Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans
Heikki Rauvala, Mikhail Paveliev Juha Kuja-Panula Natalia Kulesskaya
1 Neuroscience Center, University of Helsinki, Helsinki, Finland
2 Department of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland
Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans
Heikki Rauvala1,*, Mikhail Paveliev1, Juha Kuja-Panula1, Natalia Kulesskaya1,2
1 Neuroscience Center, University of Helsinki, Helsinki, Finland
2 Department of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland
How to cite this article:Rauvala H, Paveliev M, Kuja-Panula J, Kulesskaya N (2017) Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans. Neural Regen Res 12(5):687-691.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:Our studies have been supported by the Finnish Funding Agency for Innovation Tekes, Academy of Finland and the Sigrid Jusélius Foundation.
The current dogma in neural regeneration research implies that chondroitin sulfate proteoglycans (CSPGs) inhibit plasticity and regeneration in the adult central nervous system (CNS). We argue that the role of the CSPGs can be reversed from inhibition to activation by developmentally expressed CSPG-binding factors. Heparin-binding growth-associated molecule (HB-GAM; also designated as pleiotrophin) has been studied as a candidate molecule that might modulate the role of CSPG matrices in plasticity and regeneration. Studiesin vitroshow that in the presence of soluble HB-GAM chondroitin sulfate (CS) chains of CSPGs display an enhancing effect on neurite outgrowth. Based on thein vitrostudies, we suggest a model according to which the HB-GAM/CS complex binds to the neuron surface receptor glypican-2, which induces neurite growth. Furthermore, HB-GAM masks the CS binding sites of the neurite outgrowth inhibiting receptor protein tyrosine phosphatase sigma (PTPσ), which may contribute to the HB-GAM-induced regenerative effect.In vivostudies using two-photon imaging after local HB-GAM injection into prick-injury of the cerebral cortex reveal regeneration of dendrites that has not been previously demonstrated after injuries of the mammalian nervous system. In the spinal cord, two-photon imaging displays HB-GAM-induced axonal regeneration. Studies on the HB-GAM/CS mechanismin vitroandin vivoare expected to pave the way for drug development for injuries of brain and spinal cord.
CNS injury; axon regeneration; dendrite regeneration; proteoglycans; aggrecan; glypican; HB-GAM; pleiotrophin; PTEN
Introduction
The mature mammalian central nervous system (CNS) displays a very limited capacity to regenerate its connections after trauma. It is generally accepted that chondroitin sulfate proteoglycans (CSPGs) are major inhibitors of plasticity and regeneration in the adult CNS. CSPGs consist of a protein core to which the carbohydrate moieties designated as chondroitin sulfate (CS) chains (the designations CS chains or simply CS are used in the current text) are covalently attached. The CS chains act as the main inhibitory structures of the CSPGs. The structures and biological properties of the neuronal CSPGs are reviewed by Kwok et al. (2011).
CSPGs accumulate in glial scar that is thought to be the major area where the CS side chains of the CSPGs exert their inhibitory effect. Accordingly, chondroitinase ABC has been widely used to demonstrate the role of the CS chains in the regeneration failure after CNS injuries (Bradbury et al., 2002; Silver and Miller, 2004; Galtrey and Fawcett, 2007; Sharma et al., 2012).
In addition to their inhibitory role after injury, the CS chains are implicated in physiological regulation of CNS functions. CSPGs accumulate in perineuronal nets at the end of the critical period of development and inhibit plasticity that underlies behavioral regulation (Pizzorusso et al., 2002; Gogolla et al., 2009). Interactions of the CS chains with neurons are therefore of wide biomedical interest, regulating plasticity in the injured and non-injured CNS.
In addition to their inhibitory effects, CSPGs have beneficial effects after CNS injuries. They are, for example, implicated in modulation of immune responses and in regulation of progenitor proliferation (Rolls et al., 2009). We have therefore sought for an alternative strategy to chondroitinase ABC digestion to modulate the effects of the CSPG matrices without destroying the biologically important structures. To overcome the inhibitory effect of the adult CSPG matrices, our goal has been to recapitulate development to return the matrix structure to a growth-permissive state.
