低氘水对人肺癌细胞A549增殖和分化的影响及机制
戚冰雪,李子宜,丛峰松
(上海交通大学生命科学技术学院,上海200240)
·论著·
低氘水对人肺癌细胞A549增殖和分化的影响及机制
戚冰雪,李子宜,丛峰松
(上海交通大学生命科学技术学院,上海200240)
目的 研究低氘水(DDW)对人肺癌A549细胞增殖和分化的影响,并探讨其作用机制。方法 取A549细胞分为观察组和对照组,分别用氘含量为50 ppm的DDW配制的培养基、正常水配制的培养基培养30 d,倒置显微镜下观察两组细胞形态,用CCK-8法测定48 h内细胞生长情况。观察组和对照组细胞先分别在无血清的DDW培养基和正常水培养基中培养48 h使细胞生长同步化,然后分别加入含10%血清的DDW培养基和正常水培养基继续培养,用碘化丙啶染色和流式细胞分析仪测定两组36、48 h时处于细胞周期G1、S和G2/M期的细胞比例;取两组细胞裂解液,抽提细胞总蛋白,Western blot法检测丝裂原活化的细胞外信号调节激酶(MEK1)、表皮生长因子受体(EGFR)表达,MEK1第217和第298位丝氨酸磷酸化水平,EGFR第1016和第1110位酪氨酸磷酸化水平;比较两组肺泡Ⅰ型上皮细胞特异性蛋白标志物AQP5、T1a和肺泡Ⅱ型上皮细胞特异性蛋白标志物SPB、SPC1、SPC2、CFTR的表达。结果 对照组细胞呈立方形、细胞间紧密接触和堆积生长,观察组细胞呈纺锤形、细胞伸长、细胞间接触较松散。观察组36、48 h时的OD值均较对照组降低(P均<0.05)。血清饥饿后再刺激36 h,观察组处于G1期的细胞比例为71.75%,高于对照组的57.01%;观察组处于S期的细胞比例为22.43%,低于对照组的35.88%(P均<0.01);48 h时两组细胞周期比例比较差异无统计学意义。两组MEK1、EGFR表达及EGFR第1016位和第1110位酪氨酸磷酸化水平比较差异无统计学意义,观察组MEK1第217位和第298位丝氨酸磷酸化的水平降低(P均<0.01)。观察组SPC2水平较对照组升高(P<0.01)。结论 A549细胞用DDW长期培养,可显著抑制其细胞增殖和分化,其机制可能与降低MEK1的磷酸化水平、阻滞细胞周期G1/S期转变以及诱导A549细胞由Ⅱ型肺泡上皮细胞向Ⅰ型肺泡上皮细胞转化、提高细胞分化程度有关。
肺癌;低氘水;细胞周期;丝裂原活化的细胞外信号调节激酶;细胞分化
氘是氢的三种同位素之一[1],自然存在的氘为正常细胞生长所必需[2,3]。氘的自然含量因在地球上的采样位置不同而不同,其浓度范围为120~160 ppm[4]。氢在地球上以水的形式存在最多,氘含量低于150 ppm的水称为低氘水(DDW)。DDW可以在体外和体内抑制癌细胞生长[5,6],已有报道将其用于肺癌脑转移患者的辅助治疗,使用DDW结合常规临床治疗可以延长肿瘤患者的生存期[7]。表皮生长因子受体(EGFR)和丝裂原活化的细胞外信号调节激酶(MEK)通路是影响肿瘤细胞增殖的重要信号通路,信号通路的激活与活性位点的磷酸化水平呈正相关[8]。2016年6~9月,我们使用DDW培养人肺腺癌A549细胞,观察A549细胞EGFR和MEK1活性位点的磷酸化水平,并检测肺泡Ⅰ型上皮细胞特异性蛋白标志物AQP5、T1a和肺泡Ⅱ型上皮细胞特异性蛋白标志物SPB、SPC1、SPC2、CFTR的表达变化,探讨DDW对人肺腺癌A549细胞增殖和分化的影响及机制。
1 材料与方法
1.1 材料 A549细胞系购自中科院上海细胞库,F12K培养基、胎牛血清和0.25%的胰酶-EDTA购自Thermo Fisher公司,碘化丙啶、BCA蛋白质浓度测定试剂盒和RIPA缓冲液购自碧云天生物技术有限公司,CCK-8试剂盒购自北京博尔迈生物技术有限公司,DDW(氘含量50 ppm)购自上海齐天生物科技有限公司,用于检测MEK1、EGFR总蛋白的抗体、抗MEK1第217位丝氨酸磷酸化(MEK-pSer217)和第217位丝氨酸磷酸化(MEK1-pSer298)的抗体以及抗EGFR第1016位酪氨酸磷酸化(EGFR-pTyr1016)和第1110位酪氨酸磷酸化(EGFR-pTyr1110)的抗体购自美国Cell Signaling公司,抗AQ5、T1a、SPC1、SPC2和GADPH抗体购自美国Abcam公司,抗CFTR和SPB抗体购自美国Merk Millipore公司,耦联有辣根过氧化物酶(HRP)的二抗购自美国KPL公司,化学发光试剂购自美国GE公司,其他化学试剂购自美国Sigma公司。
