Mitomycin C induces apoptosis in human epidural scar fi broblasts after surgical decompression for spinal cord injury
Tao sui, Da-wei Ge, Lei Yang Jian Tang Xiao-jian Cao, Ying-bin Ge
1 Department of Orthopedics, the First Af filiated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
2 Department of Physiology, Nanjing Medical University, Nanjing, Jiangsu Province, China
Mitomycin C induces apoptosis in human epidural scar fi broblasts after surgical decompression for spinal cord injury
Tao sui1,#, Da-wei Ge1,#, Lei Yang1, Jian Tang1, Xiao-jian Cao1,*, Ying-bin Ge2,*
1 Department of Orthopedics, the First Af filiated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
2 Department of Physiology, Nanjing Medical University, Nanjing, Jiangsu Province, China
How to cite this article:Sui T, Ge DW, Yang L, Tang J, Cao XJ, Ge YB (2017) Mitomycin C induces apoptosis in human epidural scar fbroblasts after surgical decompression for spinal cord injury. Neural Regen Res 12(4):644-653.
Open access statement:Tis is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:Tis research was supported by the National Natural Science Foundation of China, No. 81401791, 81371968, 81672152.
Graphical Abstract

Whether endoplasmic reticulum stress pathway participates in mitomycin C-induced epidural scar fi broblast apoptosis?
Numerous studies have shown that topical application of mitomycin C aer surgical decompression ef f ectively reduces scar adhesion. However, the underlying mechanisms remain unclear. In this study, we investigated the ef f ect of mitomycin C on the proliferation and apoptosis of human epidural scar fi broblasts. Human epidural scar fi broblasts were treated with various concentrations of mitomycin C (1, 5, 10, 20, 40 μg/mL) for 12, 24 and 48 hours. Mitomycin C suppressed the growth of these cells in a dose- and time-dependent manner. Mitomycin C upregulated the expression levels of Fas, DR4, DR5, cleaved caspase-8/9, Bax, Bim and cleaved caspase-3 proteins, and it downregulated Bcl-2 and Bcl-xL expression. In addition, inhibitors of caspase-8 and caspase-9 (Z-IETD-FMK and Z-LEHD-FMK, respectively) did not fully inhibit mitomycin C-induced apoptosis. Furthermore, mitomycin C induced endoplasmic reticulum stress by increasing the expression of glucose-regulated protein 78, CAAT/enhancer-binding protein homologous protein (CHOP) and caspase-4 in a dose-dependent manner. Salubrinal signif i cantly inhibited the mitomycin C-induced cell viability loss and apoptosis, and these ef f ects were accompanied by a reduction in CHOP expression. Our results support the hypothesis that mitomycin C induces human epidural scar fi broblast apoptosis, at least in part,viathe endoplasmic reticulum stress pathway.
nerve regeneration; spinal cord injury; mitomycin C; fbroblasts; apoptosis; endoplasmic reticulum stress; surgical decompression; epidural scar; fbrosis; CAAT/enhancer-binding protein homologous protein; glucose-regulated protein 78; neural regeneration
Introduction
Early surgical decompression has proven to be an effective treatment aer spinal cord injury (Xie et al., 2015; Bakar et al., 2016). However, postoperative epidural scar adhesion and adhesive arachnoiditis are widely accepted causes of failed back surgery syndrome, which significantly contributes to poor clinical outcomes, such as nerve radicular pain and/or low back pain (Songer et al., 1995; Robertson 1996).erefore, it is important to prevent postoperative adhesion in spinal surgery.
Mitomycin C (MMC), a widely used chemotherapeutic drug, strongly inhibits fi broblast proliferation and prevents scar formation (Kumar et al., 2015; Na et al., 2015; Sun et al., 2015; Sui et al., 2016). Our previous experiments showed that topical application of MMC prevents epidural scar adhesion in adult rats aer lumbar laminectomy, and that it was safe at low concentrations (Sun et al., 2007; Su et al., 2010).
Recently, MMC was reported to have an anti-proliferative effect by triggering the apoptotic signaling pathway in fi broblasts (Liu et al., 2010). It has been reported that intrinsic and extrinsic apoptotic pathways are both involved in MMC-induced inhibition of fi broblast proliferation (Park et al., 2000; Pirnia et al., 2002). The tumor necrosis family of proteins, including the death receptors DR4, DR5 and Fas (CD95/APO-1), which are located on the plasma membrane, have been reported to be involved in the MMC-induced apoptosis of human Tenon’s fibroblasts and colon cancer cells (Hueber et al., 2002; Cheng et al., 2012).e activation of caspase-8 and caspase-9, and changes in the Bcl-2 family caused by MMC contribute to the apoptosis of human Tenon’s capsule fibroblasts (Seong et al., 2005). However, the mechanism of MMC-induced apoptosis in human epidural scar fi broblasts (HESFs) differs from that in these cells, and further studies are needed.
Materials and Methods
Materials
Primary HESFs were obtained from epidural scars after laminectomy in patients from the First Af filiated Hospital of Nanjing Medical University of China. Informed consent was acquired from all patients.is study was approved by the Ethic Committee of the First Af filiated Hospital of Nanjing Medical University in accordance with the provisions of theDeclaration of Helsinki(No. 2010-SR-088).
Cell culture
Under sterile conditions, epidural scars were dissected into 5 mm × 5 mm pieces and dissociated in 0.25% trypsin (Gibco, Grand Island, NY, USA) for 6 minutes at 37°C.e cell suspension was centrifuged at 240 ×gfor 5 minutes. Cells were maintained in Dulbecco modified Eagle Medium (Gibco) with 10% fetal bovine serum (Gibco) and penicillin (100 U/mL)/streptomycin (100 mg/L) (Gibco) at 37°C in a humidified atmosphere of 5% CO2and 95% air.
MMC treatment
HESFs seeded in 24-well plates or 10-cm dishes overnight were washed with phosphate-buffered saline (PBS; pH7.4) (Keygen, Nanjing, China) and divided into MMC and control groups. Cells in the MMC group were subdivided into five subgroups according to the concentration of MMC (Kyowa Hakko Kogoyo Co., Ltd., Tokyo, Japan) used for treatment (1, 5, 10, 20 and 40 μg/mL). Cells in the control group were treated with PBS at different time points (12, 24 and 48 hours).
To further investigate the mechanism of MMC-induced apoptosis of HESFs, HESFs were pretreated with or without caspase inhibitors, including Z-IETD-FMK (20 μM, diluted in PBS) and Z-LETD-FMK (20 μM, diluted in PBS) for 2 hours. The cells were subjected to a single application of 10 μg/mL MMC (diluted in PBS) for 24 hours in the MMC group.e control group was treated with PBS for the same period. Aer treatment, cells were immediately washed three times with PBS for subsequent experiments.