HB-GAM (pleiotrophin) as a Candidate Molecule to Modify CSPG Matrices
Heparin-binding growth-associated molecule (HB-GAM) was initially isolated by screening factors from rat brain extracts that enhance neurite outgrowth in central neurons (Rauvala, 1989). Peptide sequencing (Rauvala, 1989), molecular cloning (Li et al., 1990; Merenmies and Rauvala, 1990) and characteristics of the recombinant protein (Rauloet al., 1992) confirmed that HB-GAM is a novel glycosaminoglycan-binding protein that enhances neurite extension in CNS neurons as a substrate-bound molecule. Expression of HB-GAM displays a characteristic peak in the brain during the postnatal weeks 1–3 (Merenmies and Rauvala, 1990) corresponding to heightened plasticity of the juvenile brain (Hensch, 2004). The expression level of HB-GAM is very high in juvenile brain, up to 10–15 μg/g of wet tissue weight, but is strongly downregulated upon adulthood. HBGAM is secreted from neurons and glial cells by classic-type secretion signal mechanism, binds to CS chains of CSPGs at nanomolar Kd values (Milev et al., 1998; Sugahara and Mikami, 2007) and lines practically all fiber tracts in juvenile brain (Rauvala et al., 1994).
Taken together, characteristics of HB-GAM suggest that it could be one of the key factors that are responsible for heightened plasticity of the juvenile brain compared to the adult brain. Strong binding to the CS chains suggests that HB-GAM could modify the plasticity-inhibiting property of CSPGs. Since the potential role of HB-GAM on hostile CSPG matrices had not been previously explored, we decided to test whether HB-GAM could overcome or even reverse the CSPG inhibition in growth and regeneration of neurites.
Reversal of the CSPG Effect on Neural Regeneration by HB-GAM
To screen factors that could modify the effects of the CSPG matrices on neurite growth from primary CNS neurons, culture wells coated with aggrecan, neurocan or a CSPG mixture isolated from rat brain were tested. In all cases, the CSPG substrates strongly inhibited neurite outgrowth from primary neurons (Paveliev et al., 2016). Coating of HB-GAM together with the CSPGs was able to overcome the inhibitory effect. Interestingly, delayed addition of HB-GAM to the culture medium was able to induce neurite growth in neurons that had already been inhibited by the aggrecan substrate.
In assays where HB-GAM was added to culture medium at concentrations resembling those found in juvenile brain, no increase of neurite outgrowth but even some inhibition was observed on ordinary culture wells. However, under the same conditions robust neurite outgrowth was induced on CSPG substrates (Paveliev et al., 2016). Therefore, the CSPG substrates display an enhancing effect on neurite outgrowth when HB-GAM is present in the medium at concentrations found in juvenile brain.
Mechanism of HB-GAM-Induced Neurite Growth on CSPG Matrices
The finding that the HB-GAM effect is observed on various CSPG substrates, as those prepared using aggrecan, neurocan and a CSPG mixture from brain, suggests that HB-GAM modulates the effects of the CS chains found in various CSPGs. The findings that HB-GAM binds with high affinity to CSPGs (Kd = 0.3–8 nM) and the binding is mainly due to the CS chains (Milev et al., 1998) are consistent with this interpretation. When tested with CNS neurons plated on CSPG substrate with HB-GAM in the medium, chondroitinase ABC treatment of the substrate abrogates the neurite growth-promoting effect (Paveliev et al., 2016). It thus appears clear that neurite growth on CSPG substrate in the presence of HB-GAM depends on the CS chains that are generally regarded inhibitory. Our interpretation is that the CS side chains of the CSPG substrate are required to bind HB-GAM from the medium, and the resulting CSPG/HBGAM substrate then induces neurite growth (Figure 1).