1.2 细胞分组与处理 将A549细胞分为对照组和观察组,分别加入正常水配制的F12K培养基、DDW配制的F12K培养基中,加入终浓度为2 mmol/L的谷氨酰胺和10%胎牛血清。37 ℃、5% CO2、饱和湿度培养30 d。每天在倒置显微镜下观察细胞形态。
1.3 细胞增殖观察 将培养30 d的细胞加入0.25%的胰酶-EDTA消化,以1×105/mL接种于96孔培养板中。于12、24、36、48 h分别加入CCK-8试剂10 μL,继续培养显色90 min,用酶标仪在450 nm波长处测定吸光度OD值。每个时间点设3个复孔,实验共重复3次,取平均值。
1.4 细胞周期分析 将培养30 d的细胞加入0.25%的胰酶-EDTA消化,以1×105/mL接种到6孔培养板中。细胞贴壁后,对照组和观察组细胞分别在不含血清的正常水培养基和DDW培养基中饥饿48 h使细胞生长同步化,然后加入含10%胎牛血清的正常水培养基和DDW培养基继续培养36 h和48 h。加入胰酶消化,将细胞以2×105/mL重悬于碘化丙锭染色液中,避光染色30 min。用BD公司的FACS CaliburTM进行单个细胞内DNA含量的分析,细胞群体在细胞周期不同时相的分布用ModFit LT软件进行拟合分析。
1.5 EGFR、MEK1蛋白表达及活性位点磷酸化检测 采用Western blot方法。将培养30 d的细胞用含有蛋白酶抑制剂和磷酸脂酶抑制剂的预冷PBS洗涤,加入RIPA裂解液反复吹打裂解细胞,4 ℃离心收集上清液,用BCA试剂盒测定总蛋白浓度。每个泳道上蛋白质样品50 μg进行SDS-PAGE后用湿转法将蛋白条带转移到PVDF膜上。用含4%脱脂奶粉的PBST封闭,加入相应抗体(EGFR、MEK1抗体或针对活性位点磷酸化的抗体)4 ℃一抗孵育过夜,PBST洗涤3次后,用耦联有HRP的二抗(1∶10 000)室温孵育1 h,PBST洗涤,化学发光底物显色,化学发光扫描仪记录蛋白表达或蛋白活性位点磷酸化的条带。以目标蛋白与内参蛋白GADPH灰度值的比值计算目标蛋白的相对表达量。
1.6 肺泡上皮细胞特异性蛋白标志物检测 按照1.5中的方法提取两组细胞的总蛋白,电泳并转膜后进行Western blot分析,用抗AQ5、T1a、CFTR、SPB、SPC1、SPC2的抗体检测各蛋白标志物的表达。以目标蛋白与内参蛋白GADPH灰度值的比值计算目标蛋白的相对表达量。

2 结果
2.1 两组细胞形态比较 对照组细胞在倒置显微镜下呈立方形,细胞间接触紧密和堆积性生长;观察组细胞呈纺锤形,细胞伸长,细胞之间的接触较对照组松散。
2.2 两组细胞增殖情况比较 观察组12、24、36、48 h的OD值分别为0.33±0.12、0.38±0.01、0.42±0.02、0.50±0.06;对照组分别为0.33±0.02、0.37±0.01、0.47±0.05、0.65±0.05。观察组36、48 h时的OD值较对照组降低(P均<0.05)。
2.3 两组细胞周期分布比较 观察组36 h时处于G1期的细胞比例高于对照组,处于S期的细胞比例低于对照组(P均<0.01)。48 h时两组细胞周期比例比较差异无统计学意义。见表1。
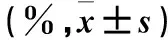
表1 两组细胞周期分布比较
注:与对照组比较,*P<0.01。
2.4 两组MEK1、EGFR磷酸化水平比较 观察组MEK1蛋白表达与对照组比较无统计学差异;与对照组比较,观察组第217位、第298位丝氨酸的磷酸化水平显著下调。观察组EGFR蛋白表达与对照组比较无统计学差异;第1016、第1110位酪氨酸的磷酸化水平也无明显变化。
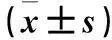
表2 两组MEK1、EGFR及其活性位点的磷酸化水平
2.5 两组肺泡上皮特异性标志物水平比较 DDW培养30 d后,观察组AQP5、T1a、SPC1、SPC2水平较对照组升高,CFTR、SPB水平无明显变化。提示观察组部分A549细胞的分化类型由Ⅱ型上皮细胞向Ⅰ型上皮细胞转化。见表3。
3 讨论
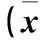
表3 两组肺泡上皮特异性标志物水平比较±s)
研究显示,氘能够影响细胞生长[8]。已有报道显示,DDW能显著延长前列腺癌和未转移肺癌患者的生存期[5,9]。然而DDW对肿瘤细胞生长抑制的分子机制目前还不清楚。细胞周期是细胞分裂经历的全过程,S期DNA进行复制,M期细胞进行分裂,S期和M期之前分别为G1生长期和G2生长期[10]。本研究显示,DDW能够抑制A549细胞从G1期向S期转化,从而抑制其生长,这种生长抑制效应是由于DDW阻滞细胞周期由G1期向G2期转变所致。
