二维炭基多孔材料的合成及应用
贺 雷 张向倩 陆安慧
二维炭基多孔材料的合成及应用
贺 雷 张向倩 陆安慧*
(大连理工大学化工学院,精细化工国家重点实验室,辽宁大连116024)
二维材料是指厚度在纳米尺度,且在两个维度(长和宽)具有较大尺寸的材料。与块体材料相比,二维材料最大的特点是具有极高的表面/体积比,有利于传质、传热和离子扩散,因而在吸附、催化以及储能等领域有广泛的应用。近年来,随着石墨烯引发的二维材料研究浪潮,二维炭基多孔材料成为全世界关注的研究热点。而二维炭基材料的孔结构是影响其性能的关键因素。本文介绍了近年来二维炭基多孔材料的合成方法,包括炭纳米片及炭-无机复合纳米片的制备,讨论了制备条件对材料孔结构的影响。在此基础上,着重介绍了二维炭基材料在吸附、多相催化及储能方面的应用。最后,对新型炭基二维材料开发中仍存在的关键科学问题进行了总结和展望。
二维材料;炭;孔结构;合成方法;吸附;催化;储能
1 Introduction
Two-dimensional(2D)materials are materials consisting of nano-scale thickness with larger width and length for the other two dimensions(the ratio of width/thickness or length/thickness larger than 50).Since the production of single layered graphene in 20041, 2D materials are nowadays under rapid development2.Besidessingle-atomic-thick graphene3and boron nitride(BN)4,other compounds with single-polyhedral-thickness containing O,S,N atoms can also be classified as 2D materials,including transition metal oxides(TMOs)5,transition metal dichalcogenides(TMDs)6, layered double hydroxides(LDH)7,8,perovskite materials9,and single-layered zeolites10-12.The ultrahigh surface-to-volume ratio feature of the 2D materials is beneficial for mass and heat transport13.Due to the unique physical structure and tunable chemical property,they are widely employed in the applications of adsorption14,heterogeneous catalysis15-17,photonic and electronic industries18-20,analysis21,and energy storage22,23.
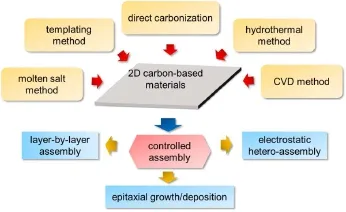
Fig.1 Synthesis strategies for 2D carbon-based materials
Among various 2D nanostructures,carbon-based materials have attracted tremendous attentions due to their broad availability, excellent electric and thermal conductivity,chemical stability,and relatively easy functionalization or hybridization24,25.Here,2D carbon-based materials are defined as the materials composed of carbon and other inorganic units with 2D structures.Carbon nanosheets(CNSs)are representative for this type of materials, which have been widely studied in energy-related and environmental applications.During the past decade,a rapid development of synthesis strategies has provided capability of preparing CNSs with controllable thickness,tunable pore size and surface area,and specific surface functional groups.So far,there are five major synthesis strategies,which will be discussed in detail in Section 2(Fig.1).To be noticed,the pore structure is the key factor that influences the performance of the carbon-based materials in many fields,such as adsorption,catalysis,and electronic devices.In general,micropores are the crucial contributors for large surface area,high adsorption capacity,and high electrical capacitance. However,for conventional porous carbons,the kinetic problems of micropores hinder their applications.It is a supplement to the conventional methods which prepare the porous carbons by introducing mesopores or even macropores into the entire structure, to improve the mass and heat transport.
This review will focus on the most recent development of 2D carbon-based porous materials.The correlation of the pore structures and the synthesis methods will be discussed.It will provide insight into ways for facile design of novel 2D carbonbased materials with certain pore structures.On this basis,the assembled structures(including carbon monolith,thin films and hybrids/composites)by 2D materials will be introduced and classified according to their composition.Furthermore,the applications of the 2D carbon-based porous materials will be introduced focusing on adsorption,heterogeneous catalysis,analysis,and electrical energy storage.

HE Lei received her PhD degree from Dalian Institute of Chemical Physics,Chinese Academy of Sciences in 2014.Since 2014, she has been working in Dalian University of Technology as a lecturer and a postdoctoral researcher.Her research interests now mainly focus on catalytic CO2conversion processes.

ZHANG Xiang-Qian received her Master degree from Dalian University of Technology in 2013.She is currently a PhD student in Prof.LU An-Hui′s group since 2014.Her research focuses on synthesis and application of porous carbon nanomaterials with designed porosity and controlled morphology.

LUAn-Hui received his PhD degree from the Institute of Coal Chemistry,Chinese Academy of Sciences in 2001.After postdoctoral work(as a Max Planck research fellow and Alexander von Humboldt fellow)in the group of Prof.F.Schüth at Max-Planck-Institut für Kohlenforschung,he was promoted to group leader in 2005.He is currently a professor at the State Key Laboratory of Fine Chemicals,School of Chemical Engineering,Dalian University of Technology since 2008.Since 2015,he has been appointed as the Dean of the School of Chemical Engineering.His research interests include synthesis of porous materials for heterogeneous catalysis,adsorption,energy storage and conversion.
2 Synthesis strategies for 2D porous carbon materials
Carbon materials with 2D structure,usually defined as CNSs, have been synthesized by various methods,including chemical vapor deposition(CVD)of gaseous precursors,templating and self-assembly method in liquid phase,molten salt route,direct carbonization,etc.The thickness,degree of graphitization,and porous structure of a CNS can be controlled by changing synthesis conditions.Furthermore,the chemical composition of CNSs can be adjusted by varying precursors,synthesis conditions,and posttreatment processes.This section provides a summary of five mainstrategies for the preparation of CNSs.Especially,the factors influencing porous structures will be discussed in detail for each strategy.Here,microporous carbon nanosheets are defined according to the ratio of micropore volume(accounts for over 60% of the total pore volume).Similarly,mesoporous carbon nanosheets are also describing the material with dominant amount of mesopores(over 60%).Besides,other carbon nanosheets, possessing hierarchical porous structures containing micropores, mesopores,or even macropores,are also introduced in this section,including the structures fabricated or assembled by carbon nanosheets.
2.1 Templating method
One of the most powerful methods for preparing porous CNSs is the templating method.In principle,a template can be any substance as long as having sheet-like structures,including soft and hard templates.Various porous carbon materials have been prepared using soft templates26.However,the sheet-like soft templates are very difficult to synthesize,which in turn leads to commonly hard-template method for preparing CNSs.Various oxide materials,including SnO2,ZnO,MgO,have been employed as hard templates27-29.Graphene oxides(GOs)have also been successfully used as shape-directing agent for the preparation of CNSs14.Typically,a templating synthetic procedure includes the preparation of carbon precursor/template composite,carbonization,and removal of the template30.To be noted,using carbonbased nanosheets(e.g.,GO)as templates will leave out the template removal process.Generally,the pore structures are mainly determined by the original structure of the templates, which could also be adjusted by the surfactants and carbonization conditions.
2.1.1 Microporous carbon nanosheets
Graphene oxides with a heavily oxygenated surface can be chosen as a shape-directing agent for preparing microporous carbon nanosheets.Its surface is negatively charged which has been confirmed by zeta potential analysis31.By means of the electrostatic interaction,surface-engineering on the GO sheets is expected to produce new hybrid products using different carbon precursors.For example,sandwich-type microporous hybrid carbon nanosheets(MHCNs)consisting of graphene and microporous carbon layers were fabricated using graphene oxides as shape-directing agent,amino acids as bridging agent,and the insitu formed poly(benzoxazine-co-resol)as carbon precursor (Fig.2)32.A strong electrostatic interaction between asparagine molecules and GO rapidly in-situ reacted with the resorcinol and formaldehyde,ending with uniform poly(benzoxazine-co-resol) layer grown on both sides of the GO sheets.The overall thickness of the nanosheets can be tuned from 20 to 200 nm according to a fitted linear correlation between the carbon precursor/GO mass ratio and the coating thickness.

Fig.2 (a)Schematic of the formation of carbon nanosheets (MHCNs)using GOs as templates;(b,c)scanning electron microscopy(SEM)images of MHCNs32;(d,e)SEM and transmission electron microscopy(TEM)images of NPCG33
Nitrogen-doped carbon nanosheets are claimed to possess excellent performances in adsorption and supercapacitors,which can be synthesized by employing nitrogen-containing carbon source in the templating process.For example,nitrogen-doped sandwichlike porous carbon nanosheets were prepared through carbonization of graphene/polyaniline hybrid materials33.The final products showed interconnected microporous structure,with the nitrogen content of 12.7%(a,atomic fraction),the surface area of 410.4 m2·g-1and the volumetric density of 0.94 g·cm-3.The content of graphene in the final products is in the range of 25%-31%(w,mass fraction).
Ionic liquids(ILs)have many excellent features,such as good solubility for chemicals,excellent thermal stability and negligibly small vapor pressure.Therefore,the ILs containing nitrile groups can be used as a heteroatom source for preparing carbon materials, which may not only facilitate the solubility of carbon precursors, but may also serve as a heteroatom source for a doping agent34.An aqueous solution of 2D nitrogen-doped microporous carbon sheets with precisely controlled thickness,can be prepared using ionic liquid functionalized graphene oxide(IL-functionalized GO) sheets as a shape-directing agent and a resorcinol/formaldehyde polymer as the carbon precursor35.The ionic liquid was selected to stabilize the isolated GO sheets and to functionalize them in an aqueous solution.This type of materials is classified as microporous carbon sheets rather than graphene or graphene-based sheets because the prepared sheets contain very small amounts of graphene,from 0.26%to 2.72%(w),and have a graphene inner layer and a carbon coating on both sides.
Recently,novel porous polymers with 2D structures have been prepared,which could also be used as self-sacrificing template. Xiang et al.36,37employed 2D covalent organic polymers(COPs) as self-sacrificing template to synthesize nitrogen-doped holey graphene-like carbons.Using different N-containing precursors, the locations of heteroatoms were precisely controlled with certainporosity structures.
2.1.2 Mesoporous carbon nanosheets
In general,micropores are the crucial contributors for large surface area.However,the ion diffusion and mass transfer in the micropores is often restricted due to the limited pore size and pore volume.Therefore,the construction of carbon nanosheets with a mesoporous domain is welcomed and should benefit for their applications.
Using mesoporous oxide nanosheets as hard templates can generate CNSs with mesoporous structure originated from the templates.For example,MgO is one of the most common hard templates for the synthesis of CNSs,which was produced by a boiling treatment for MgO particles with a subsequent calcination process27.Afterwards,the porous MgO sheets were coated with carbon source by self-assembly approach.After carbonization and removal of the MgO template,carbon nanosheets can be obtained with similar structure as the original porous MgO sheets.The carbon nanosheets had a high surface area of 883 m2·g-1as well as pillared mesoporous structures(6-8 nm).Similarly,Wang and his co-workers22have prepared a two-dimensional mesoporous carbon sheet-like framework(MCSF)material using mesoporous SiO2nanosheet as template and coal tar pitch as carbon precursor. Thus,MCSF possessed a thin carbon sheet structure and abundant mesopores with sizes centered at 2.2 and 15 nm,respectively. However,the synthesis procedure is somewhat complex and the carbon particles have a relatively low surface area(582.7 m2·g-1) and an unsatisfactory entire porosity.
Biomass resources can also be used as templates for synthesizing carbon nanosheets.Zhang and coworkers38reported a facile and scalable in-situ synthetic strategy(simultaneous templategraphitization)to fabricate porous carbon sheets(PMCSs)using biomass agar as template,in which carbon-stabilized Fe/Fe3C nanoparticles were homogeneously embedded.In the synthesis (Fig.3),the graphitic catalyst precursor(Fe(NO3)3)and template agent(Al(NO3)3)were introduced simultaneously into the agar hydrogel through the coordination of the metal precursor with the functional groups on agar,thus resulting in simultaneous realization of the template and graphitization of the carbon source under heat treatment.This PMCS possessed relatively high surface area(1023.2 m2·g-1)with the average pore diameter of 12.5 nm.
Although graphite layers have unique properties,such as excellent electric conductivity,the low surface area and nonporous structure limit their applications.To make abundant porous structures,carbon coated graphene composites have been developed.Using expandable graphite(EG)as the template,mesoporous-carbon-coated graphite nanosheet(GNS@MC)composites have been synthesized by the intercalation of resol prepolymer into the interlayers of EG under vacuum-assisted conditions,followed by the exfoliation of EG through in-situ polymerization,the growth of resol under hydrothermal conditions, and carbonization under Ar39.The carbon product possessed a specific surface area of 432.3 m2·g-1and bimodal mesopores with detectable sizes of 2.3 and 9.2 nm.Another example is the synthesis of nitrogen-doped mesoporous graphene nanosheets using GO as template and NH2CN as nitrogen source40.The as-obtained carbon nanosheets possessed high surface area with an average pore size of around 6.3 nm,which can be attributed to the abundant wrinkled structures originated from the carbonization process.The N content in the final product was 9.96%(w).