To examine the role of endoplasmic reticulum stress in MMC-induced HESF apoptosis, the endoplasmic reticulum stress inhibitor salubrinal was used. HESFs were pretreated with or without salubrinal (10 μM) for 2 hours. Then, the cells were treated with MMC (10 μg/mL) or PBS for 24 hours in the MMC group and control group, respectively.e cells were then analyzed by Cell Counting Kit-8 (CCK-8) assay, annexin V/propidium iodide double labeling and western blot assay.
Cell viability assay
HESFs treated with various concentrations of MMC (1, 5, 10,20 or 40 μg/mL) for 12, 24 or 48 hours were evaluated using the cell counting kit-8 assay (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. Cells were treated with PBS in the control group. Cells were harvested and plated at a density of 1 × 104cells/100 μL/well on a 96-well plate in 6 replicates. Cell-free culture medium was added to the blank. Aer 24 hours, the cells were subjected to various treatments, and then the CCK-8 solution (10 μL) was added to each well and incubated for 3–4 hours at 37°C.ereaer, the optical density (OD) was measured at 450 nm with an absorbance microplate reader (ELx800 Absorbance Microplate Reader, Bio-Tek, USA). Cell viability (%) was equal to (ODexperimentalgroup− ODblank)/(ODcontrolgroup−ODblank) × 100%.
Annexin V/propidium iodide double staining
Annexin V/propidium iodide double staining (BD Biosciences, CA, USA) was used to detect cell apoptosis. HESFs were plated in 60-mm dishes (4 mL, 1 × 106/well) and incubated for 24 hours. Aer treatment with different concentrations of MMC, the detached and adherent cells were collected at the indicated time points and washed twice with icecold PBS.e cells were then resuspended in binding buf f er at a concentration of 1 × 106/mL and incubated with annexin V-FITC and propidium iodide for double staining, according to the manufacturer’s instructions.e mixture was incubated in the dark for 15 minutes at room temperature and analyzed using the Beckman Coulter FC500 fl ow cytometry system and CXP soware (Beckman Coulter, Fullerton, CA,USA). The apoptosis rate in this study represents the total apoptosis rate, including early apoptosis rate and late apoptosis rate.
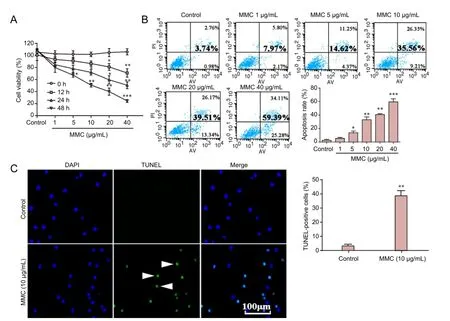
Figure 1 Efects of MMC on human epidural scar fi broblasts.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
TUNEL staining (Roche, Mannheim, Germany) was used to observe apoptotic cells. Cells were treated with 10 μg/mL MMC for 24 hours and fi xed in 3.7% paraformaldehyde for 30 minutes at room temperature. After washing with PBS, the cells were incubated with blocking solution (3% H2O2in methanol) for 10 minutes at 15–25°C. Aer rinsing with PBS, cells were permeabilized in 0.1% Triton X-100 in 0.1% sodium citrate for 2 minutes on ice, then incubated in the TUNEL reagent (green fl uorescence) for 1 hour at 37°C in a humidified atmosphere in the dark and rinsed with PBS. To visualize the total number of cells in the fi eld, slides were stained with 1 mg/L 4′,6-diamidino-2-phenylindole (blue fl uorescence) (Beyotime, Hangzhou, China) for 10 minutes.en, the apoptotic features of cells were examined by fl uorescence microscopy (Olympus BX51, Tokyo, Japan). The experiment was performed in triplicate, and a minimum of 100 cells/f i eld and at least 12 fi elds in each well were counted by a blind observer.
Western blot assay
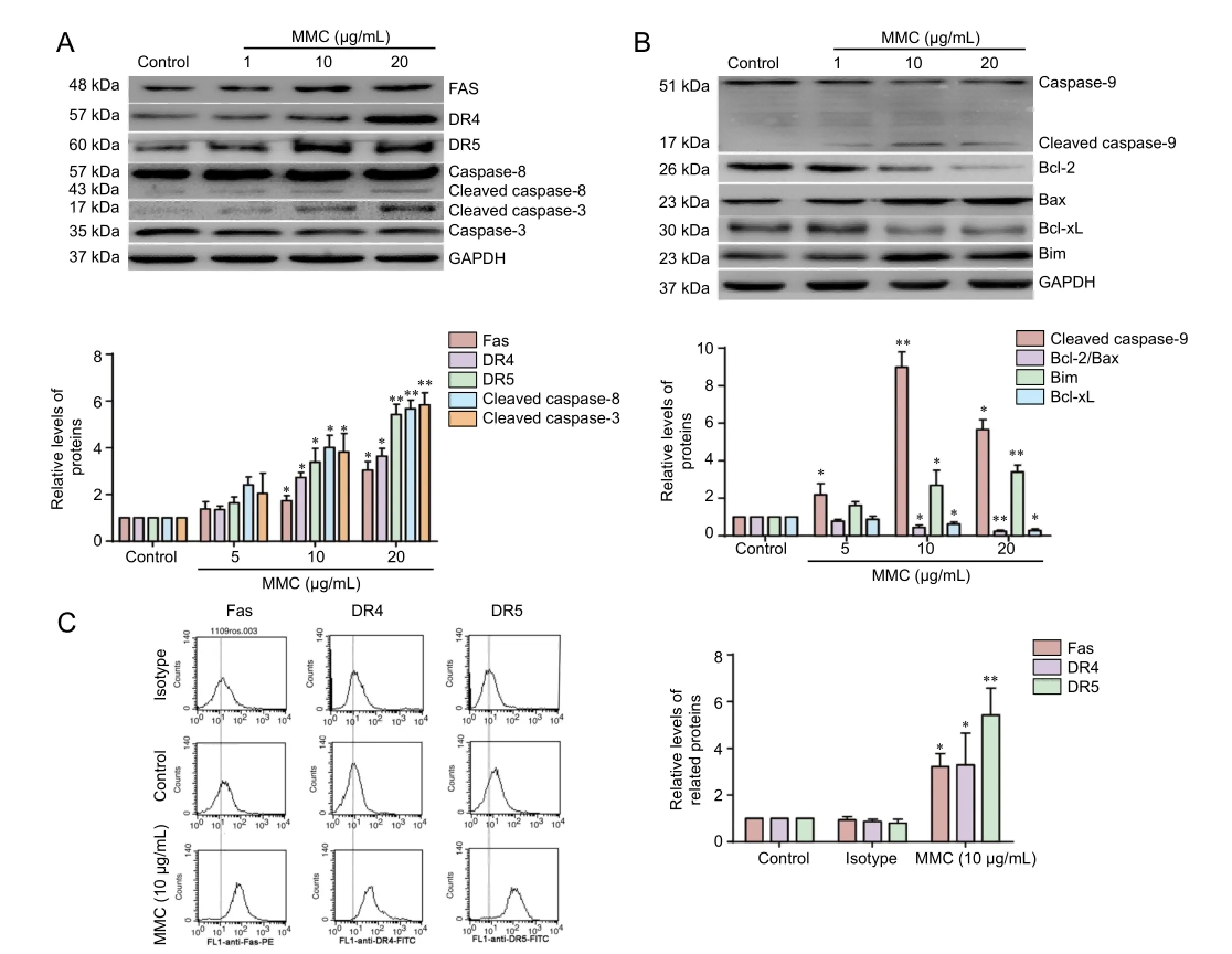
Figure 2 MMC-induced HESF apoptosis involves death receptor and mitochondrial apoptotic pathways.