HB-GAM-induced neurite outgrowth in CNS neurons has been previously shown to depend on heparan sulfate (HS) of the cell surface (Rauvala et al., 1994; Kinnunen et al., 1996), and syndecan-3 (N-syndecan) has been identified as a receptor carrying interacting HS chains (Raulo et al., 1994). We therefore wondered whether this is also the case for neurite outgrowth on CSPG substrates in the presence of HB-GAM. Indeed, neurite outgrowth on CSPG substrate in the presence of HB-GAM can be virtually abolished by heparinase treatment, but syndecan-3 knockout neurons grow neurites as the wild-type neurons (Paveliev et al., 2016). Neuron surface heparan sulfate is therefore required but syndecan-3 is not the critical heparan sulfate proteoglycan mediating neurite outgrowth on CSPG substrate.
According to our view, HB-GAM acts as a matrix-bound molecule that is able to induce neurite outgrowth. Accordingly, HB-GAM-coated beads have been used in an unbiased search of putative receptors that might explain the effect of HB-GAM bound to a CSPG substrate (Figure 1). The HBGAM-coated magnetic beads were isolated after incubation with CNS neurons, and the membrane fraction attached to the beads was analyzed by liquid chromatography-mass spectrometry. Glypican-2, an HS proteoglycan attached to the cell membrane through a glycosylphosphatidylinositol (GPI) linker, was identified as the only cell surface component with high score. Glypican-2 indeed appears as a receptor since phospholipase C, an enzyme that cleaves GPI anchors, was found to abolish neurite outgrowth on CSPG substrate in the presence of HB-GAM. Furthermore, knockdown of glypican-2 in CNS neurons clearly inhibited neurite outgrowth (Paveliev et al., 2016).
Protein tyrosine phosphatase sigma (PTPσ) has been recently identified as a transmembrane receptor binding to CS chains and mediating the inhibitory effect on neurite outgrowth (Shen et al., 2009; Coles et al., 2011; Lang et al., 2015). In solid phase binding assays, HB-GAM appears as an effective inhibitor of PTPσ binding to CS chains of the matrix: 50% inhibition in the binding of 80 nM ectodomain of PTPσ is achieved by 20 nM soluble HB-GAM. However, knockdown of PTPσ in CNS neurons does not change neurite outgrowth on CSPG substrate in the presence of HB-GAM. Therefore, downregulation of PTPσ signaling in neurons does not explain the neurite outgrowth-promoting effect of HB-GAM on CSPG matrix although it might aid in making the CSPG matrix permissive for neurite outgrowth.
Our current view of the mechanism of HB-GAM on CSPG matrix is depicted in Figure 1. HB-GAM binds from the medium to the CSPG-rich extracellular matrix, making a multi-valent interaction surface for neurons. Binding of glypican-2 to the HB-GAM/CS complex is essential to initiate neurite outgrowth. Furthermore, HB-GAM masks the CS binding sites of PTPσ reducing its inhibitory signaling, which is expected to contribute to the HB-GAM effect. HB-GAM might be viewed here in a broader context of co-signaling by neurotrophic factors and extracellular matrix bothviadirect interaction on the cell surface and interference of intracellular signaling pathways (Paveliev et al., 2007; Bespalov et al., 2011).
Dendrite Regeneration in Cerebral Cortex
To study whether thein vitroeffects on neurite outgrowth discussed above could be relevant for regenerationin vivo, we have used two-photon microscopy to follow the possible effects of injected HB-GAM in live animals in injury models. To this end, a prick-injury model of the cerebral cortex has been recently set up using transgenic mice with fluorescent dendrites (Paveliev et al., 2014). In the prick-injury model, injected HB-GAM clearly accumulates to the area of activated astrocytes which is likely due to its avid binding to the CS chains that are known to be produced at high amounts by the activated astrocytes making the glial scar. Expression of the endogenous HB-GAM is also enhanced in activated astrocytes after CNS injury, which might aid in regeneration (Takeda et al., 1995). However, detection of HB-GAM at the area of activated astrocytes in our injury model depends on the injected recombinant HB-GAM since the endogenously occurring protein was below the detection limit in immunohistochemistry and in western blotting used in the experiments (Paveliev et al., 2016).