Fig.3 (a)SEM image of PPCN;(b)X-ray diffraction(XRD)patterns of PPCN before and after acid treatment27; (c)TEM image of MCSF22;(d)mechanism of the decomposition of agar and formation of PMCS;(e)TEM image of PMCS38
Besides,the addition of GO can alter the structure of carbonmaterials.Hydrothermal carbonization(HTC)of biomass such as glucose and cellulose typically produces micrometer-sized carbon spheres.However,adding a very small amount of GO(e.g.,1:800 (mass ratio))can signif i cantly alter the morphology of its HTC product.HTC of GO and glucose resulted in thick platelets of glucose-derived carbon-coated reduced graphene oxide(r-GO) sheets at low GO concentration41.GO sheets can act as nucleation and growth sites for seeding the carbonization product of glucose. Glucose solution containing dispersed GO sheets was hydrothermally carbonized to form a brown char-like intermediate product,and f i nally converted to porous nanosheet composite by two-step chemical activation using KOH42.The composite had a relatively high packing density of 0.3 g·cm-3and large specif i c surface area of 2106 m2·g-1,as well as containing plenty of mesopores.
Nevertheless,the synthesis process for GO is tedious and expensive,which is the main limitation for its large-scale applications.Other cheap layer-structured materials with facile syntheses are widely selected as templates to prepare CNSs,such as vermiculite43,halloysite44and montmorillonite45.Take halloysite as an example,which can be selected to synthesize amorphous mesoporous carbon sheets by using polypyrrole44or furfuryl alcohol46as carbon precursors.
2.2 Molten salt route
Molten salt route is an easy and efficient method for the synthesis of carbon nanosheets with high surface area,where the molten salt acts as solvents and porogen in this process47.Depending on the nature of the salt,the carbonization temperature ranges from 100 to 1000°C48.Thus,several types of carbon nanosheets from simple porous frameworks to complex heteroatoms(nitrogen or sulfur)doping materials have been synthesized49.Their physical and chemical properties are tunable by changing the salts or carbon precursors.
2.2.1 Microporous carbon nanosheets
He and coworkers have recently synthesized a series of porous carbon nanosheets with high nitrogen content using melamine and terephthalaldehyde as carbon precursors through the Schiff-base reaction in a molten salt medium(Fig.4)50.As the mole ratio of terephthalaldehyde to melamine reaching 1.0,the nitrogen content of the obtained carbon sheet was 30.51%(w),which was much higher than that in other nitrogen-rich carbon materials.The specific surface area for all the carbons derived from the molten salt system was at least a tenfold increase compared with the sample prepared without salt.The results indicated that the salt reaction medium,possibly as a porogen,was the key factor for enlarging the specific surface area.It is believed that the ionized environment created by molten salt efficiently prevented the product from agglomeration due to the high viscosity and played a template role in directing the formation of 2D structure.Furthermore,the concentration of the carbon precursor can alsoimpact the formation of sheet-like structure51.For example,along with the decrease in the precursor concentration of glucose,the fraction of sheet structures retained in the products increased remarkably,which means that the molten salt is able to dissolve the intermediates and convert completely the carbon source to graphene layers.It has been proved that the salts played an important role in the formation of the microstructure as well as the porosity of the carbon nanosheets.Several publications attributed a template role to the intentionally added salt during the synthesis of carbons47,48,52.However,the mechanism for the formation of 2D carbon sheets is still unclear and potentially very attracting.
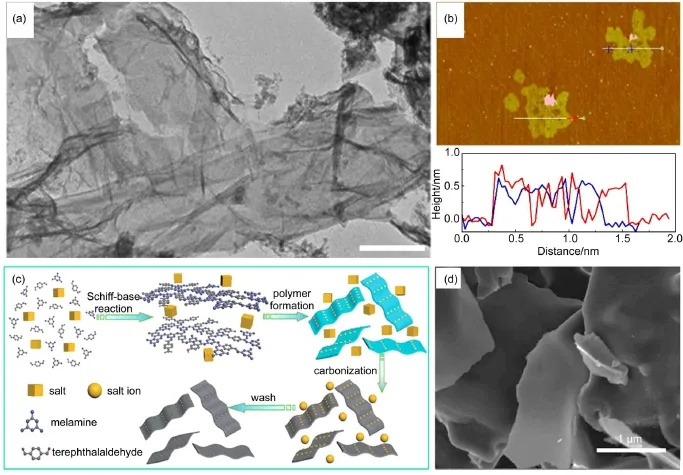
Fig.4 Typical(a)TEM and(b)atomic force microscopy(AFM)images for the graphene-like thin carbon layers synthesized in KClO3@LiCl/KCl at 900°C(scale bar:0.5 μm)49;(c)preparation strategy of carbon nanosheets using molten salt method(LiCl-KCl)and (d)the corresponding SEM image50
2.2.2 Mesoporous carbon nanosheets
The carbon nanosheets prepared from molten salt route enabled a one-step synthesis of highly porous heteroatom-doped carbons with specific surface areas up to 2000 m2·g-1.For example,Zhang and co-workers53recently fabricated the graphene-like carbon sheets with well-defined mesopores(~3.5 nm),high specific surface area(up to 2607 m2·g-1),and high pore volume(up to 3.12 cm3·g-1)using polyimide chemistry in the molten salt medium.In this process,abundant pyromellitic dianhydride and aromatic diamine undergo polycondensation following carbonization in molten KCl-ZnCl2.The in-situ formed linear aromatic polyimide with a sp2hybridized carbon skeleton can be directly coupled and rearranged into a 2D graphene-like nanosheet around the“salt scaffold”.The nitrogen atoms in amine also provided the as-obtained carbon materials with uniform foreign atoms(nitrogen content~6%(a)).Furthermore,holey carbon sheets with welldispersed and through-plane nanoholes(diameter:5-10 nm)can be produced by using different monomers.
2.2.3 Other porous carbon structures
In order to synthesize carbon nanosheets even more conveniently and economically,Fellinger and co-workers54introduced an ionothermal carbonization via hot injection in molten ZnCl2using ethylene glycol(EG)and glycerol as carbon precursors.To be noted,due to the very fast pyrolytic monomer decomposition in Schlenck-type reactor at high temperatures(up to 550°C), highly volatile but cheap organic solvents can be used as carbon precursors(such as EG).Interestingly,all organic solvents were successfully converted into carbonaceous nanomaterials with spherical,sheet-like,and branched nanofibrous morphologies and with high yields.When heteroatom-containing solvents were used, the doping levels reached up to 14%(w)nitrogen and 13%(w) sulfur.From the same group,the fabrication of vertically aligned carbon nanosheets(CNSs)and metal carbide@CNS composites were also fabricated via a salt templating induced self-assembly52. This approach allows for versatile fabrication of the carbon nanostructures and a range of hybrid materials,e.g.,metal carbide nanoparticles rigidly embedded into the graphitic carbon nanosheets.
2.3 Direct carbonization and/or activation
As described above,the templating and molten salt methods are two of the most efficient strategies for preparing 2D carbon-based materials.However,the necessity of templating removing or salt recycling somewhat complexes the preparation process.Therefore, the development of facile synthesis procedures for producing porous carbon nanosheets is still desirable and attractive.Direct carbonization is a one-step method for preparing carbon materials at high temperatures,which can be combined with the activation process at the same time.The precursors can be widely available substance,such as organic salts,nitrides,resin,biomass,etc.The porous structure of the obtained products can be tuned by using different carbon precursors,adding activators,or changing carbonization conditions.
2.3.1 Microporous carbon nanosheets
Recently,Sevilla et al.55reported an easy,one-step procedure for the synthesis of highly porous interconnected carbon nanosheets with a thickness of〈80 nm,which was based on the carbonization of an organic salt(i.e.,potassium citrate)at a temperature in the range of 750-900°C.The porosity of the carbon nanosheets essentially consisted of micropores distributed at 0.7-0.85 nm and 0.95-1.6 nm.Importantly,the micropore sizes of both systems can be enlarged by simply increasing the carbonization temperature.Furthermore,the carbon nanosheets possess specific surface areas in the range of 1400-2200 m2·g-1. Similarly,using sodium gluconate as the organic salt,highly porous carbon nanosheets were obtained after carbonization at 700-900°C56.The as-prepared carbon nanosheets have a large aspect ratio(length/thickness≈102-103),a thickness within the range of 40-200 nm,surface areas(SBET)of up to 1390 m2·g-1,and a porosity with a hierarchical organization.Importantly,the textural properties can be substantially enhanced(SBETup to 1890 m2· g-1)via an additional activation step.
Bourlinos has reported a direct synthesis of ultrathin carbon nanosheets by the solid-state pyrolysis of betaine ((CH3)3N+CH2COO-,a zwitterionic organic compound widely distributed in nature)57.The nanosheets are less than 6 nm in thickness and 1-5 μm in lateral dimensions,highly graphitized, contain polar functional groups on the surface,and possess a specific surface area(~100 m2·g-1).Moreover,the solid state pyrolysis of other betaine-based compounds(e.g.,betaine hydrochloride)can also produce carbon nanosheets of similar dimensions,however,with much lower yields(〈0.5%).
2.3.2 Mesoporous carbon nanosheets
The one-step activation and nitrogen-doping combination method is also developed for preparing nitrogen-doped graphenelike carbon nanosheets(N-CNSs)(Fig.5)58.Macroporous anion exchange resin was used as carbon precursor and nitrogen source. The combination of Ca(OH)2and NH4Cl performed as both activator and nitrogen source.The as-prepared N-CNS exhibits a porous,loose,ultrahigh pore volume(3.19 cm3·g-1)and highly wrinkled morphology.By the same research group,highly crumpled nitrogen-doped graphene-like nanosheets with a specific surface area of 1169 m2·g-1and relatively large pore volume of 2.58 cm3·g-1were prepared from a macroporous resin via simultaneous urea gasification expansion and CaCl2activation methods59.The pore sizes of the sheets were distributed narrowlyand centered in the range of 2-10 nm,corresponding to the existence of abundant mesopores.
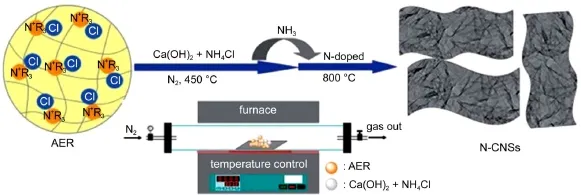
Fig.5 Schematic of the preparation process of N-CNSs58
The self-assembled organic nanosheets or biomass sources can be the carbon precursor for direct carbonization synthesis of mesoporous carbon nanosheets.Different from the templating method,the self-assembled sheet-like carbon precursor is often prepared through polymerization of monomers with a certain direction without the assistance of a template,which also simplified the preparation procedure,i.e.removal of templates can be omitted.However,only certain types of carbon naosheets can be prepared by this method due to the limitation of precursors.Yuan et al.60prepared mesoporous N-containing carbon nanosheets(NCNSs)by using polyaniline nanosheets(NSs)as a carbon precursor.The polyaniline NSs were fabricated through self-assembly by an oxidative polymerization of aniline monomer with potassium persulfate(PPS)under mild hydrothermal condition.After direct carbonization of polyaniline NSs,N-CNSs can be obtained with a well-developed mesoporous architecture(0.32 cm3·g-1), 5.9%(w)N species,a specific surface area of 352 m2·g-1,and average pore size of 5.2 nm.
2.3.3 Other porous carbon structures
By a one-step chemical activation method,highly porous, oriented,and interlinked carbon nanosheets were prepared using hydrothermally polymerized glucose spheres(pGSs)as precursor61.The unique carbon nanosheets were directly produced from pGSs after one-step activation by KOH,which displayed dramatic morphological change compared with the traditional two-step process(pre-carbonization followed by activation,yielding microporous carbon spheres).The carbon nanosheets had a surface area of 2633 m2·g-1and a pore volume of 1.86 cm3·g-1.During the one-step activation,the melt of potassium species directed the formation of oriented carbon nanosheets;the oxygen constituents in the pGSs are critical for the morphological evolution from sphere to sheet structure.
Similarly,a 3D interconnected frameworks composed of sandwiched graphene/porous carbon layers with 20 nm thickness was synthesized by using a facile one-step pyrolysis of the mixture of graphene oxide(GO)/polyaniline(PANI)hybrid and KOH62. The obtained porous carbon material exhibited a high specif i c surface area(2927 m2·g-1),hierarchical interconnected pores, moderate pore volume(1.78 cm3·g-1),short ion diffusion paths, and a nitrogen level of 6%(a).To be noted,the hierarchically connected pores(micro-,meso-and macropores)provide unimpeded channels for fast diffusion of electrolyte ions and result in an enhanced charge storage and high rate capability.
The biomass sources with nanosheet structures can also be the carbon precursors.Jin and co-workers63has developed hierarchically porous carbon nanosheets(HP-CNSs)using exfoliated waste coffee grounds by in-situ carbonization and activation processes using KOH.Despite the simple synthesis process,the HP-CNSs had a high aspect ratio nanostructure(~20 nm thickness to several micrometers in lateral size),a specific surface area of 1945.7 m2·g-1,numerous heteroatoms,and good electrical transport properties,as well as hierarchically porous characteristics(0.5-10.0 nm in pore size).