HESFs were treated with 0 (control group), 5, 10 or 20 μg/mL MMC for 24 hours.en, FAS, DR4, DR5, caspase-8, caspase-9, caspase-3 and cleaved caspase-3 protein levels were measured using western blot assay. Cells were lysedon ice with RIPA lysis buffer (Beyotime, Hangzhou, China). Protein concentrations were determined using the Bicinchoninic Acid Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). Equal amounts (25 μg/lane) of total protein were subjected to electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% skim milk in Tris-buf f ered saline and Tween 20 at room temperature for 2 hours and subsequently incubated with primary antibodies at 4°C overnight.e following primary antibodies (1:500–1:1,000) were used: mouse monoclonal FAS antibody, mouse monoclonal DR4 antibody, mouse monoclonal DR5 antibody, mouse monoclonal cleaved caspase-3 and caspase-3 antibody, mouse monoclonal cleaved caspase-8/9 and caspase-8/9 antibody (Cell Signaling Technology, Danvers, MA, USA), mouse monoclonal cleaved caspase-4 and caspase-4 antibody (MBL, Nagoya, Japan), mouse monoclonal GRP78 antibody, rabbit monoclonal CHOP antibody, rabbit polyclonal BCL-2 antibody, rabbit polyclonal Bax antibody, rabbit polyclonal Bcl-xL antibody, rabbit polyclonal Bim antibody (Cell Signaling Technology), and rabbit polyclonal GAPDH antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).e membranes were washed three times in Tris-buffered saline and Tween 20 and incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (1:5,000; Cell Signaling Technology) for 1 hour. The immune complexes were visualized by fluorography using enhanced chemiluminescence western blot detection reagents (Millipore, Bedford, USA) and visualized on X-ray fi lms (GE, Little Chalfont, Buckinghamshire, UK).e grey value of each band was measured using Lab 2.0 Software (Bio-Rad Laboratories Inc., CA, USA).e ratio of the grey value normalized to GAPDH represents the relative concentration of the protein.
Analysis of FAS, DR4 and DR5 surface expression
Cells were treated with MMC (10 μg/mL) for 24 hours and washed with PBS supplemented with 2% fetal bovine serum after detachment by trypsinization. Cells were incubated with mouse or rabbit monoclonal anti-human FAS, DR4 or DR5 antibody (similar to the western blot assay) for 20 minutes at room temperature. Cells were then washed and incubated with FITC- or phycoerythrin-conjugated goat anti-mouse or rabbit antibody (Invitrogen, Carlsbad, CA, USA) for 20 minutes at room temperature before washing and resuspension in PBS supplemented with 2% fetal bovine serum for fl ow cytometric analysis (excitation wavelength of 488 nm). Samples were analyzed on a FACSCalibur fl ow cytometer (BD Biosciences, San Jose, CA, USA). Results were expressed as mean fl uorescence intensity. Mouse and rabbit IgG isotype controls were used.
Caspase activity assay
To measure the catalytic activity of caspase family cysteine proteases, including caspase-8 and caspase-9, HESFs were lysed with a lysis buf f er (1% Triton X-100, 0.32 M sucrose, 5 mM ethylenediamine tetraacetic acid, 1 mM phenylmethyl sulfonylf l uoride, 1 g/mL leupeptin, 2 mM dithiothreitol, 10 mM Tris-HCl, pH 8.0) on ice for 30 minutes, and centrifuged at 16,000 ×gfor 20 minutes at 4°C.e supernatant fraction was collected and used for the assay. Caspase activity was determined using a fl uorometric caspase assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Ac-IETD-AFC and Ac-LEHD-AFC were used as substrates for caspase-8 and caspase-9, respectively. Caspase activity was measured with a fluorescence microplate reader (Jasco FR-777, Midland, Canada) at 495 nm.
Statistical analysis
Data are presented as the mean ± SD of triplicate experiments. Statistical differences between MMC subgroups were analyzed by one-way analysis of variance followed by Dunnett’st-test or Student’st-test using SPSS 13.0 soware (SPSS, Chicago, IL, USA). Statistical signif i cance was def i ned atP<0.05.
Results
MMC inhibited the proliferation of HESFs and induced apoptosis
Cells were treated with various concentrations of MMC (0, 1, 5, 10, 20, 40 μg/mL) for 0, 12, 24 or 48 hours and evaluated using the CCK-8 assay. As shown in Figure 1A, MMC inhibited the proliferation of these fi broblasts in a time- and dose-dependent manner.e IC50was estimated at 10 μg/mL for the 24-hour incubation. A remarkable reduction in cell viability was observed 24 hours aer treatment with MMC at a concentration of 10 μg/mL. Similar results were observed by annexin V/propidium iodide double staining. Cells treated with 0, 1, 5, 10, 20 or 40 μg/mL MMC for 24 hours underwent apoptosis in the early stage; approximately 0.98 ± 0.21%, 2.17 ± 0.94%, 4.37 ± 1.25%, 9.21 ± 3.27%, 13.34 ± 3.55% and 25.28 ± 4.59%, respectively (bottom right quadrant) (Figure 1B).e total percentage of apoptosis (bottom and top right quadrant) increased gradually with MMC concentration (Figure 1B). Additionally, to determine the cytotoxicity of MMC in HESFs, the apoptotic cells were subjected to TUNEL staining and visualized under a fluorescence microscope. As expected, the number of TUNEL-positive cells (stained with green fl uorescence) signif i cantly increased 24 hours aer the treatment with 10 μg/mL MMC (Figure 1C). These results show that MMC inhibits HESF proliferation and induces apoptosis.
Mechanism of MMC-induced HESF apoptosis
To test whether the MMC-induced apoptosis of HESFs results from the activation of the death receptor and mitochondrial apoptotic pathways, cells were treated with 0, 5, 10 or 20 μg/mL MMC for 24 hours, and FAS, DR4, DR5, caspase-8, caspase-9, caspase-3 and cleaved caspase-3 protein levels were measured using western blot assay. As shown in Figure 2A, MMC-treated cells exhibited a significant upregulation in FAS, DR4, DR5 and cleaved caspase-8 levels. Furthermore, FACS analysis showed a significant upregula-tion of cell surface receptor density for FAS, DR4 and DR5 (Figure 2C).is result is in accordance with the western blot assay.is fi nding suggests that the death receptor apoptosis pathway is involved in MMC-induced HESF apoptosis.
