慢性应激行为与大鼠海马、前额叶区细胞外信号调节蛋白激酶信号通路激活的关系研究
王凌霄,王培福,才丽娜,黄 佳,张 晨,彭代辉,方贻儒
·论著·
慢性应激行为与大鼠海马、前额叶区细胞外信号调节蛋白激酶信号通路激活的关系研究
王凌霄1,王培福1,才丽娜1,黄 佳2,张 晨2,彭代辉2,方贻儒2
目的 探讨慢性应激行为与大鼠海马、前额叶区细胞外信号调节蛋白激酶(ERK)信号通路激活的关系。方法 2011年6月—2012年8月将50只成年清洁级雄性Sprague-Dawley大鼠随机分为对照组12只、药物组12只及应激组26只。对照组大鼠不给予任何刺激,药物组和应激组大鼠给予8周应激刺激,药物组大鼠于应激刺激第1周开始给予盐酸氟西汀皮下注射。各组大鼠于造模开始前、造模结束后1 d进行糖水偏好实验和旷场试验,应激刺激结束后采用Western bloting法检测各组大鼠海马、前额叶区ERK、磷酸化细胞外信号调节蛋白激酶(P-ERK)蛋白表达情况。结果 应激刺激过程中共11只大鼠死亡,其中正常对照组2只、药物组4只、应激组5只。根据糖水偏好实验结果将应激组大鼠分为应激适应组(n=6)和应激敏感组(n=15)。造模开始前4组大鼠跨格次数、直立次数及中心格停留时间比较,差异无统计学意义(P>0.05);造模结束后1 d,药物组、应激适应组和应激敏感组大鼠跨格次数、直立次数少于对照组,中心格停留时间长于对照组(P<0.05),应激敏感组大鼠跨格次数少于药物组(P<0.05)。应激刺激结束后,4组大鼠海马、前额叶区ERK蛋白相对表达量比较,差异无统计学意义(P>0.05)。应激刺激结束后,应激敏感组大鼠海马及前额叶区P-ERK蛋白相对表达量低于药物组和应激适应组(P<0.05),而药物组和应激适应组大鼠海马及前额叶区P-ERK蛋白相对表达量比较,差异无统计学意义(P>0.05)。结论 慢性应激刺激可导致大鼠自主活动水平下降,盐酸氟西汀可在一定程度上降低慢性应激刺激对大鼠自主活动水平的影响,海马和前额叶区ERK信号通路激活可能与大鼠应激适应有关。
应激,心理学;细胞外信号调节蛋白激酶类;海马;前额叶区;大鼠
王凌霄,王培福,才丽娜,等.慢性应激行为与大鼠海马、前额叶区细胞外信号调节蛋白激酶信号通路激活的关系研究[J].实用心脑肺血管病杂志,2017,25(7):57-61.[www.syxnf.net]
WANG L X, WANG P F,CAI L N,et al.Relationship between chronic stress behaviors and ERK signal pathway activation of hippocampus and prefrontal cortex in rats[J].Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease,2017,25(7):57-61.