HB-GAM can be detected in the activated astrocyte-enriched scar area at least until day 20 after the injection, which is consistent with the long half-life of the matrix-bound HB-GAM in tissue (Dreyfus et al., 1998). Quantification of the density of dendritic tufts and the number of apical dendrites by two-photon microscopy in the cortex (until the 800 μm depth below the cortical surface that is reached by the two-photon microscopy used) reveals robust regeneration within 2–3 weeks from the start of the experiment in the core and perilesional area in HB-GAM-injected cortical injury sites compared to the IgG-injected controls (Paveliev et al., 2016). The findings in the brain trauma model thus appear consistent with the effects of HB-GAM on neurite outgrowthin vitrousing the CSPG substrates.
Substantial progress has been made to identify extracellular cues and intracellular signaling pathways that lead to axon-dendrite polarization in cultured neurons (Barnes and Polleux, 2009). Furthermore, regrowth of axons after neuronal injuries has been widely studied (Schwab and Bartholdi, 1996; Cregg et al., 2014). Although dendrite regeneration should be a relevant issue in conditions such as traumatic CNS injuries and stroke, data are apparently lacking whether and under what conditions dendrites might be able to regenerate after injury in mammalian systems. However, dendrite regeneration after nervous system injury has been studied in Drosophila during the last few years (Song et al., 2012; Stone et al., 2014; Thompson-Peer, 2016). Cell signaling mediated by phosphatase and tensin homolog (PTEN) that is important in mammalian axon regeneration is suggested to regulate dendrite regeneration in Drosophila (Song et al., 2012). One should however bear in mind that the regenerative capability in invertebrates is often different from that in mammals, for which reason it is unclear whether cell-intrinsic and extrinsic pathways found to regulate dendrite regeneration in Drosophila would also apply to vertebrates. We suggest that dendrite regeneration even in the adult mammalian CNS is possible and can be regulated by extracellular cues, as shown for HB-GAM after prick-injury in the cortex.
Axon Regeneration in Spinal Cord
Long-term imaging in transgenic mice with fluorescent axons using two-photon microscopy of spinal cord (Fenrich et al., 2012) has been used to study HB-GAM effects on axonal regeneration after transection injuries (Paveliev et al., 2016). Counting of axons that traverse the injury sites from the start of the injury until 4 weeks post-injury reveals that the numbers of axons traversing the trauma site are significantly higher in the HB-GAM-treated spinal cords compared to the IgG-treated spinal cords starting from two weeks post-injury (Paveliev et al., 2016). Furthermore, HB-GAM was found to increase the growth rate of axons. At the subcellular level, HB-GAM-treated spinal cords have axons with more branch points and varicosities compared to the control spinal cords, which agrees with previous findings that axons with complex growth terminals are more likely to regenerate compared to axons with simple growth terminals (Fenrich and Rose, 2011).
Conclusions and Remaining Questions
Our findingsin vitroandin vivoare consistent with the generally held view that the CS side chains of proteoglycans are able to strongly inhibit regenerative growth of neurites. However, the inhibition depends on CS-binding factors present in the extracellular space: under the conditions when HB-GAM is present in the medium at high concentrations resembling those found in juvenile brain, the role of the CS chains is reversed from inhibition to activation of neurite growth. We suggest that HB-GAM acts as a linker from matrix CS chains to neuron surface HS chains bound to glypican-2.
CS chains densely deposited on the glial scar are suggested to act as major inhibitors of axon regrowth (Bradbury et al., 2002; Silver and Miller, 2004; Galtrey and Fawcett, 2007; Sharma et al., 2012). From the viewpoint that CS chains are able to bind and present neurite outgrowth enhancing factor(s), such as HB-GAM, to neuron surface receptors to enhance regeneration, the glial scar might also have beneficial effects on regeneration as has been recently suggested (Anderson et al., 2016). However, in our injury models induction of endogenous HB-GAM expression by trauma (Takeda et al., 1995) was not sufficient to induce regrowth through the injury sites.