Immunodetection of caspase-9 showed a concentration-dependent decrease in the procaspase band and an increase in the cleaved caspase-9 band, indicating activation of caspase-9 in MMC-treated HESFs. In addition, the expression of cell survival proteins, Bcl-2 and Bcl-xL, was downregulated. In contrast, the expression of pro-apoptotic proteins, Bax and Bim, was upregulated (Figure 2B). In addition, the Bcl-2/Bax ratio was decreased.ese results show that MMC induces HESF apoptosis through the mitochondrial pathway.
Efects of Z-IETD-FMK and Z-LETD-FMK on MMC-induced apoptosis of HESFs
To further clarify the mechanism of MMC-induced apoptosis of HESFs, we used pharmacological inhibitors of caspase-8 (Z-IETD-FMK; 20 μM) and caspase-9 (Z-LEHD-FMK; 20 μM) to test whether inhibition of these proteases impacts the cytotoxic effect of MMC on HESFs. As shown in Figure 3A, Z-IETD-FMK and Z-LEHD-FMK strongly decreased the corresponding caspase activity.en, cells were pretreated with Z-IETD-FMK, Z-LEHD-FMK or a combination of these two drugs for 2 hours, and thereaer treated with 10 μg/mL MMC for 24 hours.e CCK-8 assay showed that Z-IETD-FMK and Z-LEHD-FMK pretreatment ef f ectively increased cell viability (25.25 ± 3.45%) compared with the MMC group (Figure 3B). However, the combination of Z-IETD-FMK and Z-LEHD-FMK did not fully inhibit MMC-induced growth inhibition (P< 0.05) (Figure 3B). Pretreatment with Z-IETD-FMK and Z-LEHD-FMK impaired MMC-mediated apoptosis (75.24 ± 7.28%), compared with the MMC group (96.28 ± 6.32%). In addition, Z-IETD-FMK and Z-LEHD-FMK significantly diminished the MMC-induced increase in cleaved caspase-3 protein levels (Figure 3D). Annexin V/propidium iodide double labeling showed that in cells treated with MMC, the rate of apoptosis was 4.33% in the control group, whereas 19.99% of cells treated with Z-IETD-FMK plus Z-LEHD-FMK were apoptotic (P< 0.05) (Figure 3C).ese results indicate that other apoptotic pathways might be involved in MMC-induced apoptosis in HESFs.
Efects of MMC on endoplasmic reticulum stress
Endoplasmic reticulum stress-associated apoptosis can be seen in several cell lines (Yoon et al., 2011; Wang et al., 2012). To examine whether the effects of MMC on HESFs are associated with endoplasmic reticulum stress signaling, we assessed levels of GRP78, caspase-4 and CHOP, which have been used as markers of endoplasmic reticulum stress (Gorman et al., 2012). As shown in Figure 4A, B, MMC induced a robust increase in the levels of GRP78, CHOP and cleaved caspase-4 in a dose-dependent manner (Figure 4A, B).ese results indicate that endoplasmic reticulum stress is involved in MMC-induced HESF apoptosis.
Efect of salubrinal on MMC-induced HESF apoptosis
To test whether endoplasmic reticulum stress is important for MMC-induced HESF apoptosis, we used the endoplasmic reticulum stress inhibitor salubrinal. Cells were pretreated with salubrinal (10 μM) for 2 hours and then incubated with 10 μg/mL MMC for 24 hours. CCK-8 assay showed that salubrinal signif i cantly increased cell viability compared with the MMC group (Figure 5A). Annexin V/propidium iodide double staining showed that in cells treated with MMC, the rate of apoptosis was 44.35 ± 5.39% in the MMC group, whereas it was only 22.88 ± 2.87% in the salubrinal pretreatment group (P< 0.01) (Figure 5C). Western blot assay showed that CHOP was significantly upregulated in MMC-treated HESFs. However, compared with the MMC group, salubrinal pretreatment downregulated CHOP protein expression (Figure 5B). Additionally, no difference in cell viability, apoptosis or CHOP expression levels was found between the control and salubrinal only groups.e results indicate that endoplasmic reticulum stress is involved in the MMC-induced apoptosis of HESFs.
Discussion
Excessive fibroblast proliferation following spinal cord decompression surgery plays a key role in epidural scar adhesion. Triggering apoptosis in HESFs is a promising approach for preventing postoperative epidural scar adhesion (Sun et al., 2016). The features of apoptosis (programmed cell death) include cell shrinkage, chromatin condensation, DNA fragmentation and caspase activation. Caspase-3, the most important executor of apoptosis, plays a crucial role in apoptosis (Brentnall et al., 2013; Huang, 2016; Yu et al., 2016). In the present study, CCK-8 assay showed that MMC inhibits cell proliferation in HESFs in a time- and dose-dependent manner. Annexin V/propidium iodide double staining and TUNEL staining were used to observe apoptotic cells. Higher concentrations of MMC enhanced apoptosis in HESFs. MMC increased cleaved caspase-3 protein levels. Collectively, these results demonstrate that MMC inhibits HESF proliferation and induces apoptosis in these cells.
Apoptotic signaling primarily proceeds in one of two classic apoptotic pathways–the mitochondrial pathway and the death receptor pathway (Lu et al., 2014; Wang et al., 2014). TRAIL, a member of the tumor necrosis factor superfamily, induces apoptosis through the action of the death domain receptors DR4 and DR5. It directly induces apoptosis through the extrinsic pathway, which involves activation of the initiator caspase-8, which in turn activates downstream caspases. A previous study showed that MMC upregulates cell surface expression of the TRAIL death receptors DR4 and DR5, and gene silencing of DR5 by short hairpin RNA reduces MMC-induced apoptosis (Cheng et al., 2012). A similar effect was found in our study. MMC upregulated DR4 and DR5 protein levels and cell surface receptor density. MMC induces apoptosis through activation of the Fas/FasL system or caspase cascades (with associated mitochondrial dysfunction), including the activation of caspase-9, release of cytosolic cytochrome c, and a decrease in Bcl-2 (Changet al., 2010; Matsunaga et al., 2010). In our present study, MMC treatment signif i cantly increased levels of Fas, cleaved caspase-8, cleaved caspase-9, and the pro-apoptotic proteins Bax and Bim. Furthermore, MMC treatment significantly decreased expression of the anti-apoptotic proteins Bcl-2 and Bcl-x. Collectively, these results clearly show that treatment with MMC leads to a rapid change in death receptor and mitochondrial apoptotic pathway-associated proteins. Based on these results, we speculate that MMC induces apoptosis in HESFs by the death receptor and mitochondrial pathways.