心理应激是抑郁症的主要发病原因之一。临床研究显示,遭受严重创伤后大部分人表现为应激适应,10%~40%的人表现为应激障碍或情绪障碍[1]。应激适应是指个体在遭受急性或慢性应激、创伤时成功适应逆境的能力[2]。目前,有关应激适应的分子机制研究较少。有研究表明,海马部位脑源性神经生长因子(brain derived neurophic factor,BDNF)水平与大鼠应激适应有关,提示神经营养因子信号通路可能参与介导应激适应[3]。
细胞外信号调节蛋白激酶(ERK)信号通路是BDNF与受体结合后激活的重要信号通路之一,其在细胞分裂、增殖、分化、存活及神经可塑中具有重要调节作用[4]。动物实验及临床研究表明,海马区ERK信号通路激活与抑郁症发病机制及抗抑郁药物作用机制相关[5-7]。近期一项研究发现,抑制腹侧被盖区ERK信号通路激活可使大鼠发生应激适应,提示特定脑区的ERK信号通路可能参与调节不同应激行为[8]。本研究立足于神经营养因子失调假说,旨在探讨慢性应激行为与大鼠海马、前额叶区ERK信号通路激活的关系。
1 材料与方法
1.1 实验动物 2011年6月—2012年8月选用清洁级雄性Sprague-Dawlwy大鼠50只,由上海复旦大学医学院动物科学实验中心提供,入组时体质量100~300 g,旷场实验(观察大鼠在3 min内穿越实验敞箱的底面块数及后肢直立次数的垂直活动得分)结果相近,购回后在实验室动物房适应性饲养3 d,维持室温22.0 ℃,人工明/暗12 h/12 h昼夜节律(光照时间9:00~21:00),单笼饲养(鼠笼40 cm×25 cm×20 cm),自由饮水及摄食。本研究中所有实验操作遵守《中华人民共和国实验动物管理条例》及《上海市实验动物管理条例》。将大鼠随机分为对照组12只、药物组12只、应激组26只。
1.2 主要试剂和实验仪器 旷场实验观察箱(自制木箱:宽100 cm,高50 cm,底面用黑线划分为25个10 cm×10 cm的方格),Fujifilm LAS-3000曝光机(日本),10%聚丙烯酰胺(PAGE)凝胶电泳及转膜装置(美国Bio-Rad公司),NC膜(美国millipore公司),戊巴比妥(上海化学试剂有限公司),蛋白裂解液(碧云天),BCA蛋白浓度测定试剂盒(BCA Protein Assay Kit),Western电化学发光剂(electrogenerated chemiluminescence,ECL)(碧云天),鼠源性ERK单克隆抗体(Santa Cruz公司);兔源性磷酸化细胞外信号调节蛋白激酶(P-ERK)1/2单克隆抗体(美国Cell Signaling公司),β-actin一抗(美国Cell Signaling公司),HRP标记鼠二抗(碧云天),HRP标记兔二抗(碧云天)。
1.3 实验方法
1.3.1 建立慢性应激模型 根据KATZ等[9]方法建立慢性应激模型。药物组和应激组大鼠均给予8周应激刺激,单笼饲养,每天给予以下1或2种刺激:禁水24 h,禁食24 h,湿垫料24 h,强迫游泳5 min,夹尾1 min,斜笼24 h,光照24 h;药物组大鼠于应激刺激第1周开始给予盐酸氟西汀10 mg/g皮下注射,持续治疗8周;对照组大鼠不给予任何刺激。