2.4 CVD method
Chemical vapor deposition(CVD)method has been widely used in producing 2D carbon materials.In this process,a gaseous carbon precursor is subject to high-temperature treatment,which induces decomposition,radical formation,and aggregation of the precursor molecules.The growth of these agglomerates through continued deposition and decomposition of the carbon precursor ultimately leads to the formation of 2D carbon materials.The gaseous precursor can be formed by vaporization,sublimation,or atomization of a solid or liquid source.There are numerous references for graphene or graphene oxide with perfect graphitic crystallinity using CVD method64-66.However,there are limited amounts of reports for carbon nanosheets using CVD method,and the porosity has not been fully discussed.It has been proved that porous carbon nanosheets derived from CVD method show advantages of a high degree of graphitization,a regular arrangement, and controllable thickness.
Wang et al.67has reported a free-standing sheet-like carbon nanostructure by inductively coupled radio-frequency plasma enhanced chemical vapor deposition(PECVD)without catalyst or special surface pretreatment.Such nanosheets with thickness of ca 1 nm were obtained without catalyst over a wide range of deposition conditions and on a variety of substrates,including metals,semiconductors and insulators.Such carbon sheets fabricated by the direct CVD and PECVD methods,seem attractive to researchers of fundamental materials chemistry,nanoscience and technology because of their potential applications innanodevices,capacitors,and chemical and bio-sensors.
Mesoporous carbon sponge consisting mostly of single-layer graphene walls has been prepared by CVD method usingAl2O3-NP as a nanosized substrate,defined as graphene mesosponge (GMS)68.After the CVD process,the color of the sample turned black with the quartz tube remaining transparent,indicating that carbon deposition only occurred on the surface of Al2O3-NP due to its catalysis.Therefore,the entire surface of Al2O3-NP can be covered by a quite thin carbon layer.As the Al2O3-NPs are not sintered,the shape of the resulting carbon coatedAl2O3-NP(Al2O3-NP/C)is similar to that of Al2O3-NP(Fig.6).The mean pore size is 5.8 nm for the obtained GMS.
Xu et al.69prepared small,thin carbon nanosheets(thickness of 2-5 nm)via CVD method using CaO as template and toluene as carbon precursor.During the CVD process,toluene is pyrolyzed to generate carbon atoms and then deposit on the nano-CaO particles.After template removal,the carbon nanosheets are obtained with a tunable thickness by changing deposition time.
CNSs synthesized by the CVD method exhibit high-quality, large-area features.However,it is very difficult to achieve precise control of the properties of the resulting carbon materials in terms of surface area,pore size,and surface functionality70.
2.5 Hydrothermal method
Hydrothermal process is widely employed to fabricate 2D carbon-based porous materials from diverse carbon precursors, especially biomass resources at relatively mild conditions.The size,shape distribution,and crystallinity of carbon nanostructures can be altered by controlling experimental conditions,including reaction temperature,reaction time,surfactant type,and precursor types,etc.
Wang et al.71reported a combined hydrothermal and activation processes with KOH that uses natural fibers(hemp bast fiber)as the precursor to achieve graphene-like carbon nanosheets.The obtained carbon nanosheets showed unique interconnected partially graphitic structure(10-30 nm in thickness)with high specific surface area(up to 2287 m2·g-1)and large volume fraction of mesoporosity(up to 58%).The macroporous voids with diameters of 1-2 μm serve as ion buffering reservoirs.The low thickness of the carbon nanosheets(10-30 nm)ensures nanoscale distances(5-15 nm)for ion diffusion.The high total content of mesopores facilitates the accessibility of the electrolyte ions to the electrode surface and allows for fast ion transport.
2D mesoporous carbon nanosheets(MCNSs)were prepared with approximately 40 nm thickness and 10 mm width72.Large numbers of mesopores with an average size of 9 nm are distributed in a disorder manner throughout the nanosheets.Such 2D carbon nanosheets with mesopores can enhance the rate performances and simultaneously maintain its high capacities and robust cycle life in lithium ion battery.The MCNSs were prepared by hydrothermal pretreatment of sodium and ethylene glycol in a closed Teflon reactor followed by a rapid pyrolysis procedure.During the hydrothermal process,the ethylene glycol solution became increasingly saturated with the metal alkoxide as it forms.And as a result of the autogenerated pressure,the excess free ethylene glycol was encapsulated into the solid metal alkoxide forming a clathrate-like structure,which can be used as ignition points in the following pyrolysis process.Therefore,nucleation of 2D MCNSs occurred around the regions rich in ethylene glycol.It was called a“popcorn effect”73.Experiments performed on non-clathrated, crystalline sodium ethoxide were not found to result in the formation of carbon nanosheets,indicating that the hydrothermal conditions are essential to produce the proper precursors.
2.6 Controlled assembly of 2D carbon-based materials
Many other 2D compounds beyond carbon are also important owing to their unique catalytic or electronic properties,such as transition metal oxides(TMOs),layered double hydroxides (LDH),boron nitride(BN),transition metal dichalcogenides (TMDs),etc.The assembly of 2D carbon nanostructures and these O,S,or N containing nanosheets can create novel properties distinct from the parent materials.In this section,the synthesis methods of the carbon-based layered hybrids are summarized and classified according to the fabrication methods.Generally,the assembly methods can be divided into three types:layer-by-layer assembly,electrostatic hetero-assembly,and epitaxial growth. 2.6.1 Layer-by-layer(LBL)assembly
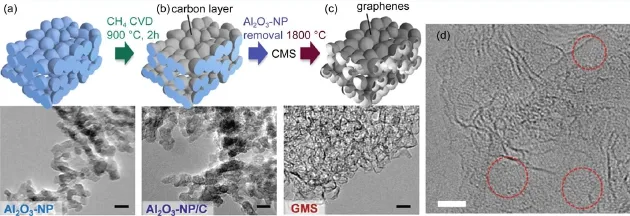
Fig.6 (a-c)Aschematic of the synthesis of GMS with TEM images at each step;(d)atomic-resolution TEM image of GMS68The scale bars are 10 nm in(a-c)and 2 nm in(d).
Layer-by-layer(LBL)assembly technique has been a premier method for the preparation of nanoscale films with tunablecomposition,thickness,and function groups74.Typically,the LBL process started from the adsorption of oppositely charged precursors onto the substrate,which therefore reversed the surface charge.Further layers can be deposited by alternating the adsorption species with opposite charge until achieving the desired thickness.
A wide variety of oxides and hydroxide are composed of primary units with layered structure,including hydrotalcites,δ-MnO2,FeOOH,etc.5,75.Among the 2D oxides/hydroxides family, layered double hydroxide(LDH)is one class of anionic materials based on the brucite-like layered structures7.With the development of synthetic methods and anion-exchange procedure,the LDH composites have been enriched during the last decade,which can be further assembled with carbon nanosheets to obtain novel 2D materials8.Chen et al.76synthesized electrically conductive poly (vinyl alcohol)(PVA)hybrid films utilizing the hydrogen-bonding layer-by-layer self-assembly method.Before the assembly,graphene oxides and single-layer LDH nanosheets were exfoliated from natural graphite oxide powders and crystallized Co-Al-NO3LDH,respectively.Utilizing the hydrogen bonding interaction of PVA/GO and PVA/LDH,multilayer hybrid with highly oriented GO and LDH were fabricated.In this process,PVA acted as a bridge to connect GO and LDH.
Dong et al.77developed a simpler way for fabricating LDH-GO hybrid using layer-by-layer assembly.Poly(diallyldimethylammonium chloride)coated ITO was employed as a substrate,which was first immersed into the Co-Al LDH dispersion for deposition following washing and drying processes.Afterwards,the substrate was immersed into GO suspension for a second layer deposition. The cycle was repeated until a desired amount of bilayers obtained.This method can be extended to the preparation of flexible electrode using PET as a substrate.
2.6.2 Electrostatic hetero-assembly
Different from graphene with a relatively weak interlayer interaction,the oxides/dichalcogenides layered materials have a high layer charge density that generates nanosheets with positive or negative charges5.Such unique property has been used for the assembly of 2D hybrids using electrostatic force.Generally,the electrostatic fabrication process is based on the exfoliation or delamination of the bulk materials to get sheet-like debris with opposite charges.
For example,LDH nanosheets(LDH-NS)with positive charge can be obtained via delamination,while GO nanosheets are negatively charged in basic solutions.Thus the electrostatic interaction between GO and LDH-NS created a self-assembly of two sheets.Wang et al.78reported the fabrication of Co-Al LDH-NS with GO using electrostatic assembly.The Co-Al LDH-NS was delaminated in formamide and GO dispersed in water.After mixing the two solutions together,the assembly process occurred automatically.Similarly,the assembled Ni-Al LDH and GO nanosheets can also be prepared using the same method79.
To simplify the synthesis procedure,the delamination process of LDH can be bypassed through modification of the LDH precursors.Long et al.80employed Cl-exchanged Ni-Fe LDH and GO as precursors.The negatively charged GO can be assembled directly into the LDH interlayer as anion.Latorre-Sanchez et al.81synthesized a hybrid containing graphene and Ni6MnO8through an electrostatic assembly of Ni-Mn LDH and GO following a calcination process.As a Ni-Mn LDH suspension can be obtained during the deposition process in methanol and showed good exfoliation property,a delamination step was unnecessary.The fabrication of Mg-Al LDH/GO hybrid aerogel was assisted by ultrasonic treatment after dispersion LDH into GO suspension82. Herein,GO played an ion-exchange role and inserted into the interlayer of Mg-Al LDH,and LDH acted as cross-linking agent for the fabrication of such aerogel.
In general,the surface charge of GO is determined by the dispersed solution.For example,the DMF-exfoliated graphene is electropositive,so that it can interact with negatively charged nanosheets.Xie′s group83reported δ-MnO2/graphene hybrid for supercapacitor using chemically integration of electropositive δ-MnO2nanosheets on graphene due to the strong electrostatic interaction.This method can be extended to prepare other 2D hybrid materials,e.g.,the fabrication of carbon nitride(C3N4)and LDH using electrostatic interactions84.
Furthermore,dichalcogenide and carbon nanosheets can be used for the fabrication of hybrid materials.Zhou et al.85reported the synthesis of MoS2-RGO hybrids with nanosheet-nanosheet structure via a lithiation-assisted exfoliation process following a hydrazine monohydrate vapor reduction technique.The MoS2was firstly exfoliated into nanosheets by electrochemical lithiation approach,and then mixed with GO following a hydrazine reduction process to get MoS2-RGO86.Due to the existence of chemical bond formed between MoS2nanosheets and graphene oxides,the agglomeration of MoS2nanosheets can be effectively restricted and the growth of Mo nanoparticles during lithiation was limited.Similarly,Liu et al.87synthesized MoS2/graphene nanocomposite using commercial bulk MoS2as precursor.The preparation process included the hydrolysis of lithiated MoS2with the assistant of untrasonication and the fabrication of MoS2and GO through electrostatic interactions.Peng et al.88developed one-pot synthesis of MoS2/GO hybrid in aqueous solution.It was found that the size of GO plays an important role in tuning the structure of MoS2/GO.
2.6.3 Epitaxial growth/deposition
Another bottom-up method for fabricating 2D carbon-based materials is the epitaxial growth of one composite on the“nanosheet substrates”.The key point is how to control the nanosheet morphology and thickness of the grown layer.For example,GO nanosheets possess negative charge in basic solution, the in-situ growth of positive charged LDH nanosheets can be achieved using GO as a support.Meanwhile,the LDH nanosheets can be highly dispersed on GO without stacking together.Garcia-Gallastegui et al.89reported the in-situ deposition of Mg-Al LDH onto GO nanosheets.Before the deposition process,GO was firstly dispersed into a basic solution containing NaOH andNa2CO3.The mixed salt solution of Mg(NO3)2and Al(NO3)3were then added.The as-obtained suspension was then aged at 333 K for 12 h,washed and dried to get the LDH/GO hybrid.Gao et al.90employed glucose reduced GO as a substrate for Ni-Al LDH growth.The existence of Ni-Al LDH efficiently separated the carbon nanosheets and enhanced the electrochemical properties.
Solvents also influenced the structure of the resulted oxides grown on graphene nanosheets.Kan and Wang reported the deposition of Fe2O3on graphene oxide employing different solvents91.They discovered that sheet-on-sheet structure can be obtained when the preparation process was carried out in isopropanol.In contrast,particle-on-sheet structure was formed when using water instead of isopropanol as the solvent.