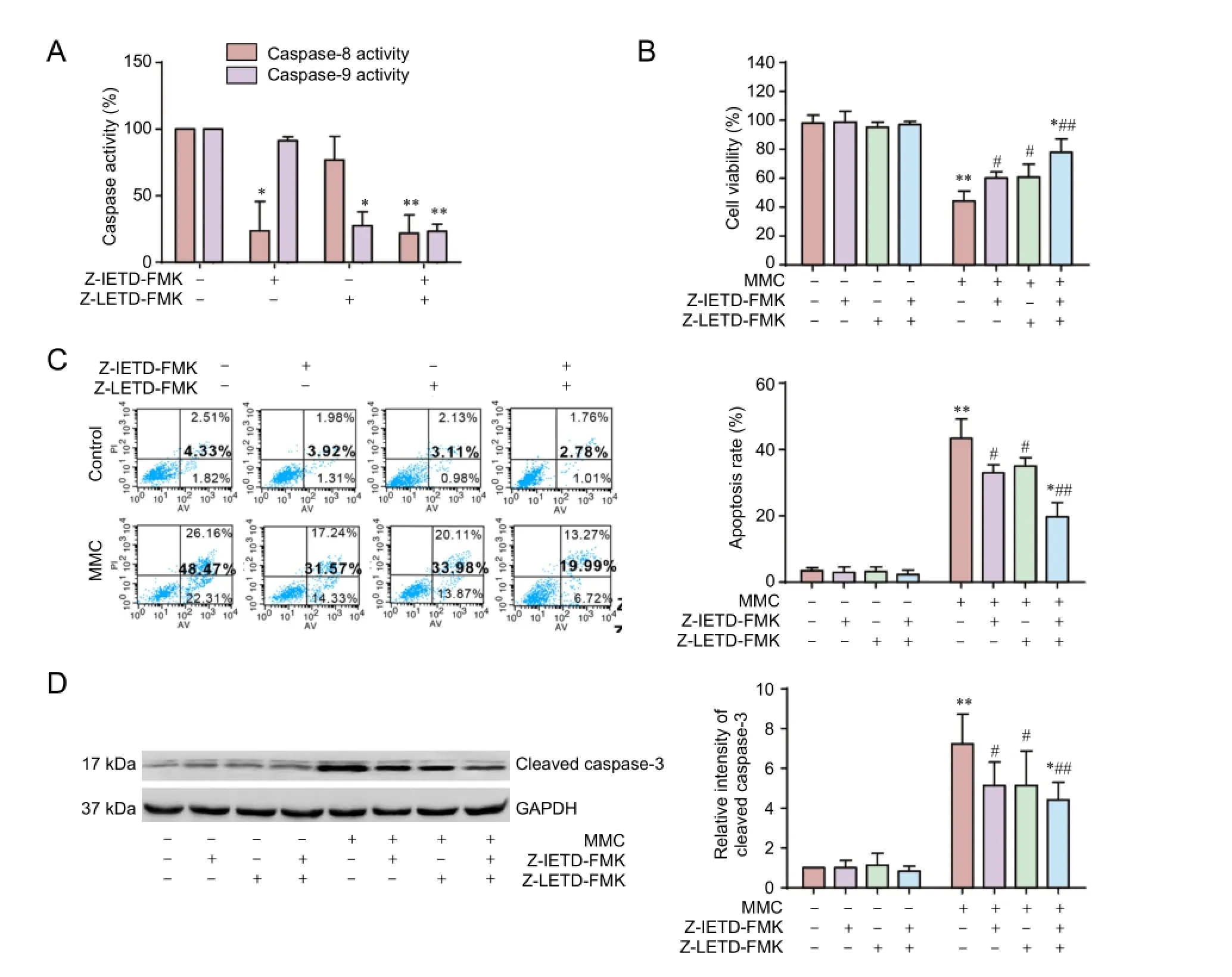
Figure 3 Z-IETD-FMK and Z-LETD-FMK partially inhibit MMC-induced apoptosis.
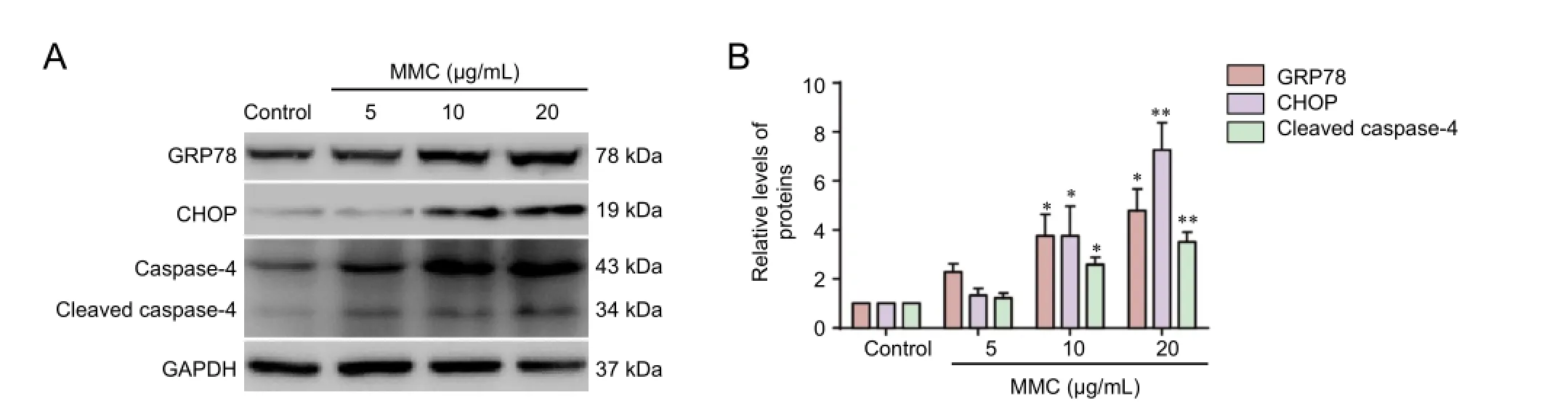
Figure 4 MMC induces endoplasmic reticulum stress.