1.3.2 旷场实验 各组大鼠于造模开始前、造模结束后1 d进行旷场实验,具体步骤如下:依次将每只大鼠轻放入箱子中心格中观察其5 min内的自主活动,记录跨格次数、直立次数、中心格停留时间。实验结束后用酒精棉球擦拭箱子去除气味。
1.3.3 糖水偏好实验[3]各组大鼠于造模开始前、造模结束后1 d进行糖水偏好实验,具体步骤如下:实验开始前给予大鼠断水断粮24 h(9:00~次日9:00),每笼给予1瓶纯水和1瓶1%蔗糖水,实验前称重,放置笼上1 h后再次称重,1 h内两个水瓶位置随机变换,实验结束后计算糖水偏爱率,糖水偏爱率=糖水消耗量/(糖水消耗量+纯水消耗量)×100%。其中以糖水偏爱率<基线值40%定义为应激敏感,以糖水偏爱率≥基线值40%定义为应激适应。
1.3.4 Western bloting法 糖水偏好实验结束后立即断头处死大鼠,快速分离海马及前额叶皮质,采用蛋白裂解液提取蛋白,储存于-80 ℃冰箱中。采用BCA蛋白浓度测定试剂盒检测蛋白水平,10%PAGE凝胶电泳分离样品蛋白质,蛋白质转移至NC膜,封闭液室温封闭1 h,采用含0.5%Tween-20的Tris缓冲盐(TBST)洗膜5 min×3次。加入鼠源性ERK单克隆抗体、兔源性P-ERK1/2单克隆抗体,4 ℃摇晃过夜,TBST洗膜5 min×3次。辣根过氧化物酶标记的山羊抗鼠IgG抗体孵育,室温1 h,TBST洗膜5 min×3次。加入Western ECL,LAS-3000曝光机显影。使用Quantity one软件计算蛋白条带灰度值,将对照组蛋白条带灰度值作为参考,分别计算药物组、应激敏感组、应激适应组大鼠海马和前额叶区ERK及P-ERK蛋白相对表达量。

2 结果
2.1 实验结果 应激刺激过程中共11只大鼠死亡,其中对照组2只、药物组4只、应激组5只。根据糖水偏好实验结果将应激组大鼠分为应激适应组(n=6)和应激敏感组(n=15)。
2.2 旷场实验结果 造模开始前4组大鼠跨格次数、直立次数及中心格停留时间比较,差异无统计学意义(P>0.05)。造模结束后1 d 4组大鼠跨格次数、直立次数及中心格停留时间比较,差异有统计学意义(P<0.05);其中药物组、应激适应组、应激敏感组大鼠跨格次数和直立次数少于对照组,中心格停留时间长于对照组,差异有统计学意义(P<0.05);应激敏感组大鼠跨格次数少于药物组,差异有统计学意义(P<0.05,见表1)。
2.3 大鼠海马及前额叶区ERK蛋白表达情况 应激刺激结束后,4组大鼠海马、前额叶区ERK蛋白相对表达量比较,差异无统计学意义(P>0.05,见表2)。
Table 2 Comparison of relative quantity expression of ERK protein in hippocampus and prefrontal cortex among the four groups of rats
