缺血性心肌病与扩张型心肌病的超声心动图特征比较与鉴别诊断
王 硕,牛铁生,李晓东
缺血性心肌病与扩张型心肌病的超声心动图特征比较与鉴别诊断
王 硕,牛铁生,李晓东
目的 探讨超声心动图对缺血性心肌病(ischemic cardiomyopathy,ICM)及扩张型心肌病(dilated cardiomyopathy,DCM) 的鉴别诊断价值。方法 收集175例因心功能不全、射血分数低且超声提示心脏扩张的患者,在纠正心衰后行选择性冠状动脉造影(selective coronary angiography,CAG),明确诊断为ICM或DCM,结果ICM组94例,DCM组81例,应用超声心动图分别对2组从心脏形态学、血流动力学及房室功能等方面进行比较。结果 ICM 组与 DCM 组多项超声指标间差异均有统计学意义(P<0.05或P<0.01)。相对于ICM ,DCM组患者心脏射血分数更低,DCM组的左房内径,右室内径,左室舒张末内径均大于ICM组,同时,DCM组的左室缩末容积及左室舒末容积均大于ICM组,同时,前者的主动脉瓣,肺动脉瓣口血流速度均小于后者。结论 ICM与DCM的心脏结构在超声的显示上有明显不同,前者心脏腔室内径相对较大,且主动脉瓣及肺动脉瓣口血流速度小于后者。
缺血性心肌病;扩张型心肌病;二维多普勒超声心动图;主动脉瓣;肺动脉瓣
在过去的几十年中,由于患者的生存期改善,因心肌梗死起源的缺血性心肌病患者已变得越来越普遍。冠状动脉旁路移植术或经皮冠状动脉介入术在缺血性心肌病患者的治疗中起着举足轻重的作用[1]。然而,此病在症状、体征上与扩张型心肌病极为相似,且部分缺血性心肌病(ischemic cardiomyopathy ,ICM)患者无典型的心绞痛或心肌梗死病史,而针对ICM的患者,通过冠状动脉旁路移植术或冠脉造影及支架置入术而达到血运重建,可以达到改善症状和生存获益的目的,尤其是对严重病例[2,3]。目前WHO标准在心肌病的分类中不包括缺血性心肌病(ischemic cardiomyopathy,ICM),但在许多临床实践和研究中仍在严重左心室功能不全患者中使用ICM或非ICM分类,因术语“ICM或非ICM”简单且容易沟通,便于了解潜在的发病机制和指导治疗方案[4]。
但是,既往的研究往往只根据病史及相关辅助检查来判定是否因冠脉缺血导致心脏扩大及射血分数下降,而并非全部经过冠脉造影明确鉴别;有相当数量的ICM仍然被误诊为DCM,造成患者诊治的延误。超声心动图作为传统的无创检查,对ICM与非ICM的鉴别诊断意义无明确定论。本研究统计了 175例因超声提示心脏扩大,为明确鉴别ICM和DCM而进行了冠脉造影的患者,回顾性分析他们的心脏超声特点,以期提高对ICM的早期诊断和治疗。
1 对象与方法
1.1 对象 由2名心内科专科医师采用Julkdine法,按常规选择投照体位对 CAG 结果进行分析,必要时加照其他体位,传统的缺血性心肌病关于血管狭窄程度的诊断标准为满足下列条件之一:(1)选择冠状动脉造影显示一支或多支血管病变,血管狭窄≥75%;(2)心梗病史;(3)冠状动脉血运重建(经皮腔内冠状动脉成形术或冠状动脉旁路移植);(4)左主干或前降支近段狭窄大于50%[5]。
