经皮冠状动脉介入治疗术6个月后应用低剂量替格瑞洛的有效性及安全性研究
宋 敏,姚朱华,门剑龙,曹明英,黎文婷,陈 颖
·论著·
经皮冠状动脉介入治疗术6个月后应用低剂量替格瑞洛的有效性及安全性研究
宋 敏1,2,姚朱华2*,门剑龙3,曹明英2,黎文婷2,陈 颖2
目的 探讨经皮冠状动脉介入治疗(PCI)术6个月后应用低剂量替格瑞洛的有效性及安全性。方法 选取2015年7月—2016年2月在天津市人民医院行PCI术6个月后复查的患者95例,根据术后服用的二磷酸腺苷受体(P2Y12受体)拮抗剂类型,将其分为替格瑞洛组(n=33)和氯吡格雷组(n=62)。其中,替格瑞洛组患者术后服用标准剂量替格瑞洛(90 mg/次,2次/d)、6个月后改为低剂量替格瑞洛(45 mg/次,2次/d),氯吡格雷组患者术后持续服用氯吡格雷(75 mg/次,1次/d),两组均联合服用阿司匹林(100 mg/次,1次/d)。比较两组患者PCI术6个月后服药1周、3个月时采用光比浊(LTA)法检测的血小板聚集率,以及随访6个月的主要不良心血管事件(MACE)和不良反应发生率。结果 PCI术6个月后服药1周、3个月,替格瑞洛组患者血小板聚集率均低于氯吡格雷组,差异有统计学意义(P<0.05);服药3个月,两组患者血小板聚集率与服药1周比较,差异无统计学意义(P>0.05)。随访期间,替格瑞洛组患者MACE发生率低于氯吡格雷组,差异有统计学意义(P<0.05);但两组患者轻度出血、呼吸困难发生率比较,差异无统计学意义(P>0.05)。结论 PCI术后6个月应用低剂量替格瑞洛的抗血小板聚集作用强于氯吡格雷,可有效减少PCI术后患者的缺血事件发生,且不增加出血风险。
血管成形术,气囊,冠状动脉;替格瑞洛;氯吡格雷;治疗结果
宋敏,姚朱华,门剑龙,等.经皮冠状动脉介入治疗术6个月后应用低剂量替格瑞洛的有效性及安全性研究[J].中国全科医学,2017,20(16):1973-1977.[www.chinagp.net]
SONG M,YAO Z H,MEN J L,et al.Efficacy and safety of low-dose ticagrelor in patients at six months after percutaneous coronary intervention[J].Chinese General Practice,2017,20(16):1973-1977.
双联抗血小板疗法(dual antiplatelet therapy,DAPT)是指阿司匹林联合一种二磷酸腺苷受体(P2Y12受体)拮抗剂可以降低血小板反应性,是急性冠脉综合征(acute coronary syndromes,ACS)和经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)术后患者防止缺血事件发生的有效治疗方案[1-2]。氯吡格雷是应用广泛的P2Y12受体拮抗剂,替格瑞洛是一种新型P2Y12受体拮抗剂,与氯吡格雷相比,替格瑞洛具有起效快、作用强的特点,且不增加患者的主要出血发生风险,但可增加次要出血发生风险[3-4]。越来越多的数据表明,亚洲人群的血栓形成、出血等不良事件的形成及抗血小板药物治疗窗与白种人不同[5];且近期研究结果显示,在我国非ST段抬高型(non-ST-elevation,NSTE)ACS患者中,低剂量替格瑞洛的抗血小板聚集作用强于氯吡格雷[6]。因此,低剂量替格瑞洛可能更适合我国人群。本研究旨在探讨行PCI术6个月后应用低剂量替格瑞洛的临床有效性及安全性,从而得出替格瑞洛在我国人群中的合适剂量。