Layered transition metal dichalcogenides,such as VS292,93, WS294,and MoS295,have attracted much attention due to their 2D structure analogous to graphene and applications in electrocatalysis and energy storage96-98.Many exfoliation methods including mechanical99or chemical exfoliation100,chemical vapor deposition101,and solution synthesis102,have been established.However, this type of material tends to form fullerene-like nanoparticles or nanotube structures instead of thin layer during processing.To overcome this tendency,the deposition of layered dichalcogenides on graphene oxides was discovered as an effective way to inhibit their growth into 3D structure103-105.The most studied method is insitu growth of dichalcogenide nanoflakes over GO or RGO for the preparation of carbon-dichalcogenide 2D hybrids.Generally,the GO sheets are used as the substrate for a deposition of dichalcogenide precursors,and followed by a thermal treatment,if necessary.Dai′s group104synthesized a MoS2/RGO hybrid in DMF solution employing a selective solvothermal method.They used a single compound(NH4)2MoS4as the source for Mo and S.The as-obtained MoS2/RGO hybrid material possessed nanoscopic fewlayer MoS2structures with large amount of exposed edges stacked onto graphene,which exhibited superior electrocatalytic activity in the hydrogen evolution reaction(HER)compared to other MoS2catalysts.Chang et al.103developed a hydrothermal route for preparing MoS2/RGO hybrid using Na2MoO4and NH2CSNH2as precursors,which showed up to 1300 mAh·g-1capacity as an anode material for Li-ion battery.Moreover,microwave-assisted synthesis approach was developed on the basis of nonhydrolytic sol-gel method using butyl mercaptan as S source and MoCl5as Mo source106.
3 Applications of 2D carbon-based materials
Due to their ultrahigh surface-to-volume ratio,unique chemical and physical properties,and tunable composition,2D carbonbased materials have been widely studied and utilized in the fields of catalysis,energy storage,water purification,electronic and photonic industries.These aspects have been summarized from different viewpoints17,107.In this section,we will focus on the applications in adsorption/separation and heterogeneous catalysis (including electronic and photonic catalysis).Others,such as energy storage,biology,and environment protection will also be briefly touched.
3.1 Adsorption and separation
Conventional porous adsorbents always have a large bulk size in all dimensions,and it therefore takes a long time for the adsorbate molecules to transfer into and out of the inner microporous network.This leads to a lack of adsorbates in the inner pores and low utilization of the overall surfaces and pores.In this case,2D materials with large surface-to-volume ratio and nanosized thickness can efficiently promote the adsorbate diffusion rate, decrease the adsorption resistance,and improve the utilization of the overall surfaces and pores108.
Tuning porous structure and functional groups are two main strategies to improve CO2adsorption.Moreover,the assembly of CNSs can produce macroscopic structures,which further improved the diffusion rate and mechanical property of the final material.Lu′s group has designed a series of 2D porous carbon sheets for CO2separation14,32,108,109.The systematic studies indicate that the porous carbon nanosheets show impressive CO2adsorption capacity under equilibrium and more better separation ability of CO2from N2under dynamic conditions than the counterpart bulk or spherical materials(Fig.7)14.The hierarchically interconnected porous structure(including macropores and mesopores)could greatly enhance the diffusion of adsorption gas molecule and maintain an excellent mechanical strength.The highest CO2adsorption capacities can reach 5.67 and 3.54 CO2molecules per nm3pore volume and per nm2surface area at 298 K and 0.1 MPa.Additionally,the monolithic materials assembled from nanosheets have a mechanical strength of up to 28.9 MPa (the highest reported for the analogues).
Beyond the construction of porous structure,the incorporation of nitrogen groups into the CNS results in improved adsorption/ desorption of CO2.Shen et al.110developed nitrogen-doped porous CNSs with a thickness of 20-40 nm(SBET=1854 m2·g-1,pore volume=0.82 cm3·g-1),which displayed CO2adsorption of 5.8 mmol·g-1at 298 K.Chen′s group developed a 2D carbonaceous polymer nanosheets by polymerizing aromatic nitrile monomers in molten zinc chloride at 400-500°C111.As-obtained nanosheets possess tunable thicknesses of 3-20 nm,well-defined microporosity,a high surface area(~537 m2·g-1),and a large micropore volume(~0.45 cm3·g-1).The CO2sorption capability reached 8.14%(w)at 298 K and 0.1 MPa,and the CO2selectivity was relatively high toward N2(25.6).
Moreover,the 2D carbon-inorganic composite materials(e.g. LDH materials86,112)showed enhanced CO2adsorption.The absolute capacity of the combined material increased 62%compared with LDH using only 7%(w)graphene oxide(GO)as a support because of its 2D structure with large amount of basic sites,which showed excellent affinity to the acid CO2molecule86.Additionally, 2D carbon materials are suitable for physical adsorption of H2, based on first principles investigation113.It has been proven that the adsorption energy on graphynes is larger than zeolite and MOFs and the H2equilibrium distance is closer to the carbon plane, which is beneficial for both H2intercalation and out-of-planediffusion.Zeolite membranes have been employed as precursors to prepare 2D carbon materials for gas separation by Zhong and coworkers114.They prepared carbon composite membrane by direct carbonization of zeolitic imidazolate framework(ZIF-L),which showed high H2/CO2selectivity with a very high H2permanence (~3.5×10-6mol·m-2·s-1·Pa-1)114.By creating such precisely controlled materials with short diffusion paths and high microporosity,instead of larger pores(little contribution to gas sorption capacity),the 2D porous carbon materials can greatly accelerate adsorption kinetics,increasing the utilization degree of the overall porosity and surface area.

Fig.7 (a,b)CO2adsorption isotherms of the porous carbon nanosheets(PCNs)for high and low CO2partial pressures at 298 K, where the solid line represents a Toth model fit to the CO2isotherms;(c)number of CO2molecules adsorbed per nm3pore volume and (d)per nm2surface area for PCNs with different thicknesses141 mmHg=133.3 Pa
Along with its excellent performance in gas adsorption,2D carbon-based materials are also considered as good adsorbents for removal of heavy metal ions,organic dyes from water.For example,the fabricated porous carbon nanosheets with Fe/Fe3C nanoparticles exhibited excellent adsorption property for methylene blue(MB),methyl orange(MO)and crystal violet(CV). The maximum adsorption capabilities for MB,MO,and CV reached 1615.9,1062.4,and 1728.3 mg·g-1,respectively38. Moreover,the possibility of magnetic separation also facilitated its application in wastewater treatment on a large scale.This multifunctional material can potentially be used as a super adsorbent to efficiently remove pollutants from wastewater.Notably, macroscopic graphene-based assemblies and architectures,e.g., graphene hydrogels and aerogels115,and graphene sponges116,117and foams118have shown promising potential as removal candidates (heavy metals,oils,and dyes)with low density,high adsorption capacity,high selectivity,good recyclability,and environmental compatibility for water treatment,owing to their combined merits of large surface area,high chemical stability,and facile functionalization.Despite the above great advantages,direct experiment data and theoretical calculations on the relevance of properties and structures are quite limited.Lu′s group108designed and synthesized two model microporous carbon materials,i.e.,carbon nanosheets(MCN)and carbon spheres(MCS)having nearly same composition,surface chemistry,and specific surface area,known morphology,but distinguishable diffusion paths(Fig.8).The results indicate that sheet structures are ideal for quick response applications in gas and liquid phase environments.
Moreover,the assembled graphene-inorganic 2D structures performed an enhanced adsorption capability,which guarantee the exposure of the active sites in aqueous solution and overcome the utilization restrictions of neat GO aerogels.Yu′s group119has synthesized a kind of FeOOH/graphene hydrogel by the metal ion induced assembly process under mild conditions,which showed a high adsorption capacity to nonpolar organic solvents and oils, with a maximum uptake capacity of 27 times its own weight and good recyclability.Additionally,it exhibited an outstanding capability for the removal of heavy metal ions,such as those presenting 139.2 and 373.8 mg·g-1adsorption capacities for Cr(VI)and Pb(II),respectively.The above results strongly confirm that porous carbon sheets exhibit improved kinetics in gas phase separation,liquid phase enrichment,and energy storage devices, due to their shorter diffusion paths and larger exposed geometrical area derived from the 2D structure.

Fig.8 (a-c)SEM and TEM images of MCN and MCS;(d,e)adsorption of Cr(VI)on MCN and MCS108
3.2 Heterogeneous catalysis
In heterogeneous catalysis,most of the reactions occur on the surface of catalysts,which means that mass and heat transfer are the most important factors beyond the catalytic reaction itself.The large surface-to-volume structure of 2D materials is beneficial for mass and heat transfer,which also provide more active sites available on the surface.Therefore,more attention has been paid for 2D materials in heterogeneous catalysis.Traditionally,carbon materials are often employed as inert support for loading active components.However,due to its good conductivity and tunable surface defects,carbon materials showed unique catalytic activity in electrocatalysis as non-metal catalysts.Also,with the development of the graphene-like C3N4material,explosive investigations have been carried out for photocatalysis processes.Notably, the applications of few layered graphene or graphene oxide have been documented in previous reviews,both as carbocatalysts or support for loading active components16,120-122.However,the applications of 2D carbon-based materials,including the assembled composites,have not been systematically discussed.In this part, we will focus on the most recent applications of 2D materials in heterogeneous catalysis based on the materials mentioned in Section 2,especially elecrocatalysis and photocatalysis.
3.2.1 Electrocatalysis
In electronic catalysis processes,the activity is usually originated from metal particles,metal oxides,dichalcogenides,or other heteroatoms instead of carbon.However,the low electric conductivity limited their applications.The strategy to solve the problem is to construct hybrid materials consisted of active components and conducting graphitic carbon.Based on the assembled materials,their electrocatalysis performances will be discussed hereafter.
Development of electrocatalysts for the oxygen evolution reaction(OER)is critical to energy conversion and storage processes.Among various catalysts,IrO2is considered as the most efficient for OER.However,the high cost and element scarcity hindered its industrial applications.Therefore,the development of non-noble-metal catalytic systems is desirable.The first row transition metal based materials are considered as promising alternatives.Long et al.80reported the fabrication of FeNi layered double oxide-GO hybrid as an active catalyst for OER.This nonnoble-metal-based electrocatalyst exhibited high activity(TOF is 1 s-1at 0.3 V),which can be attributed to the synergy effect of the catalytic active FeNi oxides and the enhanced electron transport arising from the graphene nanosheets.Lou′s group synthesized carbon coated nickel phosphide with porous nanoplate structure(Fig.9)123.Compared with NiO and Ni(OH)2,as-prepared nickel phosphides manifest superior electrocatalytic activity for OER due to their structural merits and the in-situ formed catalytically active oxidized nickel species.

Fig.9 FESEM images of Ni-Pporous nanoplates(a-c);LSV curves(d)and Tafel plots(e)of Ni-P,Ni(OH)2and NiO porous nanoplates in 1.0 mol·L-1KOH123
Hydrogen evolution reaction(HER)is another important reaction for clean energy and environment protection.Transition metal dichalcogenides(e.g.,MoS2124,WS294)are discovered as active electrocatalysts to replace noble metal Pt for HER.The computational and experimental results have confirmed that the HER activity stemmed from the sulfur edges of MoS2plates.The combination of MoS2with graphene is an efficient method to enlarge the edge exposure and improve the stability,and therefore enhance the activity in HER125.Dai′s group successfully developed MoS2/graphene hybrid using solvothermal synthesis,which exhibited superior activity with good conductivity(Tafel slope of~41 mV·decade-1)104.Leite′s group also prepared MoS2/graphene hybrid under a relatively mild condition106.The 2D material formed by steps and folded edges of MoS2layers on graphene, also showed excellent performance in HER.Zheng et al.126prepared MoS2/graphene hybrid using a novel solvent-evaporationassisted intercalation method,by which the sizes of MoS2can be controlled.Notable,this composite showed better performance for HER(overpotential of ca 140 mV and Tafel slope of 41 mV· decade-1)compared with other GO support MoS2materials.
For fuel cell application,the sluggish oxygen reduction reaction (ORR)is the rate determine step for electrochemical energy conversion,which has been attracted worldwide research interests. Nitrogen doped carbon materials are proved to be potential substitute for Pt as an efficient catalyst in ORR127.Recently,Qiao′s group128reported the application of g-C3N4@carbon in ORR based on theoretical calculation.The experimental results indicated that g-C3N4@carbon performed better efficiency than Pt/C catalyst and reliable stability.More recently,3D porous architecture was fabricated using g-C3N4nanosheets and reduced graphene oxide in aqueous solution129.As-obtained architecture possessed high surface area,multilevel porous structure,excellent electrical conductivity.It exhibited remarkable catalytic performance and excellent durability in ORR,outperformed other g-C3N4/rGO composites.The excellent performance was attributed to effective electron tunneling through g-C3N4barrier caused by ultrathin g-C3N4nanosheet.This structure leads to rich electrode-electrolytegas three-phase boundaries,and shortens the electron diffusion distance from rGO to O2.In addition,Xiang et al.36,37used 2D covalent organic polymers(COP)as precursor to synthesize nitrogen-doped graphene-like carbons,which exhibited excellent ORR electrocatalytic activity due to the precisely controlled heteroatom locations and pore sizes.
In addition to the reactions mentioned above,2D materials also showed excellent performances in other electronic assisted catalytic processes.For example,Ni-Al LDH cooperated GO hybrid was developed through in-situ co-precipitation followed a reduction procedure130.The coexistence of NiAl-LDH platelets and the graphene nanosheets effectively enhanced the electrocatalytic activity for dopamine(DA)oxidation,which may have potential application of DAdetection.