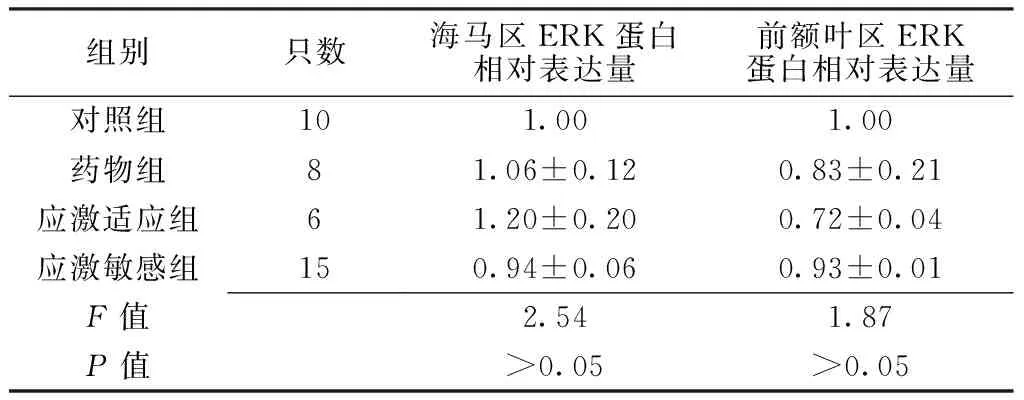
组别只数海马区ERK蛋白相对表达量前额叶区ERK蛋白相对表达量对照组101.001.00药物组81.06±0.120.83±0.21应激适应组61.20±0.200.72±0.04应激敏感组150.94±0.060.93±0.01F值2.541.87P值>0.05>0.05
注:ERK=细胞外信号调节蛋白激酶
表1 4组大鼠造模前后旷场实验结果比较
注:与对照组比较,aP<0.05;与药物组比较,bP<0.05
2.4 大鼠海马及前额叶区P-ERK蛋白表达情况 应激刺激结束后,4组大鼠海马及前额叶区P-ERK蛋白相对表达量比较,差异有统计学意义(P<0.05);其中应激敏感组大鼠海马及前额叶区P-ERK蛋白相对表达量低于药物组和应激适应组(P<0.05);药物组和应激适应组大鼠海马及前额叶区P-ERK蛋白相对表达量比较,差异无统计学意义(P>0.05,见表3、图1)。
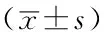
Table 3 Comparison of relative quantity expression of P-ERK protein in hippocampus and prefrontal cortex among the four groups of rats
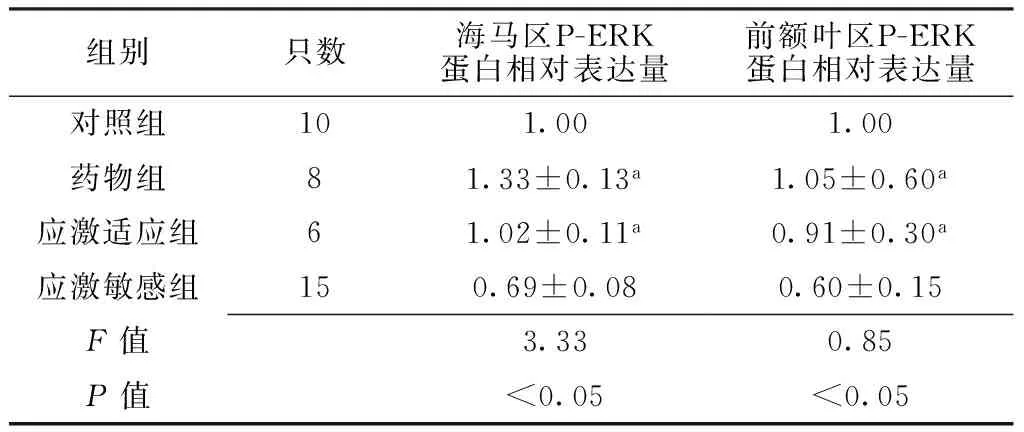
组别只数海马区P-ERK蛋白相对表达量前额叶区P-ERK蛋白相对表达量对照组101.001.00药物组81.33±0.13a1.05±0.60a应激适应组61.02±0.11a0.91±0.30a应激敏感组150.69±0.080.60±0.15F值3.330.85P值<0.05<0.05
注:P-ERK=磷酸化细胞外信号调节蛋白激酶;与应激敏感组比较,aP<0.05
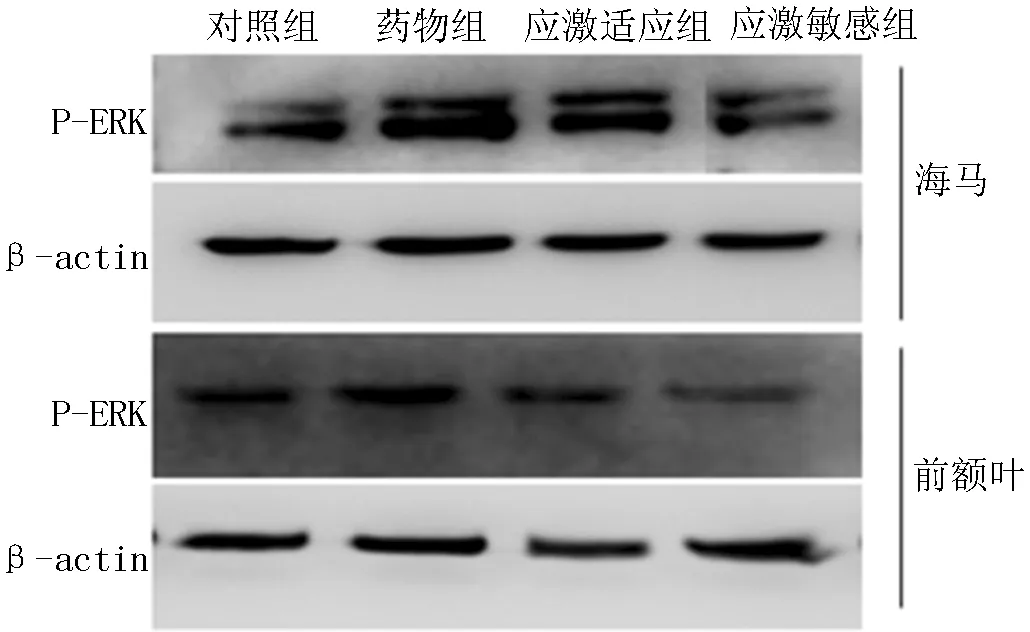
注:P-ERK=磷酸化细胞外信号调节蛋白激酶
图1 4组大鼠海马和前额叶区P-ERK蛋白电泳图
Figure 1 Electrophoretogram of P-ERK protein in hippocampus and prefrontal cortex of the four groups of rats
3 讨论
目前,有关个体应激适应能力的相关研究主要针对抵抗应激能力的社会心理因素,如积极情绪、自我调节能力、社会竞争力等[10]。近年来,应激适应的生物学机制逐渐引起临床关注。本研究通过检测不同应激行为大鼠海马及前额叶区ERK信号通路表达情况,旨在探讨不同应激反应的生物学机制。
糖水偏好实验是检测实验动物兴趣度减退程度的临床实验,旷场实验是检测实验动物自主活动水平的临床实验[11-12]。本研究结果显示,药物组、应激适应组及应激敏感组大鼠跨格次数、直立次数少于对照组,中心格停留时间长于对照组,提示慢性应激刺激可导致大鼠自主活动水平下降,与KRISHNAN等[13]研究结果相一致。
