Misuse of the metabolic modulator meldonium in sports
Giuseppe Lippi,Camilla Mattiuzzi
aSection of Clinical Biochemistry,University Hospital of Verona,Verona 37134,Italy
bMedical Direction,General Hospital of Trento,Trento 38121,Italy
Opinion
Misuse of the metabolic modulator meldonium in sports
Giuseppe Lippia,*,Camilla Mattiuzzib
aSection of Clinical Biochemistry,University Hospital of Verona,Verona 37134,Italy
bMedical Direction,General Hospital of Trento,Trento 38121,Italy
1.Biochemical structure and molecular activity of meldonium
Meldonium(commercial name Mildronate)was originally synthesized in the mid-1970s at the Institute of Organic Synthesis of the Latvian Soviet Socialist Republic Academy of Sciences.The chemical structure of this compound(3-(2,2,2-trimethylhydraziniumyl)propionate)is similar to that of 2 biologically important betaines,γ-butyrobetaine and carnitine.1Compared to γ-butyrobetaine,in meldonium,an amino group has replaced the CH2at the C-4 position(Fig.1).The primary mechanism of action of meldonium entails a competitive inhibition of γ-butyrobetaine hydroxylase,a pivotal enzyme in the metabolic pathway of carnitine biosynthesis(Fig.1).The compound also binds to,and hence inhibits,the enzyme carnitine acetyltransferase,although this latter effect appears weaker than that on γ-butyrobetaine hydroxylase.According to this biological pathway,meldonium is thought to reduce the endogenous synthesis and biological activity of carnitine.Carnitine plays a pivotal role in transportation of activated long-chain fatty acids and other products of peroxisomal β-oxidation (especially acetyl-CoA)to the mitochondria,but also modulates the acyl-CoA/CoA ratio,optimizes energy storage,and reduces the toxic effects of poorly metabolized acyl groups.2The metabolism of this compound culminates with the generation of trimethylamine-N-oxide,which is involved in the pathogenesis of both atherosclerosis and heart failure.2Recently, meldonium was also found to exert an effective inhibition of Organic Cation/Carnitine Transporter 2,a widely expressed enzyme in mammalian tissues which functions as a sodiumcoupled carnitine transporter.3
Therefore,since meldonium ultimately decreases carnitine availability in the cell,the leading metabolic consequences include a shift of fatty acid metabolism from mitochondria to peroxisomes,a reduced formation of long-chain acylcarnitines, and a protection of mitochondrial injury due to excessive fatty acid oxidation and overload of fatty acid end-products(Fig.1).3
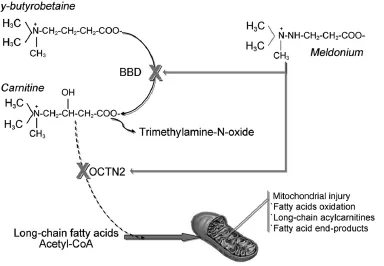
Fig.1.Metabolism of meldonium and carnitine.The administration of meldonium competitively inhibits the enzyme γ-butyrobetaine hydroxylase (BBD),which converts γ-butyrobetaine in carnitine,but also reduces carnitine adsorption by inhibiting the sodium-coupled carnitine transporter Organic Cation/Carnitine Transporter 2(OCTN2).The fina effect is a decreased transportation and metabolism of long-chain fatty acids and acetyl-CoA in the mitochondria and,consequently,a decreased risk of mitochondrial injury from fatty acid oxidation,increased generation of long-chain acylcarnitines and enhanced production of fatty acid end-products.
2.Meldonium improves post-ischaemic recovery
Due to the important regulatory activity on fatty acid metabolism,meldonium is currently used as a cardioprotective drug,especially for enhancing preconditioning-like adaptive responses.The putative effects that have been ascribed to meldonium in animal studies include prevention of atherosclerosisprogression,reduction ofinfarctsize following myocardial ischaemia,attenuation of ventricular remodelling,protection against left ventricular dysfunction,improvement of functional heart parameters,and decrease of both incidence and severity of cardiac arrhythmias.3Notably,this metabolic modulator is not currently approved for clinical use by the U.S.Food and DrugAdministration and in many countries of the European Union, and can only be prescribed in some Eastern European Counties such as Russia,Ukraine,Moldova,Belarus,Azerbaijan,and Armenia,or else it can be ordered through many virtual pharmacies available on the Web.