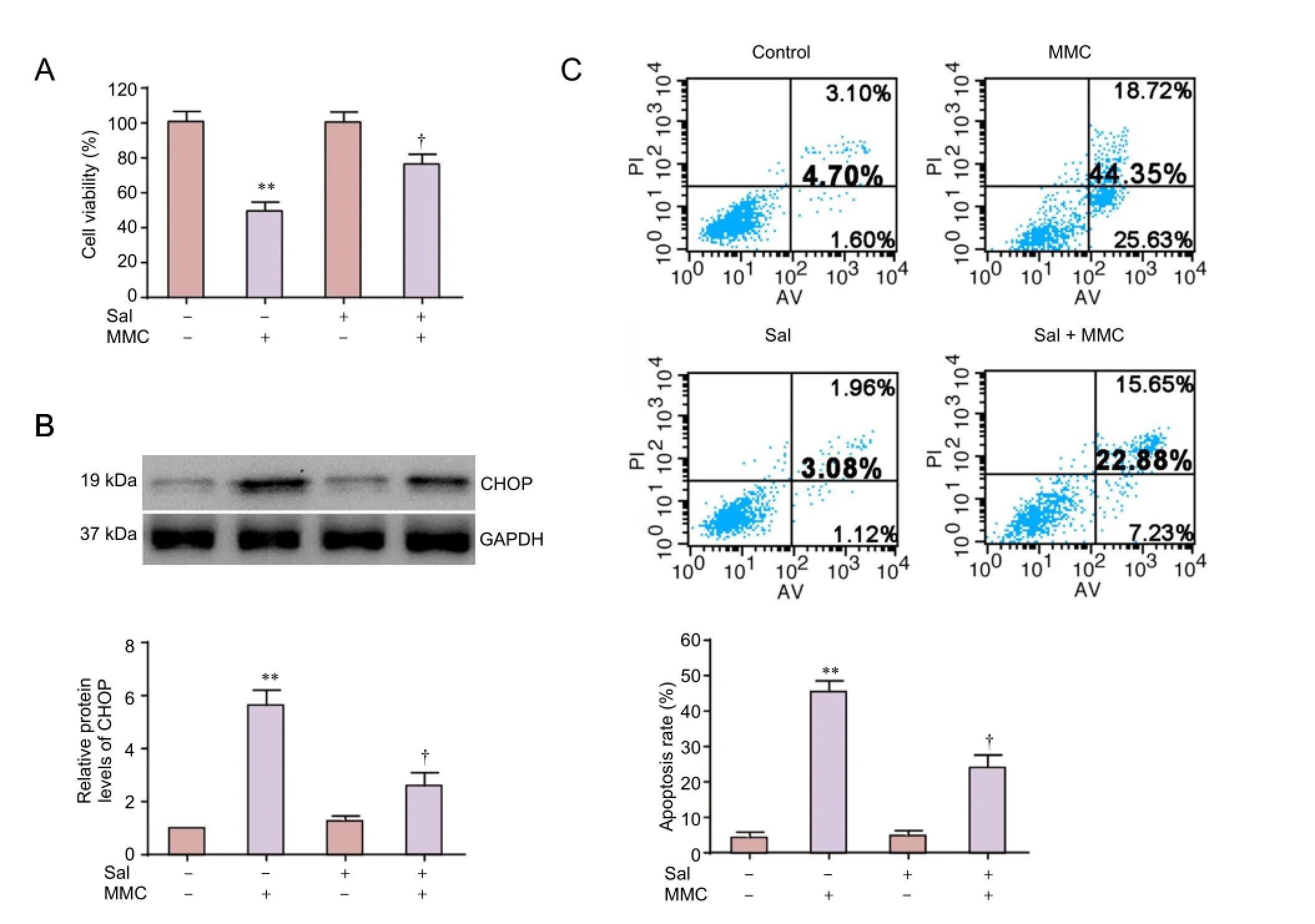
Figure 5 Sal reduces MMC-induced apoptosis in HESFs.
Recently, the endoplasmic reticulum stress pathway was identified as a third apoptotic pathway (Binetet al., 2010; Ding et al., 2012; Yang and Hu, 2015; Sun et al., 2016). Endoplasmic reticulum stress is triggered by the alteration of endoplasmic reticulum homeostasis brought about by various pathological conditions and treatments with a variety of agents. There are three distinct signaling pathways that are triggered in response to endoplasmic reticulum stress, including Ire-1, PERK and ATF-6 (Ron and Walte, 2007). Under unstressed conditions, the luminal domains of these sensors are occupied by the endoplasmic reticulum chaperone GRP78, which inhibits their activation. During endoplasmic reticulum stress, GRP78 is released from these three transmembrane proteins, thereby activating these sensors by inducing the phosphorylation and homodimerization of PERK and IRE1 and the mobilization of ATF-6 to the Golgi for activation. Activated PERK then phosphorylates eIF2α, which causes global translational attenuation. Activation ofIRE1 initiates the nonconventional splicing of Xbp-1 mRNA and promotes the synthesis of spliced Xbp-1 protein (Treglia et al., 2012). In addition, a number of molecules, including CHOP, caspase-4 and c-Jun N-terminal kinase, are activated in endoplasmic reticulum stress. CHOP and caspase-4 are indispensable in endoplasmic reticulum stress-induced apoptosis in humans (Huang et al., 2012; Lu et al., 2014). CHOP, also known as CCAAT/enhancer-binding protein, is expressed at a low level under physiological conditions, but is strongly upregulated under endoplasmic reticulum stress. Increased expression of the transcription factor CHOP leads to the downregulation of Bcl-2 and Bim and the upregulation of Bax (Puthalakath et al., 2007; Jung et al., 2015), which may cause mitochondrial dysfunction and induce apoptosis (B’Chir et al., 2014). Additionally, CHOP knockout inhibits endoplasmic reticulum stress-induced apoptosis (Watanabe et al., 2008). Our present fi ndings indicate that MMC induces transient increases in the levels of GRP78, CHOP and caspase-4 in a dose-dependent manner. Taken together, our results show that MMC-induced apoptosis of HESFs is coupled to endoplasmic reticulum stress.
Salubrinal, a selective inhibitor of eIF2α dephosphorylation, was found to be protective against endoplasmic reticulum stress-mediated apoptosis (Boyce et al., 2005). By inhibiting eIF2α dephosphorylation, salubrinal attenuates unfolded or misfolded protein synthesis and rescues cells from apoptosis. In neurons, salubrinal can reduce the load of mutant or mislocated proteins retained in the endoplasmic reticulum under conditions associated with neurodegeneration (Sokkaet al., 2007). Previous studies have also demonstrated that salubrinal reduces tunicamycin and hypoxia-induced apoptosis in the rat (Liu et al., 2012). Similar results were obtained in our analysis; the viability of MMC-treated HESFs was significantly increased after pretreatment with salubrinal. Salubrinal partially abrogated MMC-induced apoptosis. A recent study showed that salubrinal protects cardiomyocytes against apoptosis by downregulating the expression of CHOP and cleaved caspase-12 (Liu et al., 2012). In the present study, we assessed CHOP levels by western blot assay. Compared with the MMC-treated (without salubrinal) groups, salubrinal downregulated CHOP protein expression.is suggests that endoplasmic reticulum stress is involved in MMC-induced apoptosis.