The question how ligation of glypican-2 by the CS-bound HB-GAM leads to cell signaling enhancing neurite regrowth requires further investigation. Glypican-2 is a GPI-linkedplasma membrane component lacking a cytosolic tail, and it does not therefore directly communicate with the cytoskeleton of neurons to enhance neurite growth. It seems probable that glypican-2 needs to interact with a transmembrane component that regulates the cytoskeleton to enhance neurite growth, but such putative mechanism is currently unknown. On the other hand, the role of glypican-2 in neurite growth appears probable since it is highly expressed in central neurons at the stage when they extend neurites (Stipp et al., 1994), and glypicans have been found to regulate signaling of Wnts, Hedgehogs, fibroblast growth factors and bone morphogenetic proteins (Filmus et al., 2008).
We have used two-photon imaging to show the effects of injected HB-GAM in enhancing regeneration of axons and dendritesin vivoafter CNS injuries (Paveliev et al., 2016). It is intriguing that in addition to axons dendrites regenerate robustly upon local injection of excess HB-GAM. Two conclusions appear clear: (i) dendrite regeneration is possible in the adult mammalian CNS and (ii) dendrite regeneration is influenced by extracellular cues, such as HB-GAM. Dendrite regeneration resembles axon regeneration since both are influenced by the same extracellular cue. Interestingly, PTEN signaling that is a well-known enhancer of axon regeneration also enhances dendrite regeneration in Drosophila (Song et al., 2012). It therefore appears reasonable to assume that regulation of axon and dendrite regeneration in mammals have similar cues both in the extracellular and the intracellular side but it is too early to estimate how far this inference may hold.
Interestingly, there are two reports in literature that inhibition of PTEN expression leads to upregulation of HB-GAM expression both in mouse embryonic fibroblasts (Li et al., 2006) and in human mammary epithelial cells (Rahal and Simmen, 2010). We therefore tested whether the PTEN inhibitor bpV (OHpic) changes HB-GAM expression in rat primary hippocampal and cortical neurons. Indeed, PTEN inhibition leads to robust increase of HB-GAM protein levels in both of ourin vitroneuronal cultures (Kuja-Panula et al., unpublished). These findings from different cell types suggest that inhibition of PTEN generally leads to increased HB-GAM expression. PTEN inhibition leads to robust CNS regeneration, and downregulation of PTEN signaling in neurons is currently suggested as a pathway to develop regenerative therapies for CNS injuries (Gutilla and Steward, 2016). With these ideas in mind, it would be most interesting to study if elevated HBGAM expression is seen in various PTEN inhibition modelsin vivoand whether HB-GAM is a mediator of CNS regeneration in PTN deletion or knockdown.
To what extent the injected HB-GAM enhances functional recovery in different injury models still needs further investigation. However, it appears clear that the injected HBGAM enhances functional recovery in transection models and contusion models of the spinal cord at least when basic locomotor functions are assayed (Kulesskaya et al., unpublished). These findings apparently involve axon regeneration. We are currently further defining the functional recovery in the spinal cord injury models using a battery of behavioral tests. Functional significance of dendrite regeneration is unclear, and further studies are clearly warranted to unravel to what extent dendrite regeneration in the mammalian CNS would contribute to formation of synapses and functional neural circuitries.
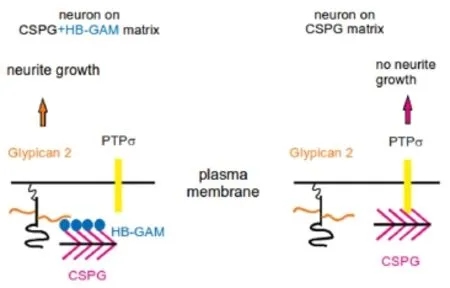
Figure 1 CSPGs and HB-GAM in regenerative neurite growth.