目前,关于抑郁症动物模型ERK蛋白表达情况的研究结果存在分歧。有研究结果显示,慢性应激动物模型及抑郁症患者脑组织ERK蛋白水平下降[14-16];也有研究结果显示,慢性强迫游泳刺激大鼠和对照大鼠海马区ERK蛋白水平间无差异[17],分析各研究结果间存在差异的可能原因为应激种类、应激强度及应激持续时间不同。本研究结果显示,4组大鼠海马和前额叶区ERK蛋白相对表达量间无差异,提示海马和前额叶区ERK蛋白表达与慢性应激行为无关。
大量研究证实,慢性应激刺激可抑制抑郁症大鼠海马、前额叶区ERK信号通路激活[16],表明局部脑组织ERK信号通路激活抑制可能与抑郁症发病机制有关。目前,关于应激适应大鼠ERK信号通路激活的研究报道较少。本研究结果显示,应激敏感组大鼠海马、前额叶区P-ERK蛋白相对表达量低于应激适应组,提示海马、前额叶区ERK信号通路激活可能参与调控不同慢性应激行为。ERK蛋白磷酸化通过激酶级联途径可激活一系列下游转录因子,如环磷酸腺苷反应元件结合蛋白(cyclic-AMP-responsive element-binding protein,CREB),CREB激活后与靶基因结合可导致BDNF表达增加,进而参与神经再生或神经可塑性的调控。有研究发现,慢性应激适应大鼠海马区BDNF mRNA表达水平高于慢性应激敏感大鼠[3];大鼠海马齿状回处BDNF过度表达可表现出应激适应行为和抗抑郁效应[18]。以上研究提示BDNF表达增加与应激适应行为有关,且大鼠海马和前额叶区ERK信号通路激活导致的不同慢性应激行为可能与调控神经再生或神经可塑性相关。
既往研究结果显示,持续给予氟西汀可使大鼠脑组织P-ERK水平升高[19],ERK信号通路阻断剂可抑制抗抑郁药物及BDNF的抗抑郁效果[20-22],提示ERK信号通路激活参与抗抑郁药物的起效机制。本研究结果显示,应激敏感组大鼠跨格次数少于药物组,提示盐酸氟西汀可在一定程度上降低慢性应激刺激对大鼠自主活动水平的影响,笔者据此推测应激适应可能与抗抑郁药物起效过程具有相似的分子信号机制,但仍有待于进一步研究证实。
综上所述,慢性应激刺激可导致大鼠自主活动水平下降,盐酸氟西汀可在一定程度上降低慢性应激刺激对大鼠自主活动水平的影响,海马和额叶区ERK信号通路激活可能与大鼠应激适应有关。
作者贡献:王凌霄、王培福、彭代辉进行实验设计与实施、资料收集整理、撰写论文;彭代辉、方贻儒指导论文修改并对文章负责;才丽娜、黄佳、张晨协助进行试验实施、评估、资料收集。
本文无利益冲突。
[1]DAVIDSON J R,STEIN D J,SHALEV A Y,et al.Posttraumatic stress disorder:acquisition,recognition,course,and treatment[J].J Neuropsychiatry Clin Neurosci,2004,16(2):135-147.