统计我院心内科2012-01至2015-12因心脏扩大、心功能不全而行冠状动脉造影(CAG)的175例患者,分别诊断为DCM和ICM。DCM 组81例,其中男63例,女18例(表1),均符合ESC制定的关于DCM的诊断标准[6]。ICM 组94例,其中男69例,女25例。均符合ACC/AHA制定的关于 ICM 诊断标准:(1)经冠脉造影证实有明确的冠脉狭窄;(2)心脏扩大;(3)反复心力衰竭;(4)除外室壁瘤、乳头肌功能不全、室间隔穿孔等冠心病并发症,除外其他心脏病或其他原因所致[7]。
1.2 仪器与方法 采用Philips IE33彩色超声显像仪,S5-1探头,频率1~5 MHz,帧频每秒(65±5)帧。受检者左侧卧位,平静呼吸,同步记录心电图。待图像稳定后,分别采集二尖瓣、乳头肌和心尖水平左室短轴及心尖四腔心、心尖三腔心、心尖两腔心切面二维灰阶动态图,采集3个心动周期的图像并存储。
1.3 常规超声心动图测值 (1)心腔测值:包括收缩末期左房内径(left atrial end-systolic diameter,LAESd)、舒张末期左室内径 (left ventricular end-diastolic diameter,LVEDd)、舒张末期右室内径 (right ventricular end-diastolic diameter,RVEDd)、舒张末期室间隔厚度(inter ventricular septal end-distolic thickness,IVSd)、舒张末期左室后壁厚度(left ventricular posterior wall end-diastolic thickness,LVPWDd)。(2)频谱参数:包括脉冲多普勒测量的二尖瓣口舒张早期峰值(E峰)、舒张晚期峰值(A峰),计算E/A。三尖瓣口舒张期峰值速度(TV),主动脉瓣口收缩期峰值速度(AV),肺动脉瓣口收缩期峰值速度(PV)。(3)心功能参数:包括左心室舒张末容积(left ventricular end-diastolic volume,EDV)、收缩末容积(left ventricular end-systolic volume,ESV)、每搏量(left ventricular stroke volume, SV)及射血分数(left ventricular ejection fraction,LVEF)。见表2。

2 结 果
2.1 心脏形态学比较 二维超声心动图显示,DCM组较ICM组各房室腔扩大更明显,心脏呈球形,DCM组LA、RV、LVD均明显大于ICM组。在ICM组,超声提示心脏虽存在低动力区,但有相当一部分患者存在节段性室壁运动减弱或矛盾运动。而在室壁厚度方面,DCM组与ICM组的IVS与LVPW相比,无显著性差异(图1、图2)。

图1 DCM(A)及ICM(B)患者左室长轴切面
DCM以全心大为主,左心室成球形扩大,右心室亦扩大; ICM主要累及左心系统,表现为左心扩大。(AO:主动脉,RV:右心室,LV:左心室,LA:左房)

图2 DCM(A)及ICM(B)患者心尖四腔心切面
DCM左右心均受累及,表现为全心大,此例患者还合并左心室心尖部血栓形成(箭头示);ICM主要累及左心系统,表现为左心扩大。(RV:右心室,RA:右房,LV:左心室,LA:左房)