研究证明,A549细胞携带致癌基因KRAS/G12S激活突变,这一突变上调MAPK/ERK信号通路,从而促进A549细胞的分裂增殖[11,12]。为了探究DDW对A549细胞生长抑制作用的信号通路,我们选择了与细胞有丝分裂密切相关的两个重要分子EGFR和MEK1上与其活性有关的关键磷酸化位点上磷酸化水平变化进行研究。结果表明,DDW培养对EGFR的表达及其第1016及第1110位酪氨酸活性位点的磷酸化水平无明显影响;对MEK1的表达水平虽无明显影响,但可显著降低其第217位和第298位丝氨酸的磷酸化水平。说明DDW可通过下调MEK/ERK信号通路抑制A549细胞增殖。
肺泡上皮根据形态和功能分为Ⅰ型上皮和Ⅱ型上皮,Ⅰ型上皮主要负责气体交换,Ⅱ型上皮通过分泌表面活性蛋白来维持肺泡的表面张力[9]。A549细胞属于Ⅱ型肺泡上皮细胞,能够产生表面活性蛋白[9,13]。本研究发现,用DDW培养基培养A549细胞30 d后,细胞发生了显著的形态学变化,由原来的立方形、细胞间接触紧密和堆积性生长变为纺锤形、细胞伸长和细胞之间接触较不紧密的生长。说明细胞的分化类型发生了变化。Western blot进一步检测肺泡上皮细胞特异性蛋白标志物表达水平,发现DDW可显著上调水通道蛋白AQP5和T1a两种Ⅰ型肺泡上皮特异性蛋白标志物的表达,同时SPC1和SPC2两种Ⅱ型上皮特异性蛋白标志物的表达也显著提高。上述细胞形态学表型和特异性标志物水平的变化说明DDW可诱导A549细胞由Ⅱ型肺泡上皮细胞向Ⅰ型肺泡上皮细胞转化,从而导致A549细胞的分化程度提高,恶性增殖能力下降。
综上所述,DDW可通过下调MAPK/ERK信号通路,阻滞A549细胞细胞周期,抑制细胞增殖;DDW还可以诱导A549细胞由Ⅱ型肺泡上皮向Ⅰ型肺泡上皮的转化。这些研究结果部分阐明了DDW抑制肺癌细胞增殖的分子机制,为DDW用于肺癌的辅助治疗提供了依据。
[1] Macrae RM. Isotopes and analogs of hydrogen--from fundamental investigations to practical applications[J]. Sci Prog, 2013,96(Pt3):237-293.
[2] Boros LG, D′Agostino DP, Katz HE, et al. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle[J]. Med Hypotheses, 2016,87:69-74.
[3] Somlyai G, Jancsó G, Jákli G, et al. Naturally occurring deuterium is essential for the normal growth rate of cells[J]. FEBS Letters, 1993,317(1-2):1-4.
[4] Ehleringer JR, Rundel PW, Nagy KA. Stable isotopes in physiological ecology and food web research[J]. Trends Ecol Evol, 1986,1(2):42-45.
[5] Gyongyi Z, Budan F, Szabo I, et al. Deuterium depleted water effects on survival of lung cancer patients and expression of Kras, Bcl2, and Myc genes in mouse lung[J]. Nutr Cancer, 2013,65(2):240-246.
[6] Cong FS, Zhang YR, Sheng HC, et al. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis[J]. Exp Ther Med, 2010,1(2):277-283.
[7] Krempels K, Somlyai I, Somlyai G. A retrospective evaluation of the effects of deuterium depleted water consumption on 4 patients with brain metastases from lung cancer[J]. Integr Cancer Ther, 2008,7(3):172-181.