Formal safety studies on using HB-GAM as a therapeutic strategy are still lacking. However, it appears likely that HB-GAM could be used at high doses to modify the CSPG matrices. One reason for this inference is that HB-GAM is endogenously expressed at high levels in the juvenile CNS although this does not necessarily mean that the adult CNS would tolerate similar high expression levels. On the other hand, we have injected HB-GAM at very high concentrations (up to 10 mg/mL) into adult mouse brain without observing any toxicity, at least in terms of TUNEL staining (Paveliev et al., 2016). Furthermore, transgenic mice overexpressing HB-GAM live a normal life span and we have not observed changes in brain histology or any adverse effects in a battery of behavioral tests (Pavlov et al., 2002). The only behavioral changes have been faster learning in water maze and decreased anxiety in elevated plus-maze, while the HB-GAM knockout mice have demonstrated an opposite behavioral phenotype. It thus appears reasonable to continue efforts in therapy development on the HB-GAM route.
Acknowledgments:We thank Seija Lågas and Erja Huttu for their help in laboratory work.
Author contributions:HR wrote the text and MP drew the figure. All authors participated in revising the manuscript.
Conflicts of interest:None declared.
Open peer reviewer:He-Zuo Lü.
Additional file:Open peer review report 1.
Anderson MA, Burda JE, Ren Y, Ao Y, O´Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195-200.
Barnes AP, Polleux F (2009) Establishment of axon-dendrite polarity in developing neurons. Ann Rev Neurosci 32:347-381.
Bespalov MM, Sidorova YA, Tumova S, Ahonen-Bishopp A, Magalhães AC, Kulesskiy E, Paveliev M, Rivera C, Rauvala H, Saarma M (2011) Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol 192:153-169.
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636-640.
Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR (2011) Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science 332:484-488.
Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J (2014) Functional regeneration beyond the glial scar. Exp Neurol 253:197-207.
Dreyfus J, Brunet-de Carvalho N, Duprez D, Raulais D, Vigny M (1998) HB-GAM/pleiotrophin: localization of mRNA and protein in the chicken developing leg. Int J Dev Biol 42:189-198.
Fenrich KK, Rose PK (2011) Axons with highly branched terminal regions successfully regenerate across spinal midline transections of the adult cat. J Comp Neurol 519:3240-3258.
Fenrich KK, Weber P, Hocine M, Zalc M, Rougon G, Debarbieux F (2012) Long-term in vivo imaging of normal and pathological mouse spinal cord with subcellular resolution using implanted glass windows. J Physiol (Lond) 590:3665-3675.
Filmus J, Capurro M, Rast J (2008) Protein family review: Glypicans. Genome Biol 9:224.
Galtrey CM, Fawcett JW (2007) The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 54:1-18.
Gogolla N, Caroni P, Luthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258-1261.
Gutilla EA, Steward O (2016) Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control? Neural Regen Res 11:1201-1203.
Hensch TK (2004) Critical period regulation. Ann Rev Neurosci 27:549-579.
Kinnunen T, Raulo E, Nolo R, Maccarana M, Lindahl U, Rauvala H (1996) Neurite outgrowth in brain neurons induced by heparin-binding growth-associated molecule (HB-GAM) depends on the specific interaction of HB-GAM with heparan sulfate at the cell surface. J Biol Chem 271:2243-2248.
Kwok JC, Dick G, Wang D, Fawcett JW (2011) Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71:1073-1089.
Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J (2015) Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 518:404-408.
Li G, Hu Y, Huo Y, Liu M, Freeman D, Gao J, Liu X, Wu DC, Wu H (2006) PTEN deletion leads to up-regulation of a secreted growth factor pleiotrophin. J Biol Chem 281:10663-10668.
Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, Milbrandt J, Deuel TF (1990) Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science 250:1690-1694.
Merenmies J, Rauvala H (1990) Molecular cloning of the 18-kDa growth-associated protein of developing brain. J Biol Chem 265:16721-16724.
Milev P, Chiba A, Häring M, Rauvala H, Schachner M, Ranscht B, Margolis RK, Margolis RU (1998) High affinity binding and overlapping localization of neurocan and phosphacan/protein-tyrosine phosphatase-zeta/beta with tenascin-R, amphoterin, and the heparin-binding growth-associated molecule. J Biol Chem 273:6998-7005.
Paveliev M, Lume M, Velthut A, Phillips M, Arumäe U, Saarma M (2007) Neurotrophic factors switch between two signaling pathways that trigger axonal growth. J Cell Sci 120:2507-2516.