3.Clinical trials using meldonium show promising results in patients with heart failure
In addition to many beneficia effects of meldonium demonstrated in animal models,3a limited number of human studies also have shown promising results.Vasiuk et al.4studied 55 patients with chronic heart failure after myocardial infarction and with a left ventricular ejection fraction of less than 40%. The patients were randomized to receive basic therapy alone, basic therapy plus trimethasidine,and basic therapy plus meldonium.A significan attenuation of glucose hypermetabolism in the ischaemic segment along with a lower functional class of chronic heart failure,and a prolonged distance for a 6 min walk test was recorded in both groups treated with trimethasidine or meldonium.Zhu et al.5carried out a randomized,double-blind,multicenter clinical study including 227 patients with acute cerebral infarction,113 of whom received 500 mg/day meldonium,whereas the remaining 114 received cinepazide.Similar improvements of functional scores(e.g., Modifie Rankin Scale,National Institutes of Health Stroke Scale score and Barthel Index)were observed between groups after 3 months of therapy,which led the authors to conclude that meldonium may be as effective as cinepazide for treating acute cerebral infarction.Statsenko et al.6studied 60 patients with myocardial infarction and symptoms of Functional Class II–III heart failure,who were randomized to receive standard therapy or standard therapy plus meldonium 1000 mg/day.After 10–14 days of therapy,patients receiving meldonium displayed major clinical improvements and more favorable changes in cardiac structural and functional parameters than those receiving only standard therapy.Smirnova et al.7studied 56 patients with cardiovascular disease,who were randomized to receive basic therapy or basic therapy plus 500 mg/day meldonium.A marked decrease of systolic blood pressure,a reduction of heart rate during heat,along with a better quality of life score(e.g., visual analogue scale)and improved adaptative responses were noticed in the group of patients treated with meldonium.
Despite the fact that meldonium is thought to be relatively safe and the number of side effects was found to be lower than 15%in clinical studies,5some potential complications have been reported,including 1 case of sudden death after peripheral embolism and ischaemic stroke(reported by a physician from the Russian Federation in a 69-year-old patient using multiple drugs other than meldonium),1 case of multi-organ failure (reported by a health professional from Ukraine in a 74-yearold patient),along with additional cases of patients with diarrhoea,anaemia,fatigue,and serum bilirubin increase.8
4.Meldonium may improve endurance performance
Due to its putative anti-ischaemic effect,there are many attractive incentives that may persuade both professional andelite athletes to misuse meldonium in sports.Such incentives include an improved peroxisomal utilization of fatty acids by the exercising muscles,a decreased production of lactate after exercise,an improved storage and utilization of glycogen,as well as the prevention of oxidative stress after intense muscular workload.These effects predictably translate into enhanced aerobic endurance and physical work potential,improved functional heart activity,ameliorated recovery after maximal and sub-maximal loads of exercise,and enhanced activation of central nervous system functions(Table 1).9

Table 1 Meldonium in sports.
5.Meldonium is misused by athletes
According to its manifold biological functions,meldonium has been included in the list of prohibited substances since 2016 by the World Anti-Doping Agency(WADA).10An assay for urine assessment of this compound has also been recently published,9based on the use of hydrophilic interaction liquid chromatography—high resolution/high accuracy mass spectrometry.The method showed adequate lower limit of detection, excellent specificit and robustness,optimal linearity and low imprecision,which made it suitable to be included in the already long list of anti-doping tests currently performed by many national and international organizations,including WADA.
Not surprisingly,the number of positive anti-doping tests for this banned compound has exponentially increased over the past few months,involving many top-class athletes(over 170 as of April 14,2016).11This evidence reflect data published in a recent study,showing that 182 positive meldonium finding were detected out of 8320 random doping control urine samples covering different sports(i.e.,2.2%),with drug concentrations comprised between 0.1 and 1428 μg/mL.9Interestingly,positive samples were found in a wide range of sport disciplines, thus suggesting that the misuse of this metabolic modulator may not be limited to certain categories of athletes.
