浅析光谱技术在200 nm以上和以下尺度范围内的不同
乌拉 郑玉祥


摘要:
一直以來光谱学在各类研究中得到了广泛的应用。不同的电磁光谱已被用于各种应用中,导致了各种光谱技术的演变。200 nm(更准确地说是185 nm)波长的光谱却仍旧是一个让光谱学家们警惕的问题。讨论和分析了为什么200 nm以下和以上的光谱技术不同的原因,并进行了相应的分析。最后提出了有效地改进方法,不仅与空气吸收有关,而且与样品的本身属性有着密切的关联。
关键词:
紫外光谱; 真空紫外线; 光谱学
中图分类号: O 433文献标志码: Adoi: 10.3969/j.issn.10055630.2016.06.010
Abstract:
Spectroscopy has been used in tremendous applications and researches since a long time. Different portions of electromagnetic spectrum have been utilized for this purpose which resulted in evolvement of various spectroscopic techniques. 200 nm (more precisely 185 nm) is still a displeasing hitch which reminds spectroscopists to be cautious while working in the region below 200 nm. In this review,we give reasons and analyze them as well on why are spectroscopic techniques different below and above 200 nm. We also present the short evolution of the techniques how did they develop and the contribution of the scientists.It's not only absorption by air which hinders but it's about the materials useable in this regime and generation and detection together state of the sample.
Keywords:
ultraviolet spectrum; vacuum ultraviolet; spectroscopy
Introduction
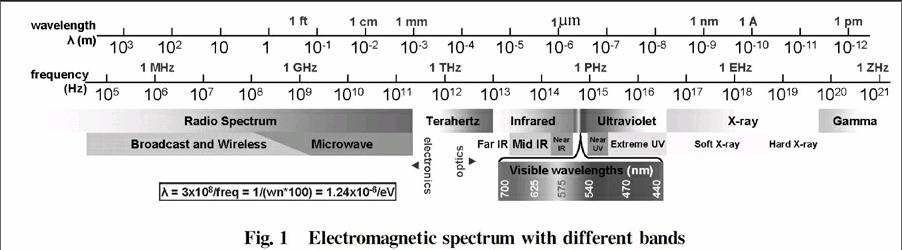
Electromagnetic spectrum is a chart of all the frequencies along with assignment of different bands as shown in Fig.1. These electromagnetic waves play an important role in a wide range of applications but the question is how? The answer is by the interaction of electromagnetic waves with mater in a loose sense. Interaction of electromagnetic waves with matter can generally be regarded as spectroscopy. Now this is a general term. Specific names are given according to either nature of waves or the technique being used in the process. For example infrared spectroscopy means electromagnetic waves from the infrared region are used in the measurement of spectra. Fourier transform infrared spectroscopy means spectroscopy is done by using Fourier transform technique. It is same for the case with spectroscopic techniques below and above 200 nm.
However our focus in this review is not to present the working principles of spectroscopy being done in this range. Instead we will try to find the fundamental reasons behind this question why are spectroscopic techniques different below and above 200 nm and circumvention means. As we can see 200 nm = 1.5 PHz lies at the end of visible band. A magnified view of the ultraviolet band is shown in Fig. 2.
The region of ultraviolet band below 200 nm is named as vacuum ultraviolet. Sometimes they are named as near UV (300~400 nm) middle UV (200~300 nm) far UV (10~200 nm) and shorter wavelengths are termed as ‘extreme UV. However their ranges and names differ in the literature. The name ‘vacuum ultraviolet refers to the regions below 200 nm regardless of their names and ranges. This name is given in order to memorize that all the spectroscopic measurements in this region (<200 nm) must be done in vacuum. This region is also called Schumann region. Victor Schumann (1841—1913) discovered the vacuum ultraviolet in 1893. He was surprised at the sudden termination of the ultraviolet spectrum near 200 nm (185 nm) and this is the reason this limit of 200 nm was considered as barrier and spectroscopic techniques are different below and above it[1].