[8] Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer[J]. Oncogene, 2007,26(22):3291-3310.
[9] Kondo H, Miyoshi K, Sakiyama S, et al. Differential regulation of gene expression of alveolar epithelial cell markers in human lung adenocarcinoma-derived A549 clones[J]. Stem Cells Int, 2015,2015:165867.
[10] Feillet C, van der Horst GT, Levi F, et al. Coupling between the circadian clock and cell cycle oscillators: implication for healthy cells and malignant growth[J]. Front Neurol, 2015,6:96.
[11] Yoon YK, Kim HP, Han SW, et al. KRAS mutant lung cancer cells are differentially responsive to MEK inhibitor due to AKT or STAT3 activation: implication for combinatorial approach[J]. Mol Carcinog, 2010,49(4):353-362.
[12] Chang F, Steelman LS, Shelton JG, et al. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review)[J]. Int J Oncol, 2003,22(3):469-480.
[13] Foster KA, Oster CG, Mayer MM, et al. Characterization of the A549 cell line as a type Ⅱ pulmonary epithelial cell model for drug metabolism[J]. Exp Cell Res, 1998,243(2):359-366.
Effects of deuterium-depleted water on proliferation and differentiation of lung cancer A549 cells
QIBingxue,LIZiyi,CONGFengsong
(SchoolofLifeSciencesandBiotechnology,ShanghaiJiaotongUniversity,Shanghai200240,China)
Objective To study the effects of deuterium-depleted water (DDW) on human lung cancer cell line A549 and to explore the underlying molecular mechanisms. Methods A549 cells were randomly divided into two groups: the observation group and the control group, which were seperately cultured in the medium prepared with 50 ppm DDW and in the medium prepared with normal water for 30 d. The morphology of cells was observed under an inverted microscope. Cell growth was measured using the colorimetric cell counting kit-8 (CCK-8) reagent to compare the growth rate of cells between the two groups. The cells in the two groups were synchronized by serum starvation and then were separately stimulated with medium containing 10% serum of DDW and medium containing normal water. At 36 and 48 h after the stimulation, cells from each group were trypsinized, stained with propidium iodide (PI) and then were analyzed by fluorescence-activated cell sorting (FACS) to determine the percentages of cells distributed in G1, S and G2/M phases during cell cycle. Total proteins were extracted from cells cultured in normal medium or DDW medium. Western blotting was conducted to detect the expression levels of MEK1 and EGFR, phosphorylation of serine 217 and serine 298 on MEK1, and phosphorylation of tyrosine 1016 and tyrosine 1110. The expression levels of several alveolus-specific protein markers (type Ⅰ alveolus markers: AQP5 and T1a, type Ⅱ alveolus markers SPB, SPC1, SPC2 and CFTR) in cells of the two groups were also compared. Results In the control group, the cells were cuboidal and in close contact with accumulation of cells. In the observation group, the cells were spindle, and intercellular contact was loose with cell elongation. The OD of the observation group was lower than that of the control group (allP<0.05). After the serum starvation, cells were stimulated for 36 h, the percentage of cells in G1phase of the observation group was 71.75%, which was higher than that of the control group (57.01%), the percentage of cells in S phase was 22.43%, which was lower than that of the control group (35.88%), (allP<0.05). There was no significant difference in cell cycle ratio between the two groups at 48 h. No significant difference was found in the expression of MEK1, EGFR and the phosphorylation of EGFR on tyrosine 1016 and 1110 between these two groups. In the observation group, the phosphorylation of MEK1 on both serine 217 and serine 298 was significantly decreased (allP<0.01). The expression level of SPC2 in the observation group was significantly increased as compared with that of the control group (P<0.01).Conclusions Long-term cultivation of A549 lung cancer cells with DDW medium significantly inhibits the cell proliferation and differentiation, which is related to decreasing phosphorylation level of MEK1, arresting the G1/S phase transformation, inducing A549 cells from type Ⅱ alveolar epithelial cells to type I alveolar epithelial cells and improving the differentiation of A549 cells.
lung carcinoma; deuterium-depleted water; cell cycle; mitogen-activated extracellular signal-regulated kinase; cell differentiation
国家自然科学基金资助项目(81272478);教育部重点实验室开放课题(AF4150013)。
戚冰雪(1988-),硕士研究生,主要研究方向为低氘水对A549细胞的增殖抑制。E-mail: 647870061@qq.com
丛峰松(1970-),副教授,主要研究方向为抗肿瘤药物的研究与开发。E-mail: fsongcong@sjtu.edu.cn
10.3969/j.issn.1002-266X.2017.06.001
R734.2
A
1002-266X(2017)06-0001-04
2016-11-14)