Paveliev M, Kislin M, Molotkov D, Yuryev M, Rauvala H, Khiroug L (2014) Acute brain trauma in mice followed by longitudinal two-photon imaging. J Vis Exp doi: 10.3791/51559
Paveliev M, Fenrich KK, Kislin M, Kuja-Panula J, Kulesskiy E, Varjosalo M, Kajander T, Mugantseva E, Ahonen-Bishopp A, Khiroug L, Kulesskaya N, Rougon G, Rauvala H (2016) HB-GAM (pleiotrophin) reverses inhibition of neural regeneration by the CNS extracellular matrix. Sci Rep 6:33916.
Pavlov I, Võikar V, Kaksonen M, Lauri SE, Hienola A, Taira T, Rauvala H (2002) Role of heparin-binding growth-associated molecule (HBGAM) in hippocampal LTP and spatial learning revealed by studies on overexpressing and knockout mice. Mol Cell Neurosci 20:330-342.
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248-1251.
Rahal OM, Simmen RC (2010) PTEN and p53 cross-regulation induced by soy isoflavone genistein promotes mammary epithelial cell cycle arrest and lobuloalveolar differentiation. Carcinogenesis 31:1491-1500.
Raulo E, Julkunen I, Merenmies J, Pihlaskari R, Rauvala H (1992) Secretion and biological activities of heparin-binding growth-associated molecule. Neurite outgrowth-promoting and mitogenic actions of the recombinant and tissue-derived protein. J Biol Chem 267:11408-11416.
Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H (1994) Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM): Identification as N-syndecan (syndecan-3). J Biol Chem 269:12999-13004.
Rauvala H (1989) An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J 8:2933-2941.
Rauvala H, Vanhala A, Castrén E, Nolo R, Raulo E, Merenmies J, Panula P (1994) Expression of HB-GAM (heparin-binding growth-associated molecules) in the pathways of developing axonal processes in vivo and neurite outgrowth in vitro induced by HB-GAM. Brain Res Dev Brain Res 79:157-176.
Rolls A, Shechter R, Schwartz M (2009) The bright side of the glial scar in CNS repair. Nat Rev Neurosci 10:235-241.
Schwab ME, Bartholdi D (1996) Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76:319-370.
Sharma K, Selzer ME, Li S (2012) Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol 237:370-378.
Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG (2009) PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326:592-596.
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146-156.
Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan LY, Jan YN (2012) Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev 26:1612-1625.
Stipp CS, Litwack ED, Lander AD (1994) Cerebroglycan: An integral membrane heparan sulfate proteoglycan that is unique to the developing nervous system and expressed specifically during neuronal differentiation. J Cell Biol 124:149-160.
Stone MC, Albertson RM, Chen L, Rolls MM (2014) Dendrite injury triggers DLK-independent regeneration. Cell Rep 6:247-253.
Sugahara K, Mikami T (2007) Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol 17:536-545.
Takeda A, Onodera H, Sugimoto A, Itoyama Y, Kogure K, Rauvala H, Shibahara S (1995) Induction of heparin-binding growth-associated molecule expression in reactive astrocytes following hippocampal neuronal injury. Neuroscience 68:57-64.
Thompson-Peer KL, De Vault L, Li T, Jan LY, Jan YN (2016) In vivo dendrite regeneration after injury is different from dendrite development. Genes Dev 30:1776-1789.
*< class="emphasis_italic">Correspondence to: Heikki Rauvala, Ph.D., heikki.rauvala@helsinki.fi.
Heikki Rauvala, Ph.D., heikki.rauvala@helsinki.fi.
orcid: 0000-0001-7809-9811 (Heikki Rauvala)
10.4103/1673-5374.206630
Accepted: 2017-05-09
- 中国神经再生研究(英文版)的其它文章
- Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial
- Genetically modifying transcription factors to promote CNS axon regeneration
- Collapsin response mediator protein-2 plays a major protective role in acute axonal degeneration
- Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury
- Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury
- MRI analysis and clinical significance of lower extremity muscle cross-sectional area after spinal cord injury