1 对象与方法
1.1 研究对象 选取2015年7月—2016年2月在天津市人民医院行PCI术6个月后复查的患者95例。纳入标准:(1)年龄18~75岁;(2)符合美国心脏协会(AHA)等制定的ACS诊断标准[7],成功行PCI术;(3)植入药物洗脱支架为Xience V、BuMA或吉威EXSEL,植入支架后心肌梗死溶栓治疗(TIMI)血流分级为3级;(4)术后服用标准剂量替格瑞洛(倍林达,英国阿斯利康公司,90 mg/片,90 mg/次,2次/d)、6个月后改为服用低剂量替格瑞洛(45 mg/次,2次/d),或术后持续服用氯吡格雷(泰嘉,深圳信立泰有限公司,25 mg/片,75 mg/次,1次/d),联合服用阿司匹林(拜耳医药保健有限公司,100 mg/次,1次/d)。排除标准:(1)服用华法林、达比加群酯等抗凝药物;(2)服用中医活血药物;(3)伴严重感染、免疫系统疾病、肿瘤或血液系统疾病;(4)贫血(血红蛋白<100 g/L);(5)肝肾功能异常;(6)血小板计数<100×109/L或>450×109/L。根据服用P2Y12受体拮抗剂类型,将纳入患者分为替格瑞洛组(n=33)和氯吡格雷组(n=62)。本研究通过天津市人民医院伦理委员会批准,纳入患者均知情同意。
1.2 研究方法
1.2.1 一般资料收集 通过查阅病历的方法收集患者的一般资料,包括性别、年龄、体质量、吸烟史、合并症、ACS类型、病变程度、用药情况及植入支架数量。其中,以吸烟≥1支/d、持续时间≥1年,或长期吸烟而戒烟时间<6个月为有吸烟史。
1.2.2 血小板聚集率检测 分别于PCI术6个月后服药1周、3个月时,抽取患者空腹静脉血3 ml置于真空管(包含质量分数为3.2%的枸橼酸钠)中,2 h内由天津医科大学总医院医学检验科专业人员采用普利生PRECIL LBY-NJ四通道血小板聚集仪检测血小板聚集率,检测方法为光比浊(LTA)法,以10 μmol/L二磷酸腺苷为诱导剂。LTA法具体操作方法参照参考文献[8],血小板聚集率以相对数表示。
1.2.3 主要不良心血管事件(MACE)和不良反应记录 通过电话随访的方式对患者进行为期6个月的随访,记录其MACE和不良反应发生情况。MACE包括心源性死亡、非致死性心肌梗死、靶血管再次血运重建、支架内血栓形成、不稳定型心绞痛(UA)、心力衰竭等;不良反应包括出血、呼吸困难。其中出血分级评估采用全球梗死相关动脉开通策略(GUSTO)分级标准:(1)严重出血,包括颅内出血或导致血流动力学不稳定且需及时干预的出血;(2)中度出血,需输血治疗但尚未引起血流动力学不稳定的出血;(3)轻度出血,未达到中度出血标准的少量出血[9]。

2 结果
2.1 两组患者一般资料 两组患者性别、年龄、体质量、吸烟史、合并高血压发生率、合并糖尿病发生率、合并血脂异常发生率、ACS类型、病变程度、β-受体阻滞剂使用率、血管紧张素转换酶抑制剂(ACEI)/血管紧张素Ⅱ受体阻滞剂(ARB)使用率、钙通道拮抗剂(CCB)使用率、质子泵抑制剂(PPI)使用率、他汀类药物使用率、植入支架数量比较,差异无统计学意义(P>0.05,见表1)。