2.2 血流动力学比较 在DCM组,主动脉瓣反流(AR)、二尖瓣反流(MR)、三尖瓣反流(TR)的发生率分别为81.48%,90.12%,77.78%。在ICM组AR、MR、TR的发生率分别为88.30%,90.43%,69.15%。两组瓣膜轻度、中度、重度返流情况,见表3。
2.3 心室收缩与舒张功能比较 DCM组与ICM组SV差异无统计学意义(P>0.05),但ICM组EF较DCM组明显增高(P<0.01)。两组A峰有显著性差异(P<0.01),但E峰及E/A无显著性差异(P>0.05)。ICM组A峰较DCM组升高(P<0.05),且PV前者亦大于后者有显著性差异(P<0.01),而TV两组无显著性差异。

表1 两组心肌病患者一般资料 ±s)
注:DCM,扩张型心肌病;ICM,缺血性心肌病

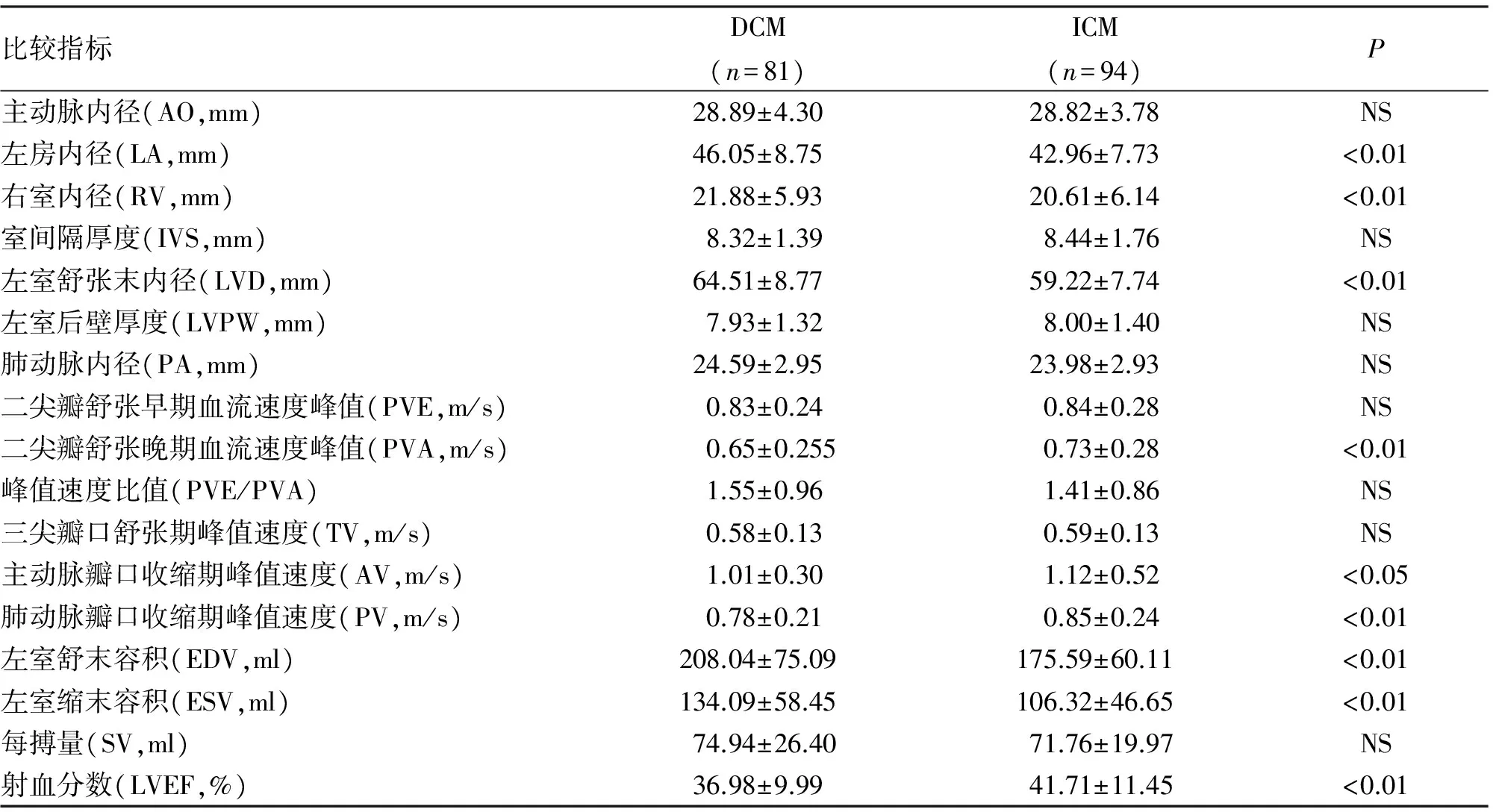
比较指标DCM(n=81)ICM(n=94)P主动脉内径(AO,mm)28.89±4.3028.82±3.78NS左房内径(LA,mm)46.05±8.7542.96±7.73<0.01右室内径(RV,mm)21.88±5.9320.61±6.14<0.01室间隔厚度(IVS,mm)8.32±1.398.44±1.76NS左室舒张末内径(LVD,mm)64.51±8.7759.22±7.74<0.01左室后壁厚度(LVPW,mm)7.93±1.328.00±1.40NS肺动脉内径(PA,mm)24.59±2.9523.98±2.93NS二尖瓣舒张早期血流速度峰值(PVE,m/s)0.83±0.240.84±0.28NS二尖瓣舒张晚期血流速度峰值(PVA,m/s)0.65±0.2550.73±0.28<0.01峰值速度比值(PVE/PVA)1.55±0.961.41±0.86NS三尖瓣口舒张期峰值速度(TV,m/s)0.58±0.130.59±0.13NS主动脉瓣口收缩期峰值速度(AV,m/s)1.01±0.301.12±0.52<0.05肺动脉瓣口收缩期峰值速度(PV,m/s)0.78±0.210.85±0.24<0.01左室舒末容积(EDV,ml)208.04±75.09175.59±60.11<0.01左室缩末容积(ESV,ml)134.09±58.45106.32±46.65<0.01每搏量(SV,ml)74.94±26.4071.76±19.97NS射血分数(LVEF,%)36.98±9.9941.71±11.45<0.01
注:DCM,扩张型心肌病;ICM,缺血性心肌病

表3 两组心肌病瓣膜反流情况
注:DCM,扩张型心肌病;ICM,缺血性心肌病;NS,无显著性差异
3 讨 论
DCM患者心肌呈弥漫性损害,故此类患者不但左室收缩功能下降,右心室收缩功能亦明显降低。在本研究中可以看出,DCM组肺动脉瓣口血流速度显著低于ICM组,右室内径明显高于ICM组,这与DCM疾病本身特点有关,DCM患者的心脏超声更容易在外观上表现为“球形心”。反之,ICM患者往往是由于缺血导致的左心室心肌收缩功能下降,进而表现为呼吸困难等心功能不全症状而就诊,此类患者心脏各腔室及血管压力升高顺序为左心室、左心房、肺动脉、右心室,这也从另一方面解释了ICM组右心室较DCM组小,且差异有统计学意义。就整体而言,通过超声测得DCM组射血分数明显低于ICM组。相应地,二尖瓣舒张晚期血流速度峰值,主动脉瓣口收缩期峰值速度,前者均显著小于后者,可见DCM组患者的左心动力及血流速度明显小于ICM。