In conclusion, we found that MMC inhibits proliferation and induces apoptosis in HESFs partiallyviathe endoplasmic reticulum stress pathway.e intracellular signal transduction mechanisms remain to be clarified. MMC might have therapeutic potential for preventing excessive postoperative scarring aer surgical decompression in spinal cord injury patients.
Author contributions:XJC and YBG designed this study. TS, DWG, and JT performed experiments. TS and LY analyzed data. TS wrote the paper. All authors approved the fnal version of the paper.
Conficts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:Tis paper was double-blinded and stringently reviewed by international expert reviewers.
B’Chir W, Chaveroux C, Carraro V, Averous J, Maurin A C, Jousse C, Muranishi Y, Parry L, Fafournoux P, Bruhat A (2014) Dual role for CHOP in the crosstalk between autophagy and apoptosis to determine cell fate in response to amino acid deprivation. Cell Signal 26:1385-1391.
Bakar D, Tanenbaum JE, Phan K, Alentado VJ, Steinmetz MP, Benzel EC, Mroz TE (2016) Decompression surgery for spinal metastases: a systematic review. Neurosurg Focus 41:E2.
Binet F, Chiasson S, Girard D (2010) Evidence that endoplasmic reticulum (ER) stress and caspase-4 activation occur in human neutrophils. Biochem Biophys Res Commun 391:18-23.
Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307:935-939.
Brentnall M, Rodriguez-Menocal L, De Guevara R L, Cepero E, Boise L H (2013) Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 14:32.
Chandler K, Cappello R (2006) Laminectomy membrane formation in dogs: is the answer still elusive? Vet J 172:1-2.
Chang SW, Chou SF, Yu SY (2010) Dexamethasone reduces mitomycin C-related inf l ammatory cytokine expression without inducing further cell death in corneal fibroblasts. Wound Repair Regen 18:59-69.
Cheng H, Hong B, Zhou L, Allen JE, Tai G, Humphreys R, Dicker DT, Liu YY, El-Deiry WS (2012) Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: evidence for the role of c-Jun N-terminal kinase activation. Cell Cycle 11:3312-3323.
Ding W, Yang L, Zhang M, Gu Y (2012) Reactive oxygen species-mediated endoplasmic reticulum stress contributes to aldosterone-induced apoptosis in tubular epithelial cells. Biochem Biophys Res Commun 418:451-456.
Gorman AM, Healy SJ, Jager R, Samali A (2012) Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacoler 134:306-316.
Gotoh T, Oyadomari S, Mori K, Mori M (2002) Nitric oxide-induced apoptosis in RAW 264.7 macrophages is mediated by endoplasmic reticulum stress pathway involving ATF6 and CHOP. J Biol Chem 277:12343-12350.
Grassner L, Wutte C, Klein B, Mach O, Riesner S, Panzer S, Vogel M, Bühren V, Strowitzki M, Vastmans J, Maier D (2016) Early decompression (< 8 h) aer traumatic cervical spinal cord injury improves functional outcome as assessed by spinal cord independence measure aer one year. J Neurotrauma 33:1658-1666.
Huang W (2016) Neuroprotective effect of tamoxifen in a model rat with aucte spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu 20:7710-7716.
Huang X, Li L, Zhang L, Zhang Z, Wang X, Zhang X, Hou L, Wu K (2012) Crosstalk between endoplasmic reticulum stress and oxidative stress in apoptosis induced by alpha-tocopheryl succinate in human gastric carcinoma cells. Br J Nutr 7:1-9.
Hueber A, Welsandt G, Jordan JF, Mietz H, Weller M, Krieglstein GK, Esser PJ (2002) Characterization of CD95 ligand (CD95L)-induced apoptosis in human tenon fi broblasts. Exp Eye Res 75:1-8.
Jung KJ, Min KJ, Bae JH, Kwon TK (2015) Carnosic acid sensitized TRAIL-mediated apoptosis through down-regulation of c-FLIP and Bcl-2 expression at the post translational levels and CHOP-dependent up-regulation of DR5, Bim, and PUMA expression in human carcinoma caki cells. Oncotarget 6:1556-1568.
Kumar V, Ali MJ, Ramachandran C (2015) Ef f ect of mitomycin-C on contraction and migration of human nasal mucosa fi broblasts: implications in dacryocystorhinostomy. Br J Ophthalmol 99:1295-1300.
Li HF, Zhao SX, Xing BP, Sun ML (2015) Ulinastatin suppresses endoplasmic reticulum stress and apoptosis in the hippocampus of rats with acute paraquat poisoning. Neural Regen Res 10:467-472.
Liu CL, Li X, Hu GL, Li RJ, He YY, Zhong W, Li S, He KL, Wang LL (2012) Salubrinal protects against tunicamycin and hypoxia induced cardiomyocyte apoptosis via the PERK-eIF2alpha signaling pathway. J Geriatr Cardiol 9:258-268.
Liu J, Ni B, Zhu L, Yang J, Cao X, Zhou W (2010) Mitomycin C-polyethylene glycol controlled-release fi lm inhibits collagen secretion and induces apoptosis of fi broblasts in the early wound of a postlaminectomy rat model. Spine J 10:441-447.
Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi A (2014) Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345:98-101.
Lu TH, Tseng TJ, Su CC, Tang FC, Yen CC, Liu YY, Yang CY, Wu CC, Chen KL, Hung DZ, Chen YW (2014) New insights into the roles of CHOP-induced apoptosis in ER stress. Toxicol Lett 224:130-140.
Matsunaga T, Tsuji Y, Kaai K, Kohno S, Hirayama R, Alpers DH, Komoda T, Hara A (2010) Toxicity against gastric cancer cells by combined treatment with 5-f l uorouracil and mitomycin C: implication in oxidative stress. Cancer Chemother Pharmacol 66:517-526.
Matsuyama S, Reed JC (2000) Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death differ 7:1155-1165.
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249-1259.
Na JH, Sung KR, Shin JA, Moon JI (2015) Antif i brotic ef f ects of pirfenidone on Tenon’s fi broblasts in glaucomatous eyes: comparison with mitomycin C and 5-f l uorouracil. Graefes Arch Clin Exp Ophthalmol 253:1537-1545.
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death differ 11:381-389.
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM (1997)e receptor for the cytotoxic ligand TRAIL. Science 276:111-113.
Park IC, Park MJ, Hwang CS, Rhee CH, Whang DY, Jang JJ, Choe TB, Hong SI, Lee SH (2000) Mitomycin C induces apoptosis in a caspases-dependent and Fas/CD95-independent manner in human gastric adenocarcinoma cells. Cancer Lett 158:125-132.
Pirnia F, Schneider E, Betticher DC, Borner MM (2002) Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and Fas-independent pathway. Cell Death differ 9:905-914.
Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337-1349.
Robertson JT (1996) Role of peridural fi brosis in the failed back: a review. Eur Spine J 5 Suppl 1:S2-6.
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519-529.
