法
于治疗前和治疗6周后对两组进行HAMD-17和BPI评定,于治疗6周末进行副反应量表(Treatment Emergent Symptom Scale,TESS)评定。其中BPI只对比部分内容:S-A、过去24小时疼痛最剧烈的程度(S-Most, S-M)、对日常生活的影响程度(I-Life, I-L)、对情绪的影响(I-Mood, I-M)和对生活兴趣的影响(I-Entertainment, I-E)。对于BPI疼痛评定,只对比组内治疗前后、组间同时间的疼痛程度和影响程度的差异[17]。HAMD-17大部分项目为5级评分:无、轻度、中度、重度、很重,分别计0~4分;部分项目为3级评分:无、轻中度、重度,分别计0分、1分、2分,总评分≤8分为无抑郁;8分<总评分≤17分为轻度抑郁;17分<总评分≤24分为中度抑郁;总评分>24分为重度抑郁。以HAMD-17评分减分率判定临床疗效:减分率≥75%为痊愈,50%≤减分率<75%为显著改善,25%≤减分率<50%为改善,减分率<25%为无效。HAMD-17评分减分率=(治疗前总评分-治疗后总评分)/治疗前总评分×100%。总显效率=(痊愈例数+显著改善例数)/该组总例数×100%,总有效率=(痊愈例数+显著改善例数+改善例数)/该组总例数×100%。由2名经过一致性培训的具有主治医师职称的医生进行评定,对研究分组及用药情况不了解。测评地点为科研接待室,环境安静舒适;每次评估耗时约40 min。
1.4 统计方法
2 结 果
2.1 研究完成情况及患者一般资料
至研究结束,有3例受试者中途退出,共44例完成:研究组退出2例女性(中途调整药物1例,消化不良1例),完成22例,其中男性10例,女性12例;平均年龄(45.10±6.70)岁,平均病程(8.64±1.37)年。对照组退出1例男性(家属反对);完成22例,其中男性11例,女性11例;平均年龄(43.20±8.10)岁,平均病程(7.62±1.71)年。两组年龄(χ2=1.043,P=0.364)、病程(χ2=1.375,P=0.162)、性别(χ2=0.628,P=0.524)差异均无统计学意义。
2.2 两组临床疗效
研究组痊愈0例,显著改善8例(36.3%),改善10例(45.5%),未愈4例(18.2%);对照组痊愈0例,显著改善0例,改善4例(18.2%),未愈18例(81.8%)。两组显著改善(χ2=4.286,P<0.01)、改善(χ2=3.901,P<0.01)、未愈(χ2=6.674,P<0.01)例数比较差异均有统计学意义。
2.3 两组HAMD-17、BPI评分比较
治疗前两组HAMD-17和BPI各项评分比较差异均无统计学意义(P均>0.05),治疗6周后,研究组HAMD-17和BPI各项评分均较治疗前低(P均<0.01),且研究组HAMD-17和BPI各项评分均低于对照组,差异均有统计学意义(P均<0.01),对照组治疗前后比较差异均无统计学意义(P均>0.05)。见表1。
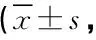
表1 两组HAMD-17、BPI评分比较分)
注:HAMD-17,汉密尔顿抑郁量表17项版;BPI,简明疼痛量表;S-M,过去24小时疼痛最剧烈的程度;S-A,过去24小时疼痛平均程度;I-L,对日常生活的影响程度;I-M,对情绪的影响;I-E,对生活兴趣的影响;t1、P1为治疗前两组比较;t2、P2为治疗6周后两组比较;t3、P3为研究组治疗前后比较;t4、P4为对照组治疗前后比较
2.4 两组不良反应比较
研究组共出现与研究药物相关的不良反应共6人、11例,其中嗜睡(2例),口干(4例),便秘(4例),出汗(1例);对照组共发生5人、13例,其中便秘(3例),嗜睡(3例),震颤(4例),口干(3例)。研究组和对照组不良反应发生率比较差异无统计学意义(27.3% vs. 22.7%,χ2=0.063,P=0.342)。
3 讨 论
抑郁伴有躯体疼痛明显增加治疗难度且复发率较高,是临床治疗的难点。本研究结果显示,伴躯体疼痛的MDD患者在接受联合黄连素治疗6周后,HAMD-17和BPI评分均较治疗前低(P均<0.01),且低于安慰剂对照组,差异有统计学意义(P均<0.01)。说明联合黄连素治疗此类人群可能更有助于改善抑郁症状和躯体疼痛,且不显著增加不良反应。
抑郁共病疼痛的病理机制复杂。研究显示,疼痛感知的神经通路与处理情感、情绪的脑区相关联[18],神经通路的活化可能会同时影响这两方面问题的临床表现;其次,抑郁症引起的认知歪曲、感知觉异常可能会让患者对疼痛更敏感或更易于出现躯体不适[19]。此外,细胞因子如炎症因子、脑源性神经营养因子等,通过影响神经元周围环境、下调G蛋白耦联受体激酶2,进而激活色氨酸代谢酶(吲哚胺2,3-二加氧酶)产生有毒的犬尿素代谢产物而发挥作用[20-21]。动物研究显示,氯胺酮通过抑制吲哚胺2,3-二加氧酶信号通路可改善疼痛抑郁共病大鼠的行为学表现[22]。黄连素同样具有良好的抗炎、抗氧化应激的活性[10-12]。此外,黄连素还可促进神经元突起再生、轴突形成、保护神经元、抗细胞凋亡、调节神经递质的生成[23-25],不仅能有效缓解大鼠慢性限制性损伤引起的坐骨神经痛[16],还可通过μ/δ阿片受体介导改善肠易激综合征模型大鼠的内脏痛[13]。黄连素在抗抑郁、抗疼痛两方面的作用相互促进、改善临床整体疗效,是发挥积极效应的生物学基础。