Victor Schumann gave explanation for the rapid ending of the ultraviolet spectrum near 185 nm. He found a number of causes out of which the first one was because of heavy absorption by air and the second was gelatine of the photographic plates. Gelatine emulsion was coated on one side of such photographic plates to paste the light sensitive silver halide crystals to the plastic used to observe ultraviolet spectrum in those times. The third problem was the quartz material of which the optics like prisms and lenses were made. To circumvent these issues He then used a vacuum spectroscope in which he put the whole setup under vacuum to eliminate the absorption by air. Similarly he used special photographic plates with poor gelatine emulsion. Such plates are called Schumann plates in which photosensitive silver halide crystals are fixed to the plastic by a very thin layer of gelatine which is almost transparent at such wavelengths.Photographic plates preexisted photographic films which have been popular in photography until recently and are being replaled rapidly with digital media.A glass plate used to be used in photographic plates while a thin plastic film was used in photographic film whose one side was coated with gelatin emulsion containing silver halide crystals.A typical photographic film is shown in Fig.3. To solve the third problem he used fluorite instead of quartz for optics because he was aware of the superiority of the fluorite over quartz for short wavelengths.
As a result He penetrated this 200 nm barrier up to 123 nm. He stopped at 123 nm because of the absorption of fluorite of which the optics were made. He was the first person to break this barrier of 200 nm. Thats why this region is also referred to as Schumann region. He wished to infiltrate the extreme ultraviolet region.
His legacy was continued by Theodore Lyman who extended his work. Based on the work of Schumann on hydrogen lines Theodore Lyman discovered the hydrogen lines series now called Lyman series. Lyman series are the series of the transitions resulting from ultraviolet emission of a hydrogen atom in which when an electron jumps from a higher (n>=2) to lowest energy level (n=1) and ‘n is the principal quantum number. He wrote several articles about Schumanns work and his legacy.
Hence spectroscopic techniques are different above and below 200 nm because of abrupt change in the behavior of the materials below and above this frequency range which ultimately requires the change of the whole setup to take spectroscopic measurements. We present these factors in detail here.
1Absorption by air
As we have mentioned before that Schumann realized very well that atmosphere becomes heavy absorber for the frequencies below 200 nm he put the whole setup under vacuum which is the best choice till today.However sometimes its not convenient to take the measurement under vacuum. As a matter of fact it costs a lot to get the system work very well in vacuum. As we know air is mixture of gases with nitrogen being 78% and oxygen 21%. Nitrogen gas is almost transparent up to 100 nm. After 100 nm absorption starts increasing and becomes intense around 85 nm. So if the spectral range is above 100 nm the system can be purged with dry nitrogen and measurements can be taken without putting the setup in vacuum. The main absorption occurs in the vacuum ultraviolet region is due to oxygen whose absorption lines starts appearing slightly before 200 nm. After 200 nm absorption lines are intensified significantly and increases quickly towards shorter wavelengths. Oxygen has numerous absorption lines in this region and only a slit 110~120 nm is empty of absorption lines of oxygen which is of course not enough. So it is actually oxygen in the air which is troublesome. The other constituents of the air are very low in proportion that they can either be neglected or their effects can be corrected numerically by knowing their absorption coefficients from the final result. So if we really want to penetrate the extreme ultraviolet region we are left with two choices either we do the experiment under vacuum or purge the system with a gas which is really transparent all the way down to the end of the spectral range. As we have just seen above nitrogen is transparent up to 100 nm but after that its unusable. Its very hard to find such materials which are transparent at such frequencies and is a topic of contemporary research. Doing the experiments in vacuum is also not free from hassles. Putting the whole spectrometer under clean vacuum is really challenging.
2Materials usable under 200 nm
Such materials do not exist which are transparent in the whole Schumann region.Different materials transmit light for only a fraction of the Schumann region. Even Schumann was aware of it and he used fluorite instead of quartz because he knew fluorite is far better than quartz at such frequencies. So he used fluorite for all the optics in his system but ultimately stopped at 123 nm because fluorite was no more transparent below it. Today we know some other materials. For example silica is transparent up to 165 nm only and calcium fluoride or lithium fluoride works in the range 110~120 nm. No other material is available to work below 120 nm. Consequently it was also not possible to design achromatic optics for this range until recently[2]. So a concave grating is preferred mainly because it introduces only one surface into the optical path with dual functionality of diffracting and focusing the light. This is very important because reflectivity of such materials rapidly decreases with decrease of wavelength. Another advantage of concave grating is its nearly linear dispersion. The disadvantage of such grating is the loss of light on both sides of the incident beam. In the same way there are difficulties for the material of the window. Either the measurements are taken without any window or materials like calcium fluoride or lithium fluoride can be used up to 120 nm[3]. Similarly thin films of ionbeamdeposited carbon have been shown to work in the range 49 to 200 nm in reflectance mode[4]. Similarly smart designs of multilayer mirrors have been presented with improved reflectivity[5] and such reflectivity can be enhanced further by addition of some other materials in the stack[6]. B/Si multilayers are found to work very well in extreme ultraviolet region[7].