DCM组TR发生率为3.70%而ICM组无此瓣膜返流,ICM组AR发生率为3.19%而DCM组无,这更验证了二者虽在形态学有相似之处,但因压力传导系统不同,瓣膜反流情况亦不同,DCM患者因右心室的扩张,更容易出现三尖瓣返流,而因右心室收缩功能不全,故而左室压力相对升高不明显,不易发生主动脉瓣反流。ICM组以左心室功能不全为主要发病根源,故而右心系统受累较少,不易发生TR。
扩张型心肌病目前被定义为左心室或双心室扩张,同时除外异常负荷的状况(如高血压、瓣膜疾病)及冠状动脉疾病,存在收缩功能不全进而造成整个心室的收缩功能障碍[8]。大量的人口研究报告表明,非缺血性心脏衰竭患者的后代患扩张型心肌病风险增加,其增加程度与其他复杂基因性状类似[9]。在诊断DCM患者的后代中进行系统的心脏筛查,发现20%~35%可能存在家族遗传病[10-12]。之后,有50种以上的基因相关疾病被报道[13]。
目前,冠状动脉粥样硬化性心脏病(coronary artery disease ,CAD)是心脏收缩性心力衰竭(heart failure ,HF)的主要原因。早期识别因冠脉狭窄所致的收缩性心力衰竭对于患者的治疗和预后至关重要。许多患者因左室收缩功能不全表现为充血性心力衰竭(心衰),其病因明显,如既往心肌梗死或心脏瓣膜病。然而,仍有大量的患者表现为收缩性心力衰竭,但病因不确定[14]。 美国心脏病学会基金会/美国心脏病协会(American College of Cardiology Foundation/American Heart Association,ACCF/AHA )指南建议对有冠心病心绞痛或心肌梗死病史的人群进行冠状动脉造影检查[15,16]。因为在这些患者中,冠脉狭窄发生率极高。此外,基于过去积累的数据,血运重建可能对曾发生心绞痛的患者带来额外的生存获益。
然而,许多患者有严重的CAD可能不表现为心绞痛或明确的既往心梗病史,而许多并非由于心肌缺血而表现为收缩性心力衰竭的患者可表现为心绞痛。这种方法在美国心脏病学会基金会/美国心脏病协会指南中,在收缩性心力衰竭的患者中实施CAG推荐等级为Ⅱa,证据等级为 C(专家意见),强调了临床数据的缺乏。因此,如何选择恰当的收缩性心力衰竭的患者并施行冠脉造影和有效的血运重建仍然是一个重要课题,目前并未有此类研究[10]。既往的国内研究只根据病史、辅助检查和临床症状来区分DCM和ICM,然后在此基础上进行心脏超声的对比,并未全部经过冠脉造影明确鉴别,因此不能保证分组的准确性。本研究所入选的病例均经过冠脉造影,结果更加真实可靠。
[1] Schuster A,Morton G,Chiribiri A,etal.Imaging in the management of ischemic ardiomyopathy: special focus on magnetic resonance[J].Am Coll Cardiol,2012, 59(4):359-370.