2.2 两组患者PCI术6个月后服药1周、3个月血小板聚集率比较 PCI术6个月后服药1周、3个月,两组患者血小板聚集率比较,差异有统计学意义(P<0.05);服药3个月,两组患者血小板聚集率与服药1周比较,差异无统计学意义(P>0.05,见表2)。
2.3 两组患者随访期间MACE和不良反应发生率比较 随访期间,两组患者MACE发生率比较,差异有统计学意义(P<0.05);两组患者均无中、重度出血,轻度出血、呼吸困难发生率比较,差异无统计学意义(P>0.05,见表3)。
3 讨论
支架内血栓形成是PCI术严重的并发症,国际指南推荐方案为阿司匹林联合一种P2Y12受体拮抗剂[1]。氯吡格雷是应用最为广泛的P2Y12受体拮抗剂,但因CYP2C19基因多态性及CYP2C19、CYP3A4水平上的药物相互作用,使得氯吡格雷的抗血小板作用出现了个体差异性,增加了发生支架内血栓的风险[10-11]。替格瑞洛是一种新型P2Y12受体拮抗剂,其母体药物及活性代谢产物(AR-C124910XX)均有抗血小板作用,起效快、作用强,不受基因型的影响,不增加主要出血发生风险,但可增加次要出血发生风险[3-4]。国际指南推荐,对于NSTE-ACS或STEMI药物洗脱支架植入术后患者,替格瑞洛90 mg/次、2次/d服用1年为一线治疗方案[12-15]。国外研究表明,低剂量替格瑞洛(60 mg/次,2次/d)的心血管获益情况与标准剂量替格瑞洛相似,但低剂量替格瑞洛可略减少出血事件的发生[16]。
与西方人群相比,亚洲人群具有较低的血栓形成倾向和较高的出血倾向[16],抗血小板药物的治疗窗也不同于西方人群[5],低剂量的替格瑞洛可能更适合亚洲人群。LI等[17]通过研究发现,服用90 mg/次、2次/d替格瑞洛在达稳态时,我国健康受试者替格瑞洛及AR-C124910XX的最大浓度及血药浓度-时间曲线下面积较西方人群高40%左右。一项近期药动学及药效学研究,选取了118例日本稳定型冠状动脉粥样硬化性心脏病(coronary artery disease,CAD)患者,发现替格瑞洛45 mg/次、2次/d对血小板聚集的抑制强于氯吡格雷75 mg/次,1次/d[18]。XUE等[6]选取我国NSTE-ACS患者进行临床试验,发现半数剂量的替格瑞洛(45 mg/次,2次/d)的血小板抑制作用与标准剂量替格瑞洛相似,明显强于氯吡格雷组。HE等[19]通过研究发现,在中国稳定型CAD患者中,1/4剂量替格瑞洛(22.5 mg/次,2次/d)的血小板抑制作用强于标准剂量的氯吡格雷。
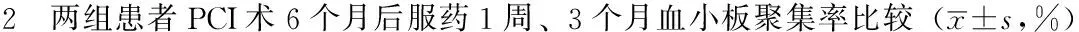
Table 2 Platelet aggregation rate in ticagrelor group and clopidogrel group measured at different time points following the reviewing
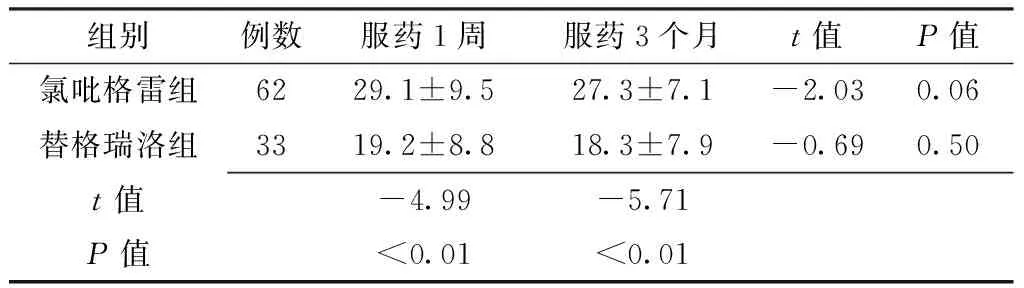
组别例数服药1周服药3个月t值P值氯吡格雷组6229.1±9.527.3±7.1-2.030.06替格瑞洛组3319.2±8.818.3±7.9-0.690.50t值-4.99-5.71P值<0.01<0.01
注:PCI术=经皮冠状动脉介入治疗术
表3 两组患者随访期间MACE和不良反应发生率比较〔n(%)〕