3Ultraviolet generation below 200 nm
The ideal light source at such frequencies must provide a continuous spectrum with constant intensity. Such a light source is presented by Lyman continuum which he described in detail in his articles according to which such light is produced by strongly condensed electric discharge in a tube. He produced light electrically in a discharge tube of quartz with tungsten electrodes. Special care must be taken to choose the gas which will not only produce the desired radiation but also transparent to the same radiation. Initially he used hydrogen at a pressure of 2~3 mmHg and by using a strong surge discharge; Lyman achieved the spectrum to 90 nm. Helium is another good candidate for this purpose. Later on some advanced techniques were developed to circumvent the limitation [811]. With two light sources in which one is filled with hydrogen and second with krypton a range of 123.6~400 nm can be easily achieved. Krypton continuum ranges from 123.6 to 170 nm and hydrogen from 170 to 400 nm. So we can use any other suitable source to extend the spectrum to any desired range. If argon source is selected spectrum can be spread to 106.7~150 nm. Similarly with neon and helium sources spectrum can go down to 60 nm for a windowless operation. It is however to ponder that the only limitation is imposed by windows material. For example lithium fluoride works in the range 110~120 nm so it imposes the practical limit on the spectrum no matter what sources are we using. Besides that many other sources for the generation of extreme ultraviolet are being discovered. Demonstration has been presented that exposure of thin films of SiO2 to intense extremely short cycle pulses results in wideband coherent extreme ultraviolet radiation having energy upto 40 electronvolts [12]. Similarly role of nonlinear optics in extreme ultraviolet has been already explored[13]. Extreme ultraviolet generation can be achieved by numerous techniques including freeelectronlaser Xray lasers highorder laser harmonics and synchrotrons[14].
4Ultraviolet detectors below 200 nm
As Schumann used poor gelatine emulsion photographic filmsto avoid high absorption by gelatine photoelectric films can be conveniently be used. Sodium salicylate has been found to give the best performance with the following advantages.
(1) It can be coated reproducibly.
(2) It is not decomposed by the radiation.
(3) It does not need any desensitizing bath before development.
Photoelectric cells are yet not so common in this range. There was time before the popularity of photodiodes; photomultipliers have been used for the detection of extreme ultraviolet [15]. However recent advances have led many new techniques to be employed for extreme ultraviolet detection out which the use of photodiodes is very common. AlGaNonSi inverted Schottky photodiodes have been reported to work in extreme ultraviolet range with a cutoff at 280 nm [16]. Siliconbased p+n junction photodiodes have been announced with an added advantage of high resistance to degradation upon excessive exposure to such radiation [17]. A smart design of curved focal plane extreme ultraviolet (EUV) detector array has been addressed which is for moonbased camera [18] since imaging is now emerging in almost all fields of spectroscopy. In short there is still scarcity of highly efficient detectors working in the range less than 200 nm.
5State of sample material to be measured under 200 nm
Sample must be in gas or vapor phase.Liquids or solids are not recommended as otherwise they must be very thin. Solids are difficult to have uniform thickness. Similarly for solutions solvents must be transparent. Saturated hydrocarbons are usually transparent. However the study of solids by extreme ultraviolet highharmonic spectroscopy has been done recently which shows the promise of this technique in new dimensions which includes solids. Ultraviolet and extreme ultraviolet spectroscopy has its applications in the exploration and study of solar related phenomena like solar corona and solarwind source areas [19].
These are the reasons why spectroscopic techniques are different above and below 20 nm. There may be some other difficulties but they are common in any spectroscopy technique. The future of vacuum ultraviolet spectroscopy is very bright and with the advancement of the technology this gap will soon be filled.