即使患者抵抗治疗抑郁伴疼痛的一线药物,联合黄连素治疗仍然能够取得良好的疗效,是该类人群潜在的治疗选择。结合黄连素的药理学特性,提示具有抗炎、抗氧化应激、促进神经发生等生物活性的药物在强异质性精神疾病的治疗中可能有较好的前景。
本研究不足之处在于:样本量较小,干预时间较短,无随访观察;对样本是否为难治性抑郁症控制不足,造成混入难治性抑郁症患者较多的一组治疗难度增大;本研究对联合用药未做严格的控制,目的是更接近实际的临床诊疗处理,但也可能使结果产生偏倚。且研究组无一例完全缓解的患者,可能需要延长观测时间以进一步检验疗效。结合动物研究有助于深入探索该药物如何发挥效应、明确作用机理。希望在将来的深入研究中更正上述问题。
[1] Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R)[J]. JAMA, 2003, 289(23): 3095-3105.
[2] World Health Organization. Depression, media centre, fact sheet [EB/OL]. http://www.who.int/mediacentre/factsheets/fs369/en/, 2012-02.
[3] Steglitz J, Buscemi J, Ferguson MJ. The future of pain research, education, and treatment: a summary of the IOM report "Relieving pain in America: a blueprint for transforming prevention, care, education, and research"[J]. Transl Behav Med, 2012, 2(1): 6-8.
[4] Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review[J]. Arch Intern Med, 2003, 163(20): 2433-2445.
[5] Katona C, Peveler R, Dowrick C, et al. Pain symptoms in depression: definition and clinical significance[J].Clin Med (Lond), 2005, 5(4): 390-395.
[6] Borsbo B, Peolsson M, Gerdle B. The complex interplay between pain intensity, depression, anxiety and catastrophising with respect to quality of life and disability[J]. Disabil Rehabil, 2009, 31(19): 1605-1613.
[7] Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review[J].Clin J Pain, 1997, 13(2): 116-137.
[8] Gerrits MM, Vogelzangs N, van Oppen P, et al. Impact of pain on the course of depressive and anxiety disorders[J].Pain, 2012, 153(2): 429-436.
[9] Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors[J].Curr Psychiatry Rep, 2013, 15(12): 421.
[10] Turk DC. Cognitive-behavioral approach to the treatment of chronic pain patients[J]. Reg Anesth Pain Med, 2003, 28(6): 573-579.
[11] Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain[J]. Best Pract Res Clin Rheumatol, 2011, 25(2): 299-309.
[12] Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews[J]. Gen Hosp Psychiatry, 2009, 31(3): 206-219.
[13] Chen C, Lu M, Pan Q, et al. Berberine improves intestinal motility and visceral pain in the mouse models mimicking diarrhea-predominant irritable bowel syndrome (IBS-D) symptoms in an opioid-receptor dependent manner[J]. PLoS One, 2015, 10(12): e0145556.