Table 3 Incidence of MACE and adverse reactions in ticagrelor group and clopidogrel group during the follow-up period
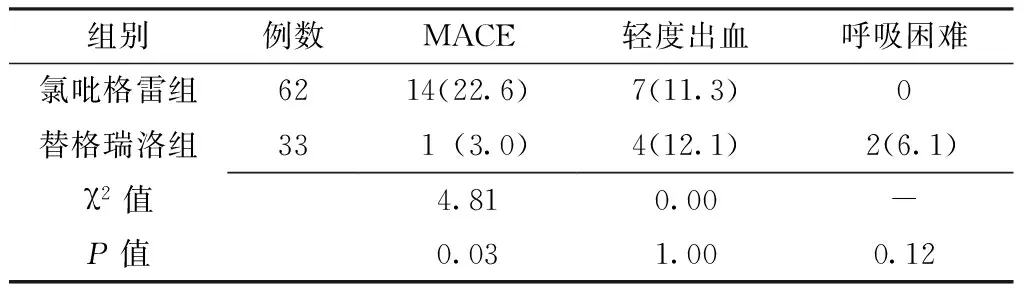
组别例数MACE轻度出血呼吸困难氯吡格雷组6214(22.6)7(11.3)0替格瑞洛组331(3.0)4(12.1)2(6.1)χ2值4.810.00-P值0.031.000.12
注:MACE=主要不良心血管事件;-代表采用Fisher确切概率法
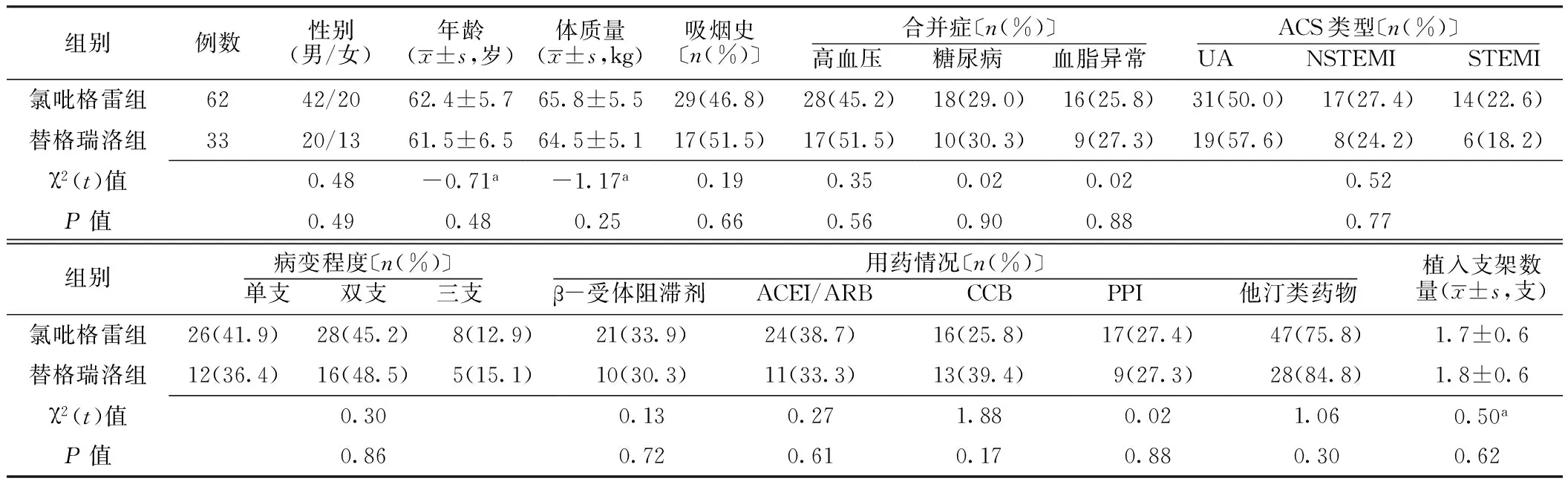
表1 两组患者一般资料比较
注:ACS=急性冠脉综合征,UA=不稳定型心绞痛,NSTEMI=非ST段抬高型心肌梗死,STEMI=ST段抬高型心肌梗死,ACEI=血管紧张素转换酶抑制剂,ARB=血管紧张素Ⅱ受体阻滞剂,CCB=钙通道拮抗剂,PPI=质子泵抑制剂;a为t值
本研究结果显示,PCI术6个月后应用替格瑞洛45 mg/次、2次/d对血小板的抑制作用大于氯吡格雷75 mg/次、1次/d,与上述研究相符。随访6个月,服用低剂量替格瑞洛患者的MACE发生率低于氯吡格雷,出血和呼吸困难发生率与氯吡格雷无差异。本研究纳入患者的植入支架为Xience V、BuMA及吉威EXSEL支架。其中,Xience V、BuMA支架采用光学相干断层成像系统(OCT)技术,植入3个月后可在支架小梁上形成很好的内膜覆盖[20];而吉威EXSEL支架植入6个月后停用双抗具有可行性[21],可为临床上PCI术后替格瑞洛的服用提供借鉴资料。但本研究也存在一定的局限性,如:(1)样本量较小;(2)采用LTA法检测血小板聚集率用以评价血小板功能,LTA法应用广泛,被认为是血小板功能检测的金标准,但目前LTA法检测血小板聚集率的影响因素较多且重复性差,很难标准化[22-23]。因此,对于PCI术后服用低剂量替格瑞洛的有效性及安全性仍需大样本、多中心、长期及多种血小板功能检测方法的临床研究予以验证,以寻求适合我国人群的替格瑞洛合适剂量。
作者贡献:宋敏参与研究的实施与可行性分析、数据收集,负责数据整理和统计学处理、结果的分析与解释、撰写论文和中英文修订,对文章整体负责,监督管理;姚朱华负责文章的构思与设计、患者入组;门剑龙参与研究的实施与可行性分析;曹明英参与数据收集,负责文章的质量控制及审校;黎文婷、陈颖参与数据收集。
本文无利益冲突。
[1]LEVINE G N,BATES E R,BLANKENSHIP J C,et al.2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention:executive summary:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions[J].Catheter Cardiovasc Interv,2012,79(3):453-495.DOI:10.1002/ccd.23438.
[2]2012 Writing Committee Members,JNEID H,ANDERSON J L,et al.2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update):a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines[J].Circulation,2012,126(7):875-910.DOI:10.1161/CIR.0b013e318256f1e0.