[2] Yusuf S, Zucker D, Peduzzi P,etal. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration[J]. Lancet, 1994,344(8922):563-570.
[3] Velazquez E J, Lee K L. Coronary-artery bypass surgery in patients with left ventricular dysfunction[J]. N Engl J Med, 2011,364:1607-1616.
[4] Maron B J, Towbin J A, Thiene G,etal. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention[J]. Circulation, 2006,113(14):1807-1816.
[5] Felker G M, Shaw L K, O'Connor C M. A standardized definition of ischemic cardiomyopathy for use in clinical research[J]. Am Coll Cardiol, 2002,39(2):210-218.
[6] Pinto Y M, Elliott P M, Arbustini E,etal. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases[J]. Eur Heart J, 2016,37(23):1850-1858.
[7] Fihn S D, Blankenship J C. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons[J]. Thorac Cardiovasc Surg,2015,149(3):e5-23.
[8] Elliott P, Andersson B, Arbustini E,etal. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases[J]. Eur Heart J,2008,29(2):270-276.
[9] Lee D S, Pencina M J, Benjamin E J,etal. Association of parental heart failure with risk of heart failure in offspring[J]. N Engl J Med,2006,355:138-147.
[10] Mahon N G, Murphy R T, MacRae C A,etal.Echocardiographic evaluation in asymptomatic relatives of patients with dilated cardiomyopathy reveals preclinical disease[J]. Ann Intern Med, 2005,143:108-115.
[11] Grunig E, Tasman J A, Kucherer H,etal. Frequency and phenotypes of familial dilated cardiomyopathy[J]. J Am Coll Cardiol, 1998,31:186-194.
[12] Michels V V, Moll P P, Miller F A,etal. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy[J]. N Engl J Med, 1992,326:77-82.
[13] Morales A, Hershberger R E. Genetic evaluation of dilated cardiomyopathy[J].Curr Cardiol Rep, 2013,15(7):375.
[14] Gheorghiade M, Sopko G.Navigating the crossroads of coronary artery disease and heart failure[J].Circulation,2006,114:1202-1213.
[15] Hunt S A, Abraham W T, Chin M H,etal. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation[J]. J Am Coll Cardiol,2009,53(15):e1-e90.
[16] Johnson R A. Palacios IDilated cardiomyopathies of the adult (first of two parts) [J]. N Engl J Med, 1982,307(17):1051-1058.
(2016-12-22收稿 2017-02-21修回)
(责任编辑 梁秋野)
Differernt echocardiographic features of ischemic cardiomyopathy and dilated cardiomyopathy: A cohort study
WANG Shuo,NIU Tiesheng,and LI Xiaodong.
Department of Cardiology, Shengjing Hospital of China Medical University,Shenyang 110004, China
Objective To investigate the value of echocardiography in the differential diagnosis of ischemic cardiomyopathy (ICM) and dilated cardiomyopathy (DCM).Methods One hundred and seventy-five cases were enrolled into this study who had been diagnosed with the symptoms of heart failure, low ejection fraction and enlargement of the heart indicated by cardiac ultrasound.After the remission of heart failure,they underwent selective coronary angiography (CAG)and were definitely diagnosed with ICM or DCM. The ICM group consisted of 94 patients and the DCM group 81 patients. Two dimensional color Doppler ultrasound echocardiography was used to find the difference in cardiac morphology, hemodynamics and ventricular function.Results There was significant difference in ultrasound echocardiography between the two groups (P<0.05 orP<0.01).Conclusions There is significant difference between ischemic cardiomyopathy and dilated cardiomyopathy in ultrasound echocardiography. In DCM, the chambers are relatively larger, and blood flow is faster.
ischemic cardiomyopathy; dilated cardiomyopathy; two dimensional Doppler echocardiography; aortic valve; pulmonary valve
王 硕,硕士,医师。
110004 沈阳,中国医科大学附属盛京医院心血管科
李晓东,E-mail:69132987@qq.com
R542.2