3.2.2 Photocatalysis
Photocatalysis has attracted much attention because it offers an alternative strategy for both clean energy and environmental protection.In a photocatalytic reaction,photo-generated electronhole pairs are formed on the catalyst surface.As opposed to an energy generation device where the charge carriers are collected by an electrode,in a photocatalytic reaction they are directly scavenged by different species presented in solution.However,the photogenerated electrons and holes in the excited states are very unstable and can easily recombine,dissipating the input energy as heat,resulting in low process efficiency.The sp2-bonded carbonatoms,and the delocalized electrons in graphene can move freely in the network with a low resistance131.The carbon vacancies or dangling bonds in graphene can influence the electronic structure of metal atoms on graphene and improve their stability.Hence,2D carbon-based materials are promising candidates in photocatalytic reactions.Notably,with the development of new synthesis methods,the graphene-like C3N4became a superstar for photocatalysis132,133.In this section,we will summarize some of the recent,the most remarkable processes of photocatalysis.
Water splitting is one of the most attracting reactions in photocatalysis to transfer solar energy into chemical energy134.Xiang et al.135prepared graphene/g-C3N4composites by combined impregnation-chemical reduction strategy.With~1.0%(w)graphene,the composite exhibited an optimum H2-production rate (451 μmol·h-1·g-1),which exceeded that of pure g-C3N4by more than 3.07 times.It showed that graphene sheets act as electronic conductive channels to separate the photogenerated charge carriers and,therefore,to enhance the photocatalytic activity of g-C3N4.To investigate the intrinsic property of g-C3N4,Cheng′s group136synthesized 2 nm g-C3N4nanosheets by thermal oxidation etching, which possessed high surface area(306 m2·g-1),large bandgap(by 0.2 eV),longer electron transport ability along the in-plane direction,and improved lifetime of photoexcited charge carriers. Therefore,the g-C3N4nanosheets exhibited an enhanced activity inhydrogenrevolution.Wang′s group137alsoreportedthe exfoliated g-C3N4nanosheets for visual light catalysis of hydrogen evolution. Recently,Schwinghammer et al.138synthesized a type of novel crystalline 2D carbon nitride nanosheets by ionothermal synthesis of the layered material poly(triazine imide),PTI,followed by onestep liquid exfoliation in water.It is the first structurally welldefined,crystalline 2D carbon nitride with the highest activity toward photocatalytic water-splitting observed up to now.
Besides carbon nitrides,other 2D materials can catalyze the water splitting photoreaction.Chang et al.139prepared MoS2/ graphene supported CdS composite,which showed H2evolution rate of 1.8 mmol·h-1in lactic acid solution at 420 nm.The layered MoS2played a role as cocatalyst for H2generation and the graphene greatly enhanced the movement of electrons.Using electrostatic assembly method,Zn-Cr-LDH were immobilized onto RGO nanosheets to form Zn-Cr-LDH-RGO nanohybrid,which showed an improved photocatalytic activity for visible light-induced O2generation with a rate of 1.20 mmol·h-1·g-1140.The high activity was attributed to the strong electronic coupling between the subnanometer-thick 2D nanostructured LDH and GO.Such structure led to the prominent increase of visible light absorption and a remarkable depression of electron-hole recombination.
Another important application of photocatalysis is the degradation of organic pollutants.The g-C3N4has been a preferred choice for the methyl orange(MO)dye degradation141.This process was mainly attributed to the selective reduction of MO induced by the photogenerated electron.The same research group also reported the degradation of rhodamine B over g-C3N4and TaON composite with high activity originated from the enhanced electron-hole separation142.Quan′s group143fabricated graphene/g-C3N4structure by a facile sonochemical approach.GO was overlaid on the surface of g-C3N4and acted as a separation center and electron acceptor.Wang et al.144fabricated ZnO with g-C3N4to hinder the electron-hole recombination and therefore increase the photocatalytic activity by 3.5 times in methyl blue(MB) degradation.Furthermore,Chen′s group145fabricated 2D porous g-C3N4nanosheets/nitrogen-doped graphene/layered MoS2(CNNS/ NRGO/MoS2)ternary nanojunction,which showed remarkable performance for the degradation of MB and removal of Cr(VI).
Apart from the above mentioned reactions,2D carbon-based materials can be used in many other photocatalysis processes.For example,the self-assembled C3N4and layered double hydroxide LDH exhibited an improved catalytic activity for photoreduction of CO2in aqueous solution as a result of the enrichment of carbonate anions in the interlayer of LDH84.Using GO nanosheet as a structural direct agent,a sandwich GO-C3N4hybrid was synthesized,which exhibited an enhanced photoreduction of CO2to CH4146.Xu et al.147reported that g-C3N4modified TiO2nanosheets (CTS)were prepared and employed as photoanode materials in dye-sensitized solar cells(DSSCs).The introduction of g-C3N4acted as the blocking layer for electron backward recombination with electrolyte,and can increase both the open circuit voltage and short-circuit photocurrent density.
3.3 Applications in electrical energy storage
Due to the consumption of fossil fuels and critical environment problems,there is an urgent requirement for clean energy conversion and storage.Lithium-ion batteries and supercapacitors are two of the most promising energy storage devices.The improvement of the electrode materials is still the main strategy to promote efficiency.2D carbon-based materials have been considered as one of the most promising alternatives as electrode materials in energy-related devices,especially the graphene-based hybrids or composites148.The reason is related to their high surface area,high conductivity,unique graphitized basal plane structure, chemical tolerance and a potentially low manufacturing cost149,150. In this section,we will briefly discuss the most recently processes of the 2D carbon-based materials in the fields of lithium-ion batteries and supercapacitors,based on the materials mentioned in Section 2.
3.3.1 Lithium-ion batteries(LIBs)
Two dimensional nanoarchitectures are of great potential in lithium-ion batteries,due to its shortened paths for fast Li+diffusion and large exposed surface for more lithium-insertion channels107.Carbon nanosheets and its 2D hybrids containing oxides,dichalcogenides,or nitrides have been widely studied as promising electrode materials for lithium-ion batteries.
The 2D mesoporous carbon materials(mesoporous carbon nanosheets,MCNSs)with 40 nm thickness and 10 μm width showed high capacities,superior rate performances,and robust cycle life at high rates as anodes for LIBs72.It is proved that the thicker 2D carbon nanosheets with mesopores can enhance the rate performances and simultaneously maintain its high capacities androbust cycle life,because they can remedy the drawbacks of thin carbon nanosheets anodes in which the diffusion of lithium ions was impeded by overlapped or folded structures.Another example was the porous carbon nanosheet materials with high nitrogen content through the Schiff-base reaction in a molten salt medium employed as anode materials in LIBs,which exhibit a high initial coulombic efficiency(ca 63.1%),a high and constant reversible capacity of 605 mAh·g-1at a current density of 100 mA·g-1even after 100 cycles,and a high-rate capability50.The excellent performance can be attributed to the high porosity,high nitrogen contentofca30%(w)andauniquetwo-dimensional(2D)structure.
Afacile in-situ solution-phase reduced method was reported for growing MoS2layers on a graphene nanosheet,which exhibited extraordinary capacity(1300 mAh·g-1),and excellent rate capability and cycling stability as an anode material for lithium ion batteries103.Zhou et al.85synthesized a MoS2nanosheet-graphene nanosheet hybrid via the combination of a lithiation assisted exfoliation process and a hydrazine monohydrate vapour reduction technique.The as-obtained nanosheet-nanosheet hybrid was more robust than particle-nanosheet hybrid and exhibited much improved cycle life in LIBs.Another graphene-like MoS2/graphene nanocomposite was prepared by hydrolysis of lithiated MoS2(LiMoS2)and displayed a flower-like architecture composed of exfoliated nanosheets87.Due to the synergetic effect of highly conductive graphene nanosheet(GNS)and MoS2,the reversible capacity of this MoS2/GNS nanocomposite is~1400 mAh·g-1in the initial cycle and remains 1351 mAh·g-1after 200 cycles at 100 mA·g-1.Furthermore,the capacity can reach 591 mAh·g-1even at a high current density of 1000 mA·g-1.Zhou et al.developed a one-step solid-phase synthesis strategy using cubic NaCl as template,which also created a 2D-confined space to achieve the construction of few-layer MoS2nanosheets robustly lain on carbon nanosheets151.This novel architecture demonstrated an outstanding long-life cycling capability at high rates.Moreover,a novel MoS2/ N-doped graphene/porous g-C3N4nanosheet multilayered nanostructure was fabricated,which exhibited excellent cycling stability(91%capacity after 100 cycles),high rate capability(83% capacity retention from 50 to 500 mA·g-1),and large capacity (more than 800 mAh·g-1at 100 mA·g-1)152.
Other 2D carbon-based composites have also been developed and showed excellent performance as anode materials in LIBs.For example,Hu et al.79applied the self-assembled Ni-Al LDH and GO hybrid as a cathode material for high-rate alkali battery.The superlattice construction enables each LDH layer being adjacent to the graphene conducting networks.Therefore,the utilization of LDH materials can be efficiently enlarged,particularly in fast charge-transfer reactions.Similarly,fabricated Ni-Mn LDH/graphene was used as an anode for Li-ion batteries,which demonstrated a maximum capacity value of 1030 mAh·g-1during the first discharge81.Using a facile solvothermal/hydrothermal preparation,Fe2O3nanosheets and nanoparticles were grown on graphene91.The sheet-on-sheet composite was found to be better suitable as an anode for Li-ion battery with a high reversible capacity of 662.4 mAh·g-1after 100 cycles at 1000 mA·g-1. Fellinger′s group52fabricated vertically aligned functional graphitic carbon nanosheets on metal carbide.The vertically aligned nanosheets exhibit remarkable lithium ion storage properties because of the large electrode electrolyte interface and strong interaction with the current collector.
3.3.2 Supercapacitors
In recent years,supercapacitors have attracted significant attention,mainly due to their high power density,long life cycle, and bridging function for the gap between traditional dielectric capacitors and batteries/fuel cells153.The capacitance and charge storage of supercapacitors mainly depend on the electrode materials.Generally,the electrode materials can be classified into three types:(1)carbon materials with large surface area;(2) conductive polymers;(3)metal oxides.In this case,many 2D carbon-based materials,including carbon nanosheets and hybrids with metal oxides,are promising candidates in supercapacitors.
Carbon nanosheets having large surface area are the most widely investigated materials for supercapacitors.Wei′s group employed MgO as template to synthesize 3D pillared-porous carbon nanosheets27.This unique structure endowed the high-rate transportation of electrolyte ions and electrons throughout the electrode matrix,resulting in excellent electrochemical performance.Similarly,using SiO2nanosheet as template,2D mesoporous carbon sheet-like framework were prepared with the pore size centered at 2.2 and 15 nm22.As-obtained material exhibited excellent rate capability and cycling stability due to its unique pore structure with short ion diffusion distance.Sevilla et al.55prepared carbon particles made up of interconnected carbon nanosheets with a thickness of〈80 nm by direct carbonization of organic salt, which behaved as high-performance supercapacitor electrodes in organic electrolyte with excellent power handling ability and superb robustness over long-term cycling.Moreover,mesoporous N-containing carbon nanosheets(N-CNSs)were prepared by direct carbonization of polyaniline nanosheets,which exhibiteda large specific capacitance of 239 F·g-1at 0.5 A·g-1,and the capacitance degradation of~4%over continuous 5000 charge-discharge cycles at 6A·g-160.By an ionic liquid-assisted method,2D nitrogen doped microporous carbon sheets were prepared with graphene as an inner layer with a microporous carbon coating on both sides,which show abundant micropores with narrow pores, short diffusion paths,high electrical conductive networks and good wettability35.It performed excellent rate capability,good long-term stability,high specific capacitance and high energy/ power density in supercapacitors.Wang et al.39developed mesoporous-carbon-coated graphite nanosheet(GNS@MC)composite,which showed a highest capacitance of 203 F·g-1at 1A· g-1in 6 mol·L-1KOH electrolyte,a good cyclic stability with 95% capacitance retention,and a high columbic efficiency of 99%after 5000 cycles.Zheng et al.61prepared graphene/activated carbon (AC)nanosheet composite as high-performance electrode material for supercapacitorusing carbonization-activation method.Besides, many sandwiched structures of carbon nanosheets have beendeveloped via different methods to improve the gravimetric and volumetric performances32,33,62,154.In summary,both the porous structure and the electronic conductivity have been modified for these carbon nanosheets in order to obtain excellent performance as electrode material in supercapacitors.