[2]FEDER A,NESTLER E J,CHARNEY D S.Psychobiology and molecular genetics of resilience[J].Nat Rev Neurosci,2009,10(6):446-457.DOI:10.1038/nrn2649.
[3]BERGSTRÖM A,JAYATISSA M N,MØRK A,et al.Stress sensitivity and resilience in the chronic mild stress rat model of depression: an in situ hybridization study[J].Brain Res,2008,1196(27):41-52.DOI:10.1016/j.brainres.2007.12.025.
[4]THOMAS G M,HUGANIR R L.MAPK cascade signalling and synaptic plasticity[J].Nat Rev Neurosci,2004,5(3):173-183.
[5]YUAN P,ZHOU R,WANG Y,et al.Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia[J].J Affect Disord,2010,124(1/2):164-169.DOI:10.1016/j.jad.2009.10.017.
[6]SHIRAYAMA Y,CHEN A C,NAKAGAWA S,et al.Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression[J].J Neurosci,2002,22(8):3251-3261.
[7]QI X,LIN W,LI J,et al.The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress[J].Behav Brain Res,2006,175(2):233-240.
[9]KATZ R J,ROTH K A,CARROLL B J.Acute and chronic stress effects on open-field activity in the rat: implications for a model of depression[J].Neurosci Biobehav Rev,1981,5(2):247-251.
[10]MASTEN A S,COATSWORTH J D.The development of competence in favorable and unfavorable environments.Lessons from research on successful children[J].Am Psychol,1998,53(2):205-220.
[11]D′AQUILA P S,PEANA A T,CARBONI V,et al.Exploratory behaviour and grooming after repeated restraint and chronic mild stress: effect of desipramine[J].Eur J Pharmacol,2000,399(1):43-47.
[12]HU H,SU L,XU Y Q,et al.Behavioral and[F-18] fluorodeoxyglucose micro positron emission tomography imaging study in a rat chronic mild stress model of depression[J].Neuroscience,2010,169(1):171-181.DOI:10.1016/j.neuroscience.2010.04.057.
[13]KRISHNAN V,HAN M H,GRAHAM D L,et al.Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions[J].Cell,2007,131(2):391-404.
[14]FENG P,GUAN Z,YANG X,et al.Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine[J].Brain Res,2003,991(1/2):195-205.
[15]DWIVEDI Y,RIZAVI H S,ROBERTS R C,et al.Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects[J].J Neurochem,2001,77(3):916-928.
[16]QI X,LIN W,LI J,et al.Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress[J].Neurobiol Dis,2008,31(2): 278-285.
[17]QI X,LIN W,LI J,et al.The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress[J].Behav Brain Res,2006,175(2):233-240.
[18]TALIAZ D,LOYA A,GERSNER R,et al.Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor[J].J Neurosci,2011,31(12):4475-4483.DOI:10.1523/JNEUROSCI.5725-10.2011.
[19]EINAT H,YUAN P,GOULD T D,et al.The role of the extracellular signal-regulated kinase signaling pathway in mood modulation[J].J Neurosci,2003,23(19):7311-7316.
[20]DUMAN C H,SCHLESINGER L,KODAMA M,et al.A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment[J].Biological Psychiatry,2007,61(5):661-670.