Seong GJ, Park C, Kim CY, Hong YJ, So HS, Kim SD, Park R (2005) Mitomycin-C induces the apoptosis of human Tenon’s capsule fi broblast by activation of c-Jun N-terminal kinase 1 and caspase-3 protease. Invest Ophthalmol Vis Sci 46:3545-3552.
Shi K, Wang D, Cao X, Ge Y (2013) Endoplasmic reticulum stress signaling is involved in mitomycin C (MMC)-induced apoptosis in human fi broblasts via PERK pathway. PLoS One 8:e59330.
Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 27:901-908.
Songer MN, Rauschning W, Carson EW, Pandit SM (1995) Analysis of peridural scar formation and its prevention aer lumbar laminotomy and discectomy in dogs. Spine (Phila Pa 1976) 20:571-580.
Su C, Yao C, Lu S, Zhang A, Cao X, Teng G, Zang F (2010) Study on the optimal concentration of topical mitomycin-C in preventing postlaminectomy epidural adhesion. Eur J Pharmacol 640:63-67.
Sui T, Liu L, Wu XT, Cao XJ (2016) Local application of mitomycin C for prevention of epidural fi brosis: study protocol for a prospective randomized controlled double-blinded trial. Clin Trials Orthop Disord 1:22-30.
Sun GZ, Gao FF, Zhao ZM, Sun H, Xu W, Wu LW, He YC (2016) Endoplasmic reticulum stress-induced apoptosis in the penumbra aggravates secondary damage in rats with traumatic brain injury. Neural Regen Res 11:1260-1266.
Sun Y, Ge Y, Fu Y, Yan L, Cai J, Shi K, Cao X, Lu C (2015) Mitomycin C induces fi broblasts apoptosis and reduces epidural fi brosis by regulating miR-200b and its targeting of RhoE. Eur J Pharmacol 765:198-208.
Sun Y, Wang LX, Wang L, Sun SX, Cao XJ, Wang P, Feng L (2007) A comparison of the ef f ectiveness of mitomycin C and 5-f l uorouracil in the prevention of peridural adhesion aer laminectomy. J Neurosurg Spine 7:423-428.
Sun Y, Zhao S, Li X, Yan L, Wang J, Wang D, Chen H, Dai J, He J (2016) Local application of rapamycin reduces epidural fi brosis aer laminectomy via inhibiting fi broblast proliferation and prompting apoptosis. J Orthop Surg Res 11:58.
Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M (2004) Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death differ 11:403-415.
Treglia AS, Turco S, Ulianich L, Ausiello P, Lofrumento DD, Nicolardi G, Miele C, Garbi C, Beguinot F, Di Jeso B (2012) Cell fate following ER stress: just a matter of “quo ante” recovery or death? Histol Histopathol 27:1-12.
Wang F, Song W, Brancati G, Segatori L (2011) Inhibition of endoplasmic reticulum-associated degradation rescues native folding in loss of function protein misfolding diseases. J Biol Chem 286:43454-43464.
Wang WJ, Wang Y, Chen HZ, Xing YZ, Li FW, Zhang Q, Zhou B, Zhang HK, Zhang J, Bian XL, Li L, Liu Y, Zhao BX, Chen Y, Wu R, Li AZ, Yao LM, Chen P, Zhang Y, Tian XY, et al. (2014) Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nat Chem Biol 10:133-140.
Wang Y, Wan B, Li D, Zhou J, Li R, Bai M, Chen F, Yu L (2012) BRSK2 is regulated by ER stress in protein level and involved in ER stress-induced apoptosis. Biochem Biophys Res Commun 423:813-818.
Watanabe Y, Tsuchiya H, Sakabe T, Matsuoka S, Akechi Y, Fujimoto Y, Yamane K, Ikeda R, Nishio R, Terabayashi K, Ishii K, Gonda K, Matsumi Y, Ashla AA, Okamoto H, Takubo K, Tomita A, Hoshikawa Y, Kurimasa A, Itamochi H, et al. (2008) CD437 induces apoptosis in ovarian adenocarcinoma cells via ER stress signaling. Biochem Biophys Res Commun 366:840-847.
Wu KY, Wang HZ, Hong SJ (2008) Mechanism of mitomycin-induced apoptosis in cultured corneal endothelial cells. Mol Vis 14:1705-1712.
Xie JB, Zhang X, Li QH, Xu ZJ (2015) Inhibition of inf l ammatory cytokines aer early decompression may mediate recovery of neurological function in rats with spinal cord injury. Neural Regen Res 10:219-224.
Yang JW, Hu ZP (2015) Neuroprotective ef f ects of atorvastatin against cerebral ischemia/reperfusion injury through the inhibition of endoplasmic reticulum stress. Neural Regen Res 10:1239-1244.
Yoon H, Kim DS, Lee GH, Kim KW, Kim HR, Chae HJ (2011) Apoptosis induced by manganese on neuronal SK-N-MC cell line: endoplasmic reticulum (er) stress and mitochondria dysfunction. Environ Health Toxicol 26:e2011017.
Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL (2006) Apoptosis-inducing factor mediates poly (ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A 103:18314-18319.
Yu YQ, Hu NC, Duan JA, Li DP, Liu C (2016) Neuroprotective ef f ects of sufentanil preconditioning on spinal cord injury in mouse models. Zhongguo Zuzhi Gongcheng Yanjiu 20:5966-5972.
Zhang R, Piao MJ, Kim KC, Kim AD, Choi JY, Choi J, Hyun JW (2012) Endoplasmic reticulum stress signaling is involved in silver nanoparticles-induced apoptosis. Int J Biochem Cell Biol 44:224-232.
Copyedited by Patel B, Pack M, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Xiao-jian Cao, Ph.D. or Ying-bin Ge, Ph.D., xiaojiancao001@163.com or ybge@njmu.edu.cn.
Xiao-jian Cao, Ph.D. or Ying-bin Ge, Ph.D., xiaojiancao001@163.com or ybge@njmu.edu.cn.
#Tese authors contributed equally to this study.
orcid: 0000-0003-3603-3448 (Xiao-jian Cao) 0000-0003-1471-9000 (Ying-bin Ge)
10.4103/1673-5374.205106
Accepted: 2017-03-12
- 中国神经再生研究(英文版)的其它文章
- Recovery of multiply injured ascending reticular activating systems in a stroke patient
- Neuroprotective mechanism of Kai Xin San: upregulation of hippocampal insulin-degrading enzyme protein expression and acceleration of amyloid-beta degradation
- Exenatide promotes regeneration of injured rat sciatic nerve
- Recombinant human fi broblast growth factor-2 promotes nerve regeneration and functional recovery after mental nerve crush injury
- Ca2+involvement in activation of extracellular-signalregulated-kinase 1/2 and m-calpain after axotomy of the sciatic nerve
- Low frequency repetitive transcranial magnetic stimulation improves motor dysfunction after cerebral infarction