The metal oxides can contribute pseudo-capacitance and thus can be used as efficient electrode materials for supercapacitors. However,its poor electronic conductivity hindered its applications.The combination of metal oxide with graphitic carbon provided a solution for this problem.Sandwich structured nanocomposites was prepared by electrostatic heteroassembly of cationic transition-metal(Co-Al,Co-Ni)LDH nanosheets and anionic GO/rGO nanosheets155.This hybrid performed as an active electrode material for supercapacitor with a high capacity up to ca 650 F·g-1as a result of hybridizing Faradaic pseudocapacitance of redoxable hydroxide nanosheets,which is approximate 6 times of increase than electric double-layer capacitance of rGO nanosheets.The Ni-Al/GO composite exhibited a maximum specific capacitance of 781.5 F·g-1and excellent cycle life.Even after 200 cycle tests,the increase of the capacitance was 22.56% compared with the initial capacitance90.Co-Al hydrotalcite and graphene hybrids are also promising electrode materials,which can be produced through a simple fabrication process77,78.Furthermore,Xie′s group83introduced the fabrication of δ-MnO2integrated on graphene planar hybrid as the working electrodes via a vacuum filtration process.On one hand,the planar structures of δ-MnO2/graphene nanosheets introduced more electrochemically active surfaces for absorption/desorption of electrolyte ions.On the other hand,it brought additional interfaces at the hybridized interlayer to facilitate charge transport during charging/discharging processes.This unique structure enables great performance improvements compared to graphene-only devices for supercapacitors.
3.4 Other applications
As 2D carbon-based materials possess unique electronic and photonic properties,many applications have been extended in the fields of drug delivery,health care,analysis,and so on.Wang et al.156prepared the GO and drug intercalated layered double hydroxide hybrid film using the anionic intercalation of LDH as precursor.The combination of LDH played a role of enhancing antibacterial effect for this material.Lin et al.157studied the potential biomedical applications of g-C3N4.They discovered that g-C3N4nanosheets can be used as efficient photosensitizers and as pH-responsive nanocarriers.As photosensitizers,g-C3N4nanosheets are able to generate reactive oxygen species(ROS)and kill cancer cells efficiently.As nanocarriers,g-C3N4nanosheets possess an ultrahigh drug-loading capacity owing to their high surface-to-volume ratio.These findings demonstrated the potential of g-C3N4for biomedical applications.
For electric fields,using a versatile and scalable process called‘patterned regrowth’,Levendorf et al.158have prepared spatially controlled lateral junctions between electrically conductive graphene and insulating h-BN.It represents an important step towards developing atomically thin integrated circuitry and enable the fabrication of electrically isolated active and passive elements embedded in continuous,one-atom-thick sheets.Another example is the enhanced field emission behavior of WS2-RGO nanocomposite159.The enhancement may be attributed to the surface protrusions of the single-to-few layer thick sheets of the nanocomposite and an overlap of the electronic structures of WS2and RGO.
For chemical analysis area,a highly efficient fluorosensor based on ultrathin g-C3N4nanosheets was developed for detecting Cu2+160. The whole detection process can be completed within 10 min and the detection limit is as lowas 0.5 nmol·L-1.Chen et al.161prepared Au hybridized g-C3N4nanosheets,which shows high specificity, good reproducibility,and long-term stability as immunosensor.Ma et al.162reported a highly sensitive and highly selective heparin sensing platform based on protonated g-C3N4nanosheets,reaching the lowest heparin detection limit of 18 ng·mL-1.
4 Perspective
To summarize up,various kinds of 2D carbon-based materials have been so far synthesized,including carbon nanosheets and many hybrids/composites containing O,N,and S atoms.The tunable porosity,thicknesses,and composition with a wide range of physical and chemical properties makes these 2D materials very promising for further scientific studies and technological applications,especially in the fields of adsorption,catalysis,electrical energy storage,etc.Although the progress has been quite impressive,the exploration is still in its infancy and several key challenges remain to be resolved.
First of all,one opportunity in the research field of 2D carbonbased materials is the construction of novel structures through the development of assembly and doping processes.The structural engineering should aim to enhance the mechanical strength with precisely controlled pore structure and active components distribution.It is influenced by several aspects:the original pore structure of the precursors,the assembly conditions of the monomers with the templates,the atmosphere and temperatures of the pyrolysis processes,etc.Interfacial engineering at the nanoscale of 2D architectures are expected to further facilitate the mass and energy transfer.Using novel 2D precursors,such as metal-organic frameworks or N-containing covalent organic polymers,the location of the heteroatoms is precisely determined. The surface defects and functional groups often play important role in many energy-related processes,which are often difficult to control.Therefore,exploring new doping and functionalization approaches are necessary to manipulate the surface fine structures.
Furthermore,novel properties should be further explored along with the possibility of creating and designing layered artificial 2D materials with“on-demand”structures.For example,as many 2D materials are soft and tenacious,flexible materials will be an attractive goal for wearable devices,such as the material for electrode in supercapacitor163.For energy related applications,such as electric industry,the urgent need is to exploit the availability of novel materials with metallic,semiconducting,and insulatingproperties.For applications in environment protection,e.g., analysis and elimination of pollutants in air or water,it is necessary to adjust the pore structure and surface properties through the functionalization and modification strategies.
Considering the ever-increasing demands for energy storage and environment protection,it is urgent and crucial to develop simple and efficient techniques for scale-up synthesis of 2D materials.However,due to the high surface energy and intersheet van der Waals attractions,2D materials are easy to overlap, restack,and agglomerate.Hence,the remained difficulty is to prepare homogeneous 2D carbon-based materials in large scale with a precisely controllable thickness and microscale lateral pore structure.At this stage,most of the reported 2D structures were prepared based on colloidal process,that is one has to work with dilute solutions.The relatively poor stability of these colloidal entities with gyration radii in the micrometer range makes the large scale preparation complicated.Therefore,the development of facile and efficient routes for 2D carbon-based materials is an urgent task in the future.
(1) Novoselov,K.S.;Falko,V.I.;Colombo,L.;Gellert,P.R.; Schwab,M.G.;Kim,K.Nature 2012,490,192.doi:10.1038/ nature11458
(2)Xu,M.S.;Liang,T.;Shi,M.M.;Chen,H.Z.Chem.Rev. 2013,113,3766.doi:10.1021/cr300263a
(3) James,D.K.;Tour,J.M.Accounts Chem.Res.2013,46,2307. doi:10.1021/ar300127r
(4) Pakdel,A.;Bando,Y.;Golberg,D.Chem.Soc.Rev.2014,43, 934.doi:10.1039/c3cs60260e
(6)Duerloo,K.A.N.;Li,Y.;Reed,E.J.Nat.Commun.2014,5, 4214.doi:10.1038/ncomms5214
(7)Guo,X.;Zhang,F.;Evans,D.G.;Duan,X.Chem.Commun. 2010,46,5197.doi:10.1039/c0cc00313a
(8)Wang,Q.;O′Hare,D.Chem.Rev.2012,112,4124. doi:10.1021/cr200434v
(9) Matsui,T.;Yamaguchi,A.;Takeoka,Y.;Rikukawa,M.;Sanui, K.Chem.Commun.2002,1094.doi:10.1039/b200965j
(10) Varoon,K.;Zhang,X.;Elyassi,B.;Brewer,D.D.;Gettel,M.; Kumar,S.;Lee,J.A.;Maheshwari,S.;Mittal,A.;Sung,C.Y.; Cococcioni,M.;Francis,L.F.;McCormick,A.V.;Mkhoyan, K.A.;Tsapatsis,M.Science 2011,334,72.doi:10.1126/ science.1208891
(11) Roth,W.J.;Nachtigall,P.;Morris,R.E.;Cejka,J.Chem.Rev. 2014,114,4807.doi:10.1021/cr400600f
(12) Zhao,Z.C.;Zhang,W.P.Acta Phys.-Chim.Sin.2016,32, 2475.[赵侦超,张维萍.物理化学学报,2016,32,2475.] doi:10.3866/PKU.WHXB201607121
(13) Zhao,Y.;Xie,Y.;Liu,Z.;Wang,X.;Chai,Y.;Yan,F.Small 2014,10,4521.doi:10.1002/smll.201401549
(14) Hao,G.P.;Jin,Z.Y.;Sun,Q.;Zhang,X.Q.;Zhang,J.T.;Lu, A.H.Energy Environ.Sci.2013,6,3740.doi:10.1039/ c3ee41906a
(15) Zaera,F.ChemSusChem 2013,6,1797.doi:10.1002/ cssc.201300398