[21]MAENG S,ZARATE C A Jr,DU J,et al.Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors[J].Biol Psychiatry,2008,63(4): 349-352.
[22]QI X,LIN W,WANG D,et al.A role for the extracellular signal-regulated kinase signal pathway in depressive-like behavior[J].Behav Brain Res,2009,199(2):203-209.DOI:10.1016/j.bbr.2008.11.051.
(本文编辑:谢武英)
Relationship between Chronic Stress Behaviors and ERK Signal Pathway Activation of Hippocampus and Prefrontal Cortex in Rats
WANGLing-xiao1,WANGPei-fu1,CAILi-na1,HUANGJia2,ZHANGChen2,PENGDai-hui2,FANGYi-ru2
1.AerospaceCentralHospital,Beijing100092,China2.MentalHealthCenterofShanghai,Shanghai200030,ChinaCorrespondingauthor:FANGYi-ru,E-mail:yirufang@aliyun.comPENGDai-hui,E-mail:pdhsh@126.com
Objective To explore the relationship between chronic stress behaviors and ERK signal pathway activation of hippocampus and prefrontal cortex in rats.Methods From June 2011 to August 2012,A total of clear 50 male Sprague-Dawley rats were randomly divided into A group(n=12),B group(n=12)and C group(n=26),thereinto rats of A group did not
any stress stimulation,rats of B group and C group received stress stimulation for 8 weeks,meanwhile rats of B group received subcutaneous injection of fluoxetine hydrochloride at the first week of stress stimulation.Sucrose preference test and open-field test were carried out before modeling and after 1 day of modeling,Western bloting method was used to detect the protein expressions of ERK and P-ERK of hippocampus and prefrontal cortex after stress stimulation.Results A total of 11 rats died during the stress stimulation,including 2 rats in A group,4 rats in B group and 5 rats in C group.Rats of C group were divided into C1 group(with stress adaptation,n=6)and C2 group(with stress sensitive,n=15) according to sucrose preference test.No statistically significant differences of striding grid times,upright times or central square stays was found among the four groups(P>0.05);after 1 day of modeling,striding grid times,upright times of B group,C1 group and C2 group were statistically significantly less than those of A group,central square stays of B group,C1 group and C2 group was statistically significantly longer than that of control group,meanwhile striding grid times of C2 group was statistically significantly less than that of B group(P<0.05).No statistically significant differences of relative quantity expression of ERK protein of hippocampus or prefrontal cortex was found among the four groups after stress stimulation(P>0.05).After stress stimulation,relative quantity expression of P-ERK protein of hippocampus and prefrontal cortex of C2 group was statistically significantly lower than that of B group and C1 group(P<0.05),while no statistically significant differences of relative quantity expression of P-ERK protein of hippocampus or prefrontal cortex was found between B group and C1 group(P>0.05).Conclusion Chronic stress stimulation can reduce the autonomous activity level of rats,fluoxetine hydrochloride can reduce the impact of chronic stress stimulation on autonomous activity level of rats to some extent,and ERK signal pathway activation of hippocampus and prefrontal cortex may correlated with stress adaptation in rats.
Stress,psychological;Extracellular signal-regulated kinases;Hippocampus;Prefrontal cortex;Rat
国家高技术研究发展计划“863”计划项目(2006AA02Z430);国家自然科学基金重大计划项目(91232719);国家临床重点专科-上海市精神卫生中心(卫生部医政司2011-873);上海市自然科学基金资助项目(09ZR1427200);上海市卫生局公共卫生海外人才项目(GWHW201208);上海交通大学科学基金项目(11XJ21006)
方贻儒,E-mail:yirufang@aliyun.com 彭代辉,E-mail:pdhsh@126.com
R 395
A
10.3969/j.issn.1008-5971.2017.07.013
2017-04-15;
2017-07-11)
1.100092北京市,航天中心医院
2.200030上海市精神卫生中心