6Conclusion
Materials behave differently above and below 200 nm so different spectroscopic techniques are needed. Absorption by air increases significantly below 200 nm so the whole spectroscopic setup must be placed under high vacuum. Materials transparency below 200 nm is not stabilized and hence such materials are either hard to find or they cover fraction of the region below 200 nm. Ultraviolet generation and detection below 200 nm is also challenging. Sample whose characterization is required must be in gas or vapor phase.
参考文献:
[1]LYMAN T. The extension of the spectrum beyond the Schumann region[J]. Proceedings of the National Academy of Sciences of the United States of America 1915 1(6): 368371.
[2]WANGY X YUN W B JACOBSEN C. Achromatic Fresnel optics for wideband extremeultraviolet and Xray imaging[J]. Nature 2003 424(6944): 5053.
[3]Calcium Fluoride: Optical material for applications from deep UV to IR[EB/OL]. [20160517]. http:∥www.hellmamaterials.com/text/986/en/ausblenden/hellmamaterialscalciumfluoride.html.
[4]LARRUQUERTJ, KESKIKUHA R A M. Reflectance measurements and optical constants in the extreme ultraviolet of thin films of ionbeamdeposited carbon[J]. Optics Communications 2000 183(5/6): 437443.
[5]SINGHM, BRAAT J J. Design of multilayer extremeultraviolet mirrors for enhanced reflectivity[J]. Applied Optics 2000 39(13): 21892197.
[6]SINGH M BRAAT J J M. Improved theoretical reflectivities of extreme ultraviolet mirrors[C]∥Proceedings of SPIE 3997 Emerging Lithographic Technologies IV. Santa Clara CA: SPIE 2000.
[7]RAVETM F, BRIDOU F RAYNAL A et al. B/Si multilayers for soft xray and extreme ultraviolet optics[J]. Journal of Applied Physics 2001 89(2): 11451150.
[8]TANAKAY, ZELIKOFF M. Continuous emission spectrum of xenon in the vacuum ultraviolet region[J]. Journal of the Optical Society of America 1954 44(3): 254255.
[9]WILKINSONP G, TANAKA Y. New xenonlight source for the vacuum ultraviolet[J]. Journal of the Optical Society of America 1955 45(5): 344349.
[10]WILKINSON P G. New krypton light source for the vacuum ultraviolet[J]. Journal of the Optical Society of America 1955 45(12): 10441046.
[11]TANAKAY JURSA A S LEBLANC F J. Continuous emission spectra of rare gases in the vacuum ultraviolet region. II. Neon and helium[J]. Journal of the Optical Society of America 1958 48(5): 304308.
[12]LUUT T GARG M KRUCHININ S Y et al. Extreme ultraviolet highharmonic spectroscopy of solids[J]. Nature 2015 521(7553): 498502.
[13]SEKIKAWAT, KOSUGE A KANAI T et al. Nonlinear optics in the extreme ultraviolet[J]. Nature 2004 432(7017): 605608.
[14]CANOVA F POLETTO L. Optical technologies for extremeultraviolet and soft xray coherent sources: springer series in optical sciences[M]. Berlin Heidelberg: Springer 2015.
[15]MICHELSD J, HUNTER W R. Detectors for the extreme ultraviolet. I: photomultipliers used in the dc output current mode[J]. Applied Optics 1967 6(3): 385390.
[16]MALINOWSKIP E, DUBOZ J Y DE MOOR P et al. Extreme ultraviolet detection using AlGaNonSi inverted Schottky photodiodes[J]. Applied Physics Letters 2011 98(14): 141104.
[17]SHIL SARUBBI F NIHTIANOV S N et al. High performance siliconbased extreme ultraviolet (EUV) radiation detector for industrial application[C]∥Proceeding of the 35th annual conference of IEEE industrial electronics. Porto Portugal: IEEE 2009: 18911896.
[18]NIQ SONG K LIU S et al. Curved focal plane extreme ultraviolet detector array for a EUV camera on CHANG E lander[J]. Optics Express 2015 23(24): 3075530766.
[19]MOSESJ D, KO Y K LAMING J M et el. Ultraviolet and extreme ultraviolet spectroscopy of the solar corona at the Naval Research Laboratory[J]. Applied Optics 2015 54(31):F222F231.
(編辑:张磊)
——《光谱学与光谱分析》已全文上网
——《光谱学与光谱分析》已全文上网
——《光谱学与光谱分析》已全文上网