(16) Navalon,S.;Dhakshinamoorthy,A.;Alvaro,M.;Garcia,H. Chem.Rev.2014,114,6179.doi:10.1021/cr4007347
(17) Sun,Y.;Gao,S.;Lei,F.;Xie,Y.Chem.Soc.Rev.2015,44, 623.doi:10.1039/c4cs00236a
(18)Teramura,K.;Iguchi,S.;Mizuno,Y.;Shishido,T.;Tanaka,T. Angew.Chem.Int.Ed.2012,51,8008.doi:10.1002/ anie.201201847
(19) Chen,Y.C.;Hsu,C.Y.;Lin,R.Y.Y.;Ho,K.C.;Lin,J.T. ChemSusChem 2013,6,20.doi:10.1002/cssc.201200609
(20)Tang,H.;Hessel,C.M.;Wang,J.;Yang,N.;Yu,R.;Zhao,H.; Wang,D.Chem.Soc.Rev.2014,43,4281.doi:10.1039/ c3cs60437c
(21) Zhang,L.;Wang,J.;Zhu,J.;Zhang,X.;San Hui,K.;Hui,K. N.J.Mater.Chem.A 2013,1,9046.doi:10.1039/c3ta11755c
(22)Wang,Q.;Yan,J.;Wei,T.;Feng,J.;Ren,Y.;Fan,Z.;Zhang, M.;Jing,X.Carbon 2013,60,481.doi:10.1016/j. carbon.2013.04.067
(23) Stephenson,T.;Li,Z.;Olsen,B.;Mitlin,D.Energy Environ. Sci.2014,7,209.doi:10.1039/c3ee42591f
(24)Sun,Q.;Guo,C.Z.;Wang,G.H.;Li,W.C.;Bongard,H.J.; Lu,A.H.Chemistry 2013,19,6217.doi:10.1002/ chem.201300307
(25) Guo,S.;Dong,S.Chem.Soc.Rev.2011,40,2644. doi:10.1039/c0cs00079e
(26) Liu,D.;Hu,Y.Y.;Zeng,C.;Qu,D.Y.Acta Phys.-Chim.Sin. 2016,32,2826.[刘 丹,胡艳艳,曾 超,屈德宇.物理化学学报,2016,32,2826.]doi:10.3866/PKU.WHXB201609141
(27) Fan,Z.;Liu,Y.;Yan,J.;Ning,G.;Wang,Q.;Wei,T.;Zhi,L.; Wei,F.Adv.Energy Mater.2012,2,419.doi:10.1002/ aenm.201100654
(28)Yan,Y.;Cheng,Q.;Pavlinek,V.;Saha,P.;Li,C.J.Solid State Electrochem.2013,17,1677.doi:10.1007/s10008-013-2025-3
(29)Wang,H.Q.;Zhao,Z.B.;Chen,M.;Xiao,N.;Li,B.B.;Qiu, J.S.New Carbon Mater.2014,29,280.doi:10.1016/S1872-5805(14)60137-2
(30) Lee,J.;Kim,J.;Hyeon,T.Adv.Mater.2006,18,2073. doi:10.1002/adma.200501576
(31) Konkena,B.;Vasudevan,S.J.Phys.Chem.Lett.2012,3,867. doi:10.1021/jz300236w
(32)Hao,G.P.;Lu,A.H.;Dong,W.;Jin,Z.Y.;Zhang,X.Q.; Zhang,J.T.;Li,W.C.Adv.Energy Mater.2013,3,1421. doi:10.1002/aenm.201300383
(33)Wang,Q.;Yan,J.;Fan,Z.Electrochim.Acta 2014,146,548. doi:10.1016/j.electacta.2014.09.036
(34)Guo,D.C.;Mi,J.;Hao,G.P.;Dong,W.;Xiong,G.;Li,W.C.; Lu,A.H.Energy Environ.Sci.2013,6,652.doi:10.1039/c2ee23127a
(35) Jin,Z.Y.;Lu,A.H.;Xu,Y.Y.;Zhang,J.T.;Li,W.C.Adv. Mater.2014,26,3700.doi:10.1002/adma.201306273
(36) Xiang,Z.;Cao,D.;Huang,L.;Shui,J.;Wang,M.;Dai,L.Adv. Mater.2014,26,3315.doi:10.1002/adma.201306328
(37) Xiang,Z.;Xue,Y.;Cao,D.;Huang,L.;Chen,J.F.;Dai,L. Angew.Chem.Int.Ed.2014,53,2433.doi:10.1002/ anie.201308896
(38) Zhang,S.;Zeng,M.;Li,J.;Li,J.;Xu,J.;Wang,X.J.Mater. Chem.A 2014,2,4391.doi:10.1039/c3ta14604a
(39)Wang,L.;Mu,G.;Tian,C.;Sun,L.;Zhou,W.;Tan,T.;Fu,H. ChemSusChem 2012,5,2442.doi:10.1002/cssc.201200529
(40)Wen,Z.;Wang,X.;Mao,S.;Bo,Z.;Kim,H.;Cui,S.;Lu,G.; Feng,X.;Chen,J.Adv.Mater.2012,24,5610.doi:10.1002/ adma.201201920
(41) Krishnan,D.;Raidongia,K.;Shao,J.;Huang,J.ACS Nano 2014,8,449.doi:10.1021/nn404805p
(42)Zheng,C.;Zhou,X.;Cao,H.;Wang,G.;Liu,Z.J.Power Sources 2014,258,290.doi:10.1016/j.jpowsour.2014.01.056
(43) Xu,C.;Ning,G.;Zhu,X.;Wang,G.;Liu,X.;Gao,J.;Zhang, Q.;Qian,W.;Wei,F.Carbon 2013,62,213.doi:10.1016/j. carbon.2013.05.059
(44) Liu,Y.;Cai,Q.;Li,H.;Zhang,J.J.Appl.Polymer Sci.2013, 128,517.doi:10.1002/app.38208
(45) Fan,X.;Yu,C.;Yang,J.;Ling,Z.;Hu,C.;Zhang,M.;Qiu,J. Adv.Energy Mater.2015,5,1401761.doi:10.1002/ aenm.201570035
(46)Huang,Z.H.;Wang,A.;Kang,F.;Chuan,X.Mater.Lett. 2010,64,2444.doi:10.1016/j.matlet.2010.07.078
(47) Deng,X.;Zhao,B.T.;Zhu,L.;Shao,Z.P.Carbon 2015,93, 48.doi:10.1016/j.carbon.2015.05.031
(48) Liu,X.;Fechler,N.;Antonietti,M.Chem.Soc.Rev.2013,42, 8237.doi:10.1039/c3cs60159e
(49) Liu,X.;Antonietti,M.Carbon 2014,69,460.doi:10.1016/j. carbon.2013.12.049
(50) He,B.;Li,W.C.;Lu,A.H.J.Mater.Chem.A 2015,3,579. doi:10.1039/c4ta05056h
(51) Liu,X.;Giordano,C.;Antonietti,M.Small 2014,10,193. doi:10.1002/smll.201300812
(52) Zhu,J.;Sakaushi,K.;Clavel,G.;Shalom,M.;Antonietti,M.; Fellinger,T.P.J.Am.Chem.Soc.2015,137,5480. doi:10.1021/jacs.5b01072
(53) Zhang,P.;Qiao,Z.A.;Zhang,Z.;Wan,S.;Dai,S.J.Mater. Chem.A 2014,2,12262.doi:10.1039/c4ta02307b
(54) Chang,Y.;Antonietti,M.;Fellinger,T.P.Angew.Chem.Int. Ed.2015,54,5507.doi:10.1002/anie.201411685
(55) Sevilla,M.;Fuertes,A.B.ACS Nano 2014,8,5069. doi:10.1021/nn501124h
(56) Fuertes,A.B.;Sevilla,M.ACS Appl.Mater.Interfaces 2015, 7,4344.doi:10.1021/am508794f
(57) Bourlinos,A.;Steriotis,T.;Zboril,R.;Georgakilas,V.;Stubos, A.J.Mater.Sci.2009,44,1407.doi:10.1007/s10853-009-3263-8
(58)Peng,H.;Ma,G.;Sun,K.;Mu,J.;Lei,Z.J.Mater.Chem.A 2014,2,17297.doi:10.1039/c4ta03929g
(59) Peng,H.;Ma,G.;Sun,K.;Zhang,Z.;Yang,Q.;Ran,F.;Lei, Z.J.Mater.Chem.A 2015,3,13210.doi:10.1039/c5ta03034j
(60) Hou,L.;Lian,L.;Li,D.;Pang,G.;Li,J.;Zhang,X.;Xiong, S.;Yuan,C.Carbon 2013,64,141.doi:10.1016/j. carbon.2013.07.045
(61) Zheng,X.;Lv,W.;Tao,Y.;Shao,J.;Zhang,C.;Liu,D.;Luo, J.;Wang,D.W.;Yang,Q.H.Chem.Mater.2014,26,6896. doi:10.1021/cm503845q
(62)Yan,J.;Wang,Q.;Lin,C.;Wei,T.;Fan,Z.Adv.Energy Mater. 2014,4,1400500.doi:10.1002/aenm.201400500
(63)Yun,Y.S.;Park,M.H.;Hong,S.J.;Lee,M.E.;Park,Y.W.; Jin,H.J.ACS Appl.Mater.Interfaces 2015,7,3684. doi:10.1021/am5081919
(64)DeArco,L.G.;Yi,Z.;Kumar,A.;Chongwu,Z.IEEE Transactions on Nanotechnology 2009,8,135. doi:10.1109/tnano.2009.2013620
(65) Reina,A.;Jia,X.;Ho,J.;Nezich,D.;Son,H.;Bulovic,V.; Dresselhaus,M.S.;Kong,J.Nano Lett.2009,9,30. doi:10.1021/nl801827v
(66) Zhao,M.Q.;Liu,X.F.;Zhang,Q.;Tian,G.L.;Huang,J.Q.; Zhu,W.C.;Wei,F.ACS Nano 2012,6,10759.doi:10.1021/ nn304037d
(67)Wang,J.;Zhu,M.;Outlaw,R.A.;Zhao,X.;Manos,D.M.; Holloway,B.C.Carbon 2004,42,2867.doi:10.1016/j. carbon.2004.06.035
(68) Nishihara,H.;Simura,T.;Kobayashi,S.;Nomura,K.; Berenguer,R.;Ito,M.;Uchimura,M.;Iden,H.;Arihara,K.; Ohma,A.;Hayasaka,Y.;Kyotani,T.Adv.Funct.Mater.2016, 26,6418.doi:10.1002/adfm.201602459
(69) Xu,B.;Zheng,D.F.;Jia,M.Q.;Liu,H.;Cao,G.P.;Qiao,N.; Wei,Y.P.;Yang,Y.S.Mater.Lett.2015,143,159. doi:10.1016/j.matlet.2014.12.102
(70)Tao,Y.;Endo,M.;Inagaki,M.;Kaneko,K.J.Mater.Chem. 2011,21,313.doi:10.1039/c0jm01830a
(71)Wang,H.;Xu,Z.;Kohandehghan,A.;Li,Z.;Cui,K.;Tan,X.; Stephenson,T.J.;King′ondu,C.K.;Holt,C.M.B.;Olsen,B. C.;Tak,J.K.;Harfield,D.;Anyia,A.O.;Mitlin,D.ACS Nano 2013,7,5131.doi:10.1021/nn400731g
(72) Li,J.;Yao,R.;Bai,J.;Cao,C.ChemPlusChem 2013,78,797. doi:10.1002/cplu.201300158
(73) Choucair,M.;Thordarson,P.;Stride,J.A.Nat.Nanotech. 2009,4,30.doi:10.1038/2008.365
(74)Wang,Y.;Angelatos,A.S.;Caruso,F.Chem.Mater.2008,20, 848.doi:10.1021/cm7024813
(75) Chen,P.;Xu,K.;Li,X.;Guo,Y.;Zhou,D.;Zhao,J.;Wu,X.; Wu,C.;Xie,Y.Chem.Sci.2014,5,2251.doi:10.1039/ c3sc53303d
(76) Chen,D.;Wang,X.;Liu,T.;Wang,X.;Li,J.ACS Appl.Mater. Interfaces 2010,2,2005.doi:10.1021/am100307v
(77)Dong,X.Y.;Wang,L.;Wang,D.;Li,C.;Jin,J.Langmuir 2012,28,293.doi:10.1021/la2038685
(78) Wang,L.;Wang,D.;Dong,X.Y.;Zhang,Z.J.;Pei,X.F.; Chen,X.J.;Chen,B.;Jin,J.Chem.Commun.2011,47,3556. doi:10.1039/c0cc05420h
(79) Hu,J.;Lei,G.;Lu,Z.;Liu,K.;Sang,S.;Liu,H.Chem. Commun.2015,51,9983.doi:10.1039/c5cc01767j
(80) Long,X.;Li,J.;Xiao,S.;Yan,K.;Wang,Z.;Chen,H.;Yang, S.Angew.Chem.Int.Ed.2014,53,7584.doi:10.1002/ anie.201402822
(81) Latorre-Sanchez,M.;Atienzar,P.;Abellan,G.;Puche,M.; Fornes,V.;Ribera,A.;Garcia,H.Carbon 2012,50,518. doi:10.1016/j.carbon.2011.09.007
(82) Fang,Q.;Chen,B.J.Mater.Chem.A 2014,2,8941. doi:10.1039/c4ta00321g
(83) Peng,L.;Peng,X.;Liu,B.;Wu,C.;Xie,Y.;Yu,G.Nano Lett. 2013,13,2151.doi:10.1021/nl400600x
(84)Hong,J.;Zhang,W.;Wang,Y.;Zhou,T.;Xu,R. ChemCatChem 2014,6,2315.doi:10.1002/cctc.201402195
(85)Zhou,X.;Wan,L.J.;Guo,Y.G.Chem.Commun.2013,49, 1838.doi:10.1039/c3cc38780a
(86) Zeng,Z.;Yin,Z.;Huang,X.;Li,H.;He,Q.;Lu,G.;Boey,F.; Zhang,H.Angew.Chem.Int.Ed.2011,50,11093. doi:10.1002/anie.201106004
(87) Liu,Y.C.;Zhao,Y.P.;Jiao,L.F.;Chen,J.J.Mater.Chem.A 2014,2,13109.doi:10.1039/c4ta01644k
(88) Peng,J.;Weng,J.Carbon 2015,94,568.doi:10.1016/j. carbon.2015.07.035
(89) Garcia-Gallastegui,A.;Iruretagoyena,D.;Gouvea,V.; Mokhtar,M.;Asiri,A.M.;Basahel,S.N.;Al-Thabaiti,S.A.; Alyoubi,A.O.;Chadwick,D.;Shaffer,M.S.P.Chem.Mater. 2012,24,4531.doi:10.1021/cm3018264
(90)Gao,Z.;Wang,J.;Li,Z.;Yang,W.;Wang,B.;Hou,M.;He,Y.; Liu,Q.;Mann,T.;Yang,P.;Zhang,M.;Liu,L.Chem.Mater. 2011,23,3509.doi:10.1021/cm200975x
(91) Kan,J.;Wang,Y.Sci.Rep.2013,3,3502.doi:10.1038/ srep03502
(92) Feng,J.;Sun,X.;Wu,C.;Peng,L.;Lin,C.;Hu,S.;Yang,J.; Xie,Y.J.Am.Chem.Soc.2011,133,17832.doi:10.1021/ ja207176c
(93) Feng,J.;Peng,L.;Wu,C.;Sun,X.;Hu,S.;Lin,C.;Dai,J.; Yang,J.;Xie,Y.Adv.Mater.2012,24,1969.doi:10.1002/ adma.201104681
(94) Cheng,L.;Huang,W.;Gong,Q.;Liu,C.;Liu,Z.;Li,Y.;Dai, H.Angew.Chem.Int.Ed.2014,53,7860.doi:10.1002/ anie.201402315
(95) Ganatra,R.;Zhang,Q.ACS Nano 2014,8,4074.doi:10.1021/ nn405938z
(96) Chen,D.;Chen,W.;Ma,L.;Ji,G.;Chang,K.;Lee,J.Y.Mater. Today 2014,17,184.doi:10.1016/j.mattod.2014.04.001
(97)Wang,H.;Feng,H.B.;Li,J.H.Small 2014,10,2165. doi:10.1002/smll.201303711
(98)Tan,C.L.;Zhang,H.Chem.Soc.Rev.2015,44,2713. doi:10.1039/c4cs00182f