[3]KOWALCZYK M,BANACH M,MIKHAILIDIS D P,et al.Ticagrelor——a new platelet aggregation inhibitor in patients with acute coronary syndromes.An improvement of other inhibitors?[J].Med Sci Monit,2009,15(12):MS24-30.
[4]CANNON C P,HUSTED S,HARRINGTON R A,et al.Safety,tolerability,and initial efficacy of AZD6140,the first reversible oral adenosine diphosphate receptor antagonist,compared with clopidogrel,in patients with non-ST-segment elevation acute coronary syndrome:primary results of the DISPERSE-2 trial[J].J Am Coll Cardiol,2007,50(19):1844-1851.DOI:10.1016/j.jacc.2007.07.053.
[5]LEVINE G N,JEONG Y H,GOTO S,et al.Expert consensus document:World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI[J].Nat Rev Cardiol,2014,11(10):597-606.DOI:10.1038/nrcardio.2014.104.
[6]XUE H J,SHI J,LIU B,et al.Comparison of half- and standard-dose ticagrelor in Chinese patients with NSTE-ACS[J].Platelets,2016,27(5):440-445.DOI:10.3109/09537104.2015.1135890.
[7]FIHN S D,GARDIN J M,ABRAMS J,et al.2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines,and the American College of Physicians,American Association for Thoracic Surgery,Preventive Cardiovascular Nurses Association,Society for Cardiovascular Angiography and Interventions,and Society of Thoracic Surgeons[J].J Am Coll Cardiol,2012,60(24):e44-164.DOI:10.1161/CIR.0b013e3182776f83.