[14] Shen JD, Ma LG, Hu CY, et al. Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice[J].Neurosci Lett, 2016, 614: 77-82.
[15] Zhu X, Sun Y, Zhang C, et al. Effects of berberine on a rat model of chronic stress and depression via gastrointestinal tract pathology and gastrointestinal flora profile assays[J].Mol Med Rep, 2017, 15(5): 3161-3171.
[16] Kiim HJ. Berberine ameliorates allodynia induced by chronic constriction injury of the sciatic nerve in rats[J]. J Med Food, 2015, 18(8): 909-915.
[17] Ping G, East L, Arthur A. A preoperative education intervention to reduce anxiety and improve recovery among Chinese cardiac patients: a randomized controlled trial[J]. Int J Nurs Stud, 2012, 49(2): 129-137.
[18] Meerwijk EL, Ford JM, Weiss SJ. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain[J]. Brain Imaging Behav, 2013, 7(1): 1-14.
[19] Gupta A, Silman AJ, Ray D, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study[J].Rheumatology (Oxford), 2007, 46(4): 666-671.
[20] 覃加敏, 陆永经, 蔡伦, 等. 疼痛与抑郁共病的炎症机制[J]. 医学研究生学报, 2015, 9(8): 893-896.
[21] 刘迪, 唐倩倩, 曹君利, 等. 脑源性生长因子参与疼痛-抑郁共病的研究进展[J]. 中国药理学通报, 2015, 13 (1): 26-30.
[22] 韩金凤, 徐宁, 潘薇, 等. 氯胺酮对疼痛抑郁共病大鼠行为学及吲哚胺2,3二加氧酶信号通路的影响[J]. 临床麻醉学杂志, 2016, 32(12): 1200-1203.
[23] Han AM, Heo H, Kwon YK. Berberine promotes axonal regeneration in injured nerves of the peripheral nervous system[J]. J Med Food, 2012, 15(4): 413-417.
[24] Peng WH, Lo KL, Lee YH, et al. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice[J]. Life Sci, 2007, 81(11): 933-938.
[25] Hu Y, Ehli EA, Hudziak JJ, et al. Berberine and evodiamine influence serotonin transporter (5-HTT) expression via the 5-HTT-linked polymorphic region[J]. Pharmacogenomics J, 2012, 12(5): 372-378.
(本文编辑:陈 霞)
Combined berberine in treating physical pain of major depressive disorder patients: a randomize double-blind placebo-control study
YangChenghao,MaYanyan,AnXuguang,LiJie*
(TianjinAndingHospital,Tianjin300222,China*Correspondingauthor:LiJie,E-mail:tjlijie3827@163.com)
Objective To evaluate the efficacy and safety of augmentation treatment with berberine in major depressive disorder (MDD) patients with physical pain who were resistance to TCAs and SNRIs therapy. This study also aimed to explore new methods for anti-depressant treatment.Methods 47 patients who met the criteria of MDD in Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) with TCAs and SNRIs treatment resistance from Tianjin Anding Hospital were selected. They were randomly divided into the study group (n=24) and the control group (n=23).The study group and the control group were treated with berberine and placebo respectively on the basis of the existing drug treatment. The two groups were treated for 6 weeks. The Hamilton Depression Scale-17 item (HAMD-17) and Brief Pain Inventory (BPI) were assessed at the baseline and at the end of the 6thweek. The safety was assessed with Treatment Emergent Symptom Scale (TESS) after treatment for 6 weeks.Results At the end of the 6thweek, the study group had significantly higher rate of improvement and rate of effective than the control group (χ2=4.286, 3.901,P<0.01). The scores of HAMD-17 and BPI of the study group were lower than before treatment and lower than those of the control group(P<0.01). There was no significant difference in the incidence of adverse events between the two groups (27.3% vs. 22.7%,χ2=0.063,P=0.342).Conclusion Augmentation treatment with berberine to MDD patients with physical pain who were therapy-resistant to TCAs and SNRIs therapy may help to improve symptoms of depression and physical pain without increase the incidence of adverse reactions.
Berberine; Therapy resistance; Major depressive disorder; Physical pain; Brief Pain Inventory
天津市卫生行业重点攻关项目(13KG118);天津市自然科学基金一般项目(16JCYBJC24200)
R749.4
A
10.11886/j.issn.1007-3256.2017.04.006
2017-05-07)