(99) Brivio,J.;Alexander,D.T.L.;Kis,A.Nano Lett.2011,11, 5148.doi:10.1021/nl2022288
(100)Zhou,K.G.;Mao,N.N.;Wang,H.X.;Peng,Y.;Zhang,H.L. Angew.Chem.,Int.Ed.2011,50,10839.doi:10.1002/ anie.201105364
(101)Lee,Y.H.;Zhang,X.Q.;Zhang,W.;Chang,M.T.;Lin,C.T.; Chang,K.D.;Yu,Y.C.;Wang,J.T.W.;Chang,C.S.;Li,L.J.; Lin,T.W.Adv.Mater.2012,24,2320.doi:10.1002/ adma.201104798
(102)Altavilla,C.;Sarno,M.;Ciambelli,P.Chem.Mater.2011,23, 3879.doi:10.1021/cm200837g
(103)Chang,K.;Chen,W.Chem.Commun.2011,47,4252. doi:10.1039/c1cc10631g
(104)Li,Y.;Wang,H.;Xie,L.;Liang,Y.;Hong,G.;Dai,H.J.Am. Chem.Soc.2011,133,7296.doi:10.1021/ja201269b
(105)Kumar,N.A.;Dar,M.A.;Gul,R.;Baek,J.B.Mater.Today 2015,18,286.doi:10.1016/j.mattod.2015.01.016
(106) Firmiano,E.G.S.;Cordeiro,M.A.L.;Rabelo,A.C.; Dalmaschio,C.J.;Pinheiro,A.N.;Pereira,E.C.;Leite,E.R. Chem.Commun.2012,48,7687.doi:10.1039/c2cc33397j
(107) Liu,J.H.;Liu,X.W.Adv.Mater.2012,24,4097.doi:10.1002/ adma.201104993
(108) Jin,Z.Y.;Xu,Y.Y.;Sun,Q.;Lu,A.H.Small 2015,11,5151. doi:10.1002/smll.201501692
(109)Hao,G.P.;Li,W.C.;Qian,D.;Lu,A.H.Adv.Mater.2010,22, 853.doi:10.1002/adma.200903765
(110)Shen,W.Z.;Hu,T.P.;Fan,W.B.RSC Adv.2014,4,9126. doi:10.1039/c3ra47946c
(111) Zhang,M.;Liu,L.;He,T.;Wu,G.T.;Chen,P.Chem.-Asian J. 2016,11,1849.doi:10.1002/asia.201600396
(112) Iruretagoyena,D.;Shaffer,M.S.P.;Chadwick,D.Adsorption 2014,20,321.doi:10.1007/s10450-013-9595-3
(113)Bartolomei,M.;Carmona-Novillo,E.;Giorgi,G.Carbon 2015,95,1076.doi:10.1016/j.carbon.2015.08.118
(114)Zhong,Z.;Yao,J.;Low,Z.X.;Chen,R.;He,M.;Wang,H. Carbon 2014,72,242.doi:10.1016/j.carbon.2014.01.072
(115)Chen,Y.Q.;Chen,L.B.;Bai,H.;Li,L.J.Mater.Chem.A 2013,1,1992.doi:10.1039/c2ta00406b
(116)Nguyen,D.D.;Tai,N.H.;Lee,S.B.;Kuo,W.S.Energy Environ.Sci.2012,5,7908.doi:10.1039/c2ee21848h
(117)Liu,Y.;Ma,J.K.;Wu,T.;Wang,X.R.;Huang,G.B.;Qiu,H. X.;Li,Y.;Wang,W.;Gao,J.P.ACS Appl.Mater.Interfaces 2013,5,10018.doi:10.1021/am4024252
(118) Niu,Z.Q.;Chen,J.;Hng,H.H.;Ma,J.;Chen,X.D.Adv. Mater.2012,24,4144.doi:10.1002/adma.201200197
(119)Cong,H.P.;Ren,X.C.;Wang,P.;Yu,S.H.ACS Nano 2012,6,2693.doi:10.1021/nn300082k
(120) Machado,B.F.;Serp,P.Catal.Sci.Technol.2012,2,54. doi:10.1039/c1cy00361e
(121) Cheng,Y.;Fan,Y.;Pei,Y.;Qiao,M.Catal.Sci.Technol.2015, 5,3903.doi:10.1039/c5cy00630a
(122) Fan,X.B.;Zhang,G.L.;Zhang,F.B.Chem.Soc.Rev.2015, 44,3023.doi:10.1039/c5cs00094g
(123)Yu,X.Y.;Feng,Y.;Guan,B.Y.;Lou,X.W.;Paik,U.Energy Environ.Sci.2016,9,1246.doi:10.1039/c6ee00100a
(124) Jaramillo,T.F.;Jorgensen,K.P.;Bonde,J.;Nielsen,J.H.; Horch,S.;Chorkendorff,I.Science 2007,317,100. doi:10.1126/science.1141483
(125)Lu,Q.P.;Yu,Y.F.;Ma,Q.L.;Chen,B.;Zhang,H.Adv.Mater. 2016,28,1917.doi:10.1002/adma.201503270
(126)Zheng,X.L.;Xu,J.B.;Yan,K.Y.;Wang,H.;Wang,Z.L.; Yang,S.H.Chem.Mater.2014,26,2344.doi:10.1021/ cm500347r
(127) Gong,K.P.;Du,F.;Xia,Z.H.;Durstock,M.;Dai,L.M. Science 2009,323,760.doi:10.1126/science.1168049
(128) Zheng,Y.;Jiao,Y.;Chen,J.;Liu,J.;Liang,J.;Du,A.;Zhang, W.;Zhu,Z.;Smith,S.C.;Jaroniec,M.;Lu,G.Q.;Qiao,S.Z. J.Am.Chem.Soc.2011,133,20116.doi:10.1021/ja209206c
(129) Tian,J.;Ning,R.;Liu,Q.;Asiri,A.M.;Al-Youbi,A.O.;Sun, X.ACS Appl.Mater.Interfaces 2014,6,1011.doi:10.1021/ am404536w
(130) Li,M.X.;Zhu,J.E.;Zhang,L.L.;Chen,X.;Zhang,H.M.; Zhang,F.Z.;Xu,S.L.;Evans,D.G.Nanoscale 2011,3,4240. doi:10.1039/c1nr10592b
(131) Novoselov,K.S.;Geim,A.K.;Morozov,S.V.;Jiang,D.; Zhang,Y.;Dubonos,S.V.;Grigorieva,I.V.;Firsov,A.A. Science 2004,306,666.doi:10.1126/science.1102896
(132) Wang,X.;Blechert,S.;Antonietti,M.ACS Catal.2012,2, 1596.doi:10.1021/cs300240x
(133) Zheng,Y.;Liu,J.;Liang,J.;Jaroniec,M.;Qiao,S.Z.Energy Environ.Sci.2012,5,6717.doi:10.1039/c2ee03479d
(134)Wang,Y.;Wang,X.;Antonietti,M.Angew.Chem.Int.Ed. 2012,51,68.doi:10.1002/anie.201101182
(135) Xiang,Q.;Yu,J.;Jaroniec,M.J.Phys.Chem.C 2011,115, 7355.doi:10.1021/jp200953k
(136) Niu,P.;Zhang,L.;Liu,G.;Cheng,H.M.Adv.Funct.Mater. 2012,22,4763.doi:10.1002/adfm.201200922
(137) Yang,S.;Gong,Y.;Zhang,J.;Zhan,L.;Ma,L.;Fang,Z.; Vajtai,R.;Wang,X.;Ajayan,P.M.Adv.Mater.2013,25,2452. doi:10.1002/adma.201204453
(138)Schwinghammer,K.;Mesch,M.B.;Duppel,V.;Ziegler,C.; Senker,J.;Lotsch,B.V.J.Am.Chem.Soc.2014,136,1730. doi:10.1021/ja411321s
(139)Chang,K.;Mei,Z.W.;Wang,T.;Kang,Q.;Ouyang,S.X.;Ye, J.H.ACS Nano 2014,8,7078.doi:10.1021/nn5019945
(140) Gunjakar,J.L.;Kim,I.Y.;Lee,J.M.;Lee,N.S.;Hwang,S.J. Energy Environ.Sci.2013,6,1008.doi:10.1039/c3ee23989f
(141) Yan,S.C.;Li,Z.S.;Zou,Z.G.Langmuir 2009,25,10397. doi:10.1021/la900923z
(142) Yan,S.C.;Lv,S.B.;Li,Z.S.;Zou,Z.G.Dalton Trans.2010, 39,1488.doi:10.1039/b914110c
(143) Liao,G.;Chen,S.;Quan,X.;Yu,H.;Zhao,H.J.Mater.Chem. 2012,22,2721.doi:10.1039/c1jm13490f
(144) Wang,Y.;Shi,R.;Lin,J.;Zhu,Y.Energy Environ.Sci.2011,4, 2922.doi:10.1039/c0ee00825g
(145) Hou,Y.;Wen,Z.;Cui,S.;Guo,X.;Chen,J.Adv.Mater.2013, 25,6291.doi:10.1002/adma.201303116
(146) Ong,W.J.;Tan,L.L.;Chai,S.P.;Yong,S.T.Chem. Commun.2015,51,858.doi:10.1039/c4cc08996k
(147) Xu,J.;Wang,G.;Fan,J.;Liu,B.;Cao,S.;Yu,J.J.Power Sources 2015,274,77.doi:10.1016/j.jpowsour.2014.10.033
(148) Miller,J.R.;Outlaw,R.A.;Holloway,B.C.Science 2010, 329,1637.doi:10.1126/science.1194372
(149) Han,S.;Wu,D.;Li,S.;Zhang,F.;Feng,X.Small 2013,9, 1173.doi:10.1002/smll.201203155
(150) Raccichini,R.;Varzi,A.;Passerini,S.;Scrosati,B.Nat.Mater. 2015,14,271.doi:10.1038/nmat4170
(151) Zhou,J.W.;Qin,J.;Zhang,X.;Shi,C.S.;Liu,E.Z.;Li,J.J.; Zhao,N.Q.;He,C.N.ACS Nano 2015,9,3837.doi:10.1021/ nn506850e
(152) Hou,Y.;Li,J.Y.;Wen,Z.H.;Cui,S.M.;Yuan,C.;Chen,J.H. Nano Energy 2014,8,157.doi:10.1016/j.nanoen.2014.06.003
(153) Wang,G.;Zhang,L.;Zhang,J.Chem.Soc.Rev.2012,41,797. doi:10.1039/c1cs15060
(154) Luo,H.;Liu,Z.;Chao,L.;Wu,X.;Lei,X.;Chang,Z.;Sun,X. J.Mater.Chem.A 2015,3,3667.doi:10.1039/c4ta05843g
(155) Ma,R.Z.;Liu,X.H.;Liang,J.B.;Bando,Y.;Sasaki,T.Adv. Mater.2014,26,4173.doi:10.1002/adma.201400054
(156)Wang,Y.;Zhang,D.;Bao,Q.;Wu,J.;Wan,Y.J.Mater.Chem. 2012,22,23106.doi:10.1039/c2jm35144g
(157) Lin,L.S.;Cong,Z.X.;Li,J.;Ke,K.M.;Guo,S.S.;Yang,H. H.;Chen,G.N.J.Mater.Chem.B 2014,2,1031.doi:10.1039/ c3tb21479f
(158) Levendorf,M.P.;Kim,C.J.;Brown,L.;Huang,P.Y.; Havener,R.W.;Muller,D.A.;Park,J.Nature 2012,488,627. doi:10.1038/nature11408
(159) Rout,C.S.;Joshi,P.D.;Kashid,R.V.;Joag,D.S.;More,M. A.;Simbeck,A.J.;Washington,M.;Nayak,S.K.;Late,D.J. Sci.Rep.2013,3,3282.doi:10.1038/srep03282
(160) Tian,J.;Liu,Q.;Asiri,A.M.;Al-Youbi,A.O.;Sun,X.Analyt. Chem.2013,85,5595.doi:10.1021/ac400924j
(161) Chen,L.;Zeng,X.;Si,P.;Chen,Y.;Chi,Y.;Kim,D.H.;Chen, G.Analyt.Chem.2014,86,4188.doi:10.1021/ac403635f
(162) Ma,T.Y.;Tang,Y.;Dai,S.;Qiao,S.Z.Small 2014,10,2382. doi:10.1002/smll.201303827
(163) Xiao,F.;Yang,S.;Zhang,Z.;Liu,H.;Xiao,J.;Wan,L.;Luo, J.;Wang,S.;Liu,Y.Sci.Rep.2015,5,9359.doi:10.1038/ srep09359
Two-Dimensional Carbon-Based Porous Materials: Synthesis and Applications
HE Lei ZHANG Xiang-Qian LUAn-Hui*
(State Key Laboratory of Fine Chemicals,School of Chemical Engineering,Dalian University of Technology, Dalian 116024,Liaoning Province,P.R.China)
Two-dimensional(2D)materials possess nanoscale thickness with large aspect ratios on the other two dimensions.The ultrahigh surface-to-volume ratio of 2D materials is the most important property different from their bulk counterparts,and is beneficial for mass and heat transport,and ion diffusion.Among the various 2D materials,carbon-based materials have attracted tremendous attentions since the first explosive research on graphene.Therefore,they provide opportunities for applications in adsorption,catalysis,and electrical energy storage.The porous structure of such carbon materials is a key influence on the properties of these 2D materials. This review focuses on recent developments in synthesis strategies for 2D carbon-based materials,especially the preparation of carbon nanosheets and carbon-inorganic hybrids/composites nanosheets.The main factors influencing the porous structure of the material are discussed for each method.Applications of the materials are introduced,mainly in the fields of adsorption,heterogeneous catalysis,and electrical energy storage.Finally, the leading-edge issues of novel 2D carbon-based materials for the future are discussed.
Two-dimensional material;Carbon;Porous structure;Synthesis strategy;Adsorption; Catalysis;Electrical energy storage
O643
Ma,R.;Sasaki,T.Adv.Mater.2010,22,5082.
10.1002/ adma.201001722
doi:10.3866/PKU.WHXB201612201
Received:October 21,2016;Revised:December 20,2016;Published online:December 20,2016.
*Corresponding author.Email:anhuilu@dlut.edu.cn;Tel:+86-411-84986112.
The project was supported by the National Natural Science Foundation of China(21225312,21403027,21506022).国家自然科学基金(21225312,21403027,21506022)资助项目