[8]ZHANG H Z,KIM M H,JEONG Y H.Predictive values of post-clopidogrel platelet reactivity assessed by different platelet function tests on ischemic events in East Asian patients treated with PCI[J].Platelets,2014,25(4):292-299.DOI:10.3109/09537104.2013. 815341.
[9]李靖,王乐丰.急性冠脉综合征抗栓治疗出血情况研究现状[J].国际心血管病杂志,2009,36(5):270-273.DOI:10.3969/j.issn.1673-6583.2009.05.005. LI J,WANG L F.Bleeding complications of anti-thrombotic therapy in patients with acute coronary syndrome[J].International Journal of Cardiovascular Disease,2009,36(5):270-273.DOI:10.3969/j.issn.1673-6583.2009.05.005.
[10]NORGARD N B,DINICOLANTONIO J J.P2Y12 antagonists in non-ST-segment elevation acute coronary syndromes:latest evidence and optimal use[J].Ther Adv Chronic Dis,2015,6(4):204-218.DOI:10.1177/2040622315584113.
[11]GEISLER T,LANGER H,WYDYMUS M,et al.Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation[J].Eur Heart J,2006,27(20):2420-2425.DOI:10.1093/eurheartj/ehl275.
[12]AMSTERDAM E A,WENGER N K,BRINDIS R G,et al.2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes:a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines[J].J Am Coll Cardiol,2014,64(24):2713-2714.DOI:10.1016/j.jacc.2014.10.01.
[13]ROFFI M,PATRONO C,COLLET J P,et al.2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation.Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology(ESC)[J].G Ital Cardiol(Rome),2016,17(10):831-872.DOI:10.1714/2464.25804.
[14]O′GARA P T,KUSHNER F G,ASCHEIM D D,et al.2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines[J].J Am Coll Cardiol,2013,62(16):e147-239.DOI:10.1016/j.jacc.2013.05.019.
[15]Task Force on the Management of ST-segment Elevation Acute Myocardial Infarction of the European Society of Cardiology(ESC),STEG P G,JAMES S K,et al.ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation[J].Eur Heart J,2012,33(20):2569-2619.DOI:10.1093/eurheartj/ehs215.
[16]BONACA M P,BRAUNWALD E,SABATINE M.Long-term use of ticagrelor in patients with prior myocardial infarction[J].N Engl J Med,2015,372(19):1791-1800.DOI:10.1056/NEJMoa1500857.
[17]LI H,BUTLER K,YANG L,et al.Pharmacokinetics and tolerability of single and multiple doses of ticagrelor in healthy Chinese subjects:an open-label,sequential,two-cohort,single-centre study[J].Clin Drug Investig,2012,32(2):87-97.DOI:10.2165/11595930-000000000-00000.
[18]HIASA Y,TENG R,EMANUELSSON H.Pharmacodynamics,pharmacokinetics and safety of ticagrelor in Asian patients with stable coronary artery disease[J].Cardiovasc Interv Ther,2014,29(4):324-333.DOI:10.1007/s12928-014-0277-1.
[19]HE M J,LIU B,SUN D H,et al.One-quarter standard-dose ticagrelor better than standard-dose clopidogrel in Chinese patients with stable coronary artery disease:a randomized,single-blind,crossover clinical study[J].Int J Cardiol,2016,215:209-213.DOI:10.1016/j.ijcard.2016.04.087.
[20]凌寒.开通“罪犯血管”,贵在自主创新——访哈尔滨医科大学附属第二医院心血管病医院院长兼心内科主任于波教授[J].中国当代医药,2012,19(30):1-3.DOI:10.3969/j.issn.1674-4721.2012.30.001. LING H.The opening of the "criminal blood vessels" is mainly in independent innovation——visited professor Yu Bo who is the dean and director of cardiology in the Second Affiliated Hospital of Cardiovascular Hospital of Harbin Medical University[J].China Modern Medicine,2012,19(30):1-3.DOI:10.3969/j.issn.1674-4721.2012.30. 001.
[21]HAN Y,JING Q,XU B,et al.Safety and efficacy of biodegradable polymer-coated sirolimus-eluting stents in "real-world" practice:18-month clinical and 9-month angiographic outcomes [J].JACC Cardiovasc Interv,2009,2(4):303-309.DOI:10.1016/j.jcin.2008.12.013.
[22]ISRAELS S J.Laboratory testing for platelet function disorders[J].Int J Lab Hematol,2015,37(Suppl 1):S18-24.DOI:10.1111/ijlh.12346.
[23]PANICCIA R,ANTONUCCI E,MAGGINI N,et al.Assessment of platelet function on whole blood by multiple electrode aggregometry in high-risk patients with coronary artery disease receiving antiplatelet therapy[J].Am J Clin Pathol,2009,131(6):834-842.DOI:10.1309/AJCPTE3K1SGAPOIZ.
(本文编辑:王凤微)
Efficacy and Safety of Low-dose Ticagrelor in Patients at Six Months after Percutaneous Coronary Intervention
SONGMin1,2,YAOZhu-hua2*,MENJian-long3,CAOMing-ying2,LIWen-ting2,CHENYing2
1.GraduateSchool,TianjinMedicalUniversity,Tianjin300070,China2.DeparmentofCardiology,TianjinPeople′sHospital,Tianjin300121,China3.MedicalLaboratory,TianjinMedicalUniversityGeneralHospital,Tianjin300052,China*Correspondingauthor:YAOZhu-hua,Chiefphysician,Mastersupervisor;E-mail:tjyzhpci@163.com
Objective To evaluate the efficacy and safety of low-dose ticagrelor in patients at six months after percutaneous coronary intervention(PCI).Methods Nine-five patients underwent postoperative review at 6 months after PCI in Tianjin People′s Hospital between July 2015 and February 2016 were selected as the participants and divided into the ticagrelor group(n=33) and clopidogrel group(n=62) based on the P2Y12 adenosine diphosphate-receptor antagonist type that the postoperatively orally taken drug belongs to.Patients in the ticagrelor group
standard dose of ticagrelor(90 mg,twice daily),and those in the clopidogrel group took clopidogrel(75 mg,once daily) after PCI.At six months after PCI,the ticagrelor group changed to low-dose ticagrelor(45 mg,twice daily),but the clopidogrel group continued to take the same dose of clopidogrel.Both groups were given aspirin(100 mg,once daily).Light transmission aggregometry(LTA) was used to measure the platelet aggregation rate at the time that they completed 1-week,and 3-month administration following the reviewing.Comparisons were made between the two groups′ two measurement results of platelet aggregation rate,major adverse cardiac events(MACE) and adverse reactions occurred during the 6-month follow-up.Results The platelet aggregation rates measured at the time that the patients completed 1-week,and 3-month administration following the reviewing in ticagrelor group were all dramatically lower than those in clopidogrel group(P<0.05).In both groups,little difference was observed between the two measurement results of platelet aggregation rate(P>0.05).The follow-up results showed that,the incidence of MACE was significantly lower in the ticagrelor group than that in the clopidogrel group(P<0.05),but there were no significant difference in the incidence of mild hemorrhage and dyspnea between the two groups(P>0.05).Conclusion Inhibitory effect on platelet aggregation of low-dose ticagrelor administered at 6 months after PCI was significantly stronger than that of clopidogrel.Low-dose ticagrelor can reduce the incidence of ischemic events in the patients after PCI,without increasing the risk of bleeding.
Angioplasty,balloon,coronary;Ticagrelor;Clopidogrel;Treatment outcome
R 543.3
A
10.3969/j.issn.1007-9572.2017.16.014
2017-01-10;
2017-04-17)
1.300070 天津市,天津医科大学研究生院
2.300121 天津市人民医院心内科
3.300052 天津市,天津医科大学总医院医学检验科
*通信作者:姚朱华,主任医师,硕士生导师;E-mail:tjyzhpci@163.com