6.Concluding remarks
In the long history of doping in sports,competitive athletes have searched for illegal advantages by misusing various modulators of aerobic performance,such as erythropoiesis stimulating substances(e.g.,erythropoietin and analogues),blood transfusions,and also metabolic modulators,such as hypoxiainducible factor 1(HIF-1)and cobalt chloride.12Meldonium is currently claimed to be a relatively safe agent in patientsneeding anti-ischaemic therapy,but there is no compelling evidence that it may be effective in improving athletic performance,nor that its administration may be safe in healthy subjects.Therefore,action should be taken to prevent the misuse of meldonium and to identify athletes who may jeopardize their health by attempting to artificialy enhance performance.
Authors’contributions
GL drafted the manuscript;CM helped draft the manuscript. Both authors have read and approved the f nal version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
Neither of the authors declare competing financia interests.
1.Sahartova O,Shatz V,Kalvins I.HPLC analysis of mildronate and its analogues in plasma.J Pharm Biomed Anal 1993;11:1045–7.
2.Vaz FM,Wanders RJ.Carnitine biosynthesis in mammals.Biochem J 2002;361:417–29.
3.Dambrova M,Makrecka-Kuka M,Vilskersts R,Makarova E,Kuka J, Liepinsh E,et al.Pharmacological effects of meldonium:biochemical mechanisms and biomarkers of cardiometabolic activity.Pharmacol Res 2016;doi:10.1016/j.phrs.2016.01.019[Epub ahead of print].
4.Vasiuk I,Iushchuk EN,Shkol’nik EL,Khadzegova AB,Sadulaeva IA, Vit’ko NK,et al.Comparative trial of effica y of trimethasidine MB and 3-(2,2,2-trimethylhydrasine)propionate dihydrate in chronic heart failure. Ter Arkh 2007;79:51–8.
5.Zhu Y,Zhang G,Zhao J,Li D,Yan X,Liu J,et al.Effica y and safety of mildronate for acute ischemic stroke:a randomized,double-blind, active-controlled phase II multicenter trial.Clin Drug Investig 2013;33: 755–60.
6.Statsenko ME,Shilina NN,Turkina SV.Use of meldonium in the combination treatment of patients with heart failure in the early postinfarction period.Ter Arkh 2014;86:30–5.
7.Smirnova MD,Svirida ON,Vitsenia MV,Kuzmina AE,Lankin VZ, Tikhaze AK,et al.Using meldonium to improve the adaptation of patients with cardiovascular disease to the effects of heat and correction of associated oxidative stress.Kardiologiia 2014;54:53–9.
8.Common mildronate side effects.Available at:http://patientsville.com/ medication/mildronate_side_effects.htm;[accessed 14.06.2016].
9.Görgens C,Guddat S,Dib J,Geyer H,Schänzer W,Thevis M.Mildronate (Meldonium)in professional sports—monitoring doping control urine samples using hydrophilic interaction liquid chromatography—high resolution/high accuracy mass spectrometry.Drug TestAnal 2015;7:973–9.
10.Thevis M,Kuuranne T,Walpurgis K,Geyer H,Schänzer W.Annual banned-substance review:analytical approaches in human sports drug testing.Drug Test Anal 2016;8:7–29.
11.World Anti-Doping Agency.WADA statement on meldonium notice issued to stakeholders.Available at:https://www.wada-ama.org/en/media/news/ 2016-04/wada-statement-on-meldonium-notice-issued-to-stakeholders; [accessed 14.06.2016].
12.Lippi G,Franchini M,Salvagno GL,Guidi GC.Biochemistry,physiology, and complications of blood doping:facts and speculation.Crit Rev Clin Lab Sci 2006;43:349–91.
Received 3 June 2016;accepted 14 June 2016 Available online 24 June 2016
Peer review under responsibility of Shanghai University of Sport.
*Corresponding author.
E-mail address:giuseppe.lippi@univr.it(G.Lippi)
http://dx.doi.org/10.1016/j.jshs.2016.06.008
2095-2546/©2017 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Journal of Sport and Health Science2017年1期
Journal of Sport and Health Science2017年1期
- Journal of Sport and Health Science的其它文章
- Building a healthy China by enhancing physical activity: Priorities,challenges,and strategies
- Exercise is...?:A commentary response
- Exergaming:Hope for future physical activity?or blight on mankind?
- Running slow or running fast;that is the question: The merits of high-intensity interval training
- Fairness in Olympic sports:How can we control the increasing complexity of doping use in high performance sports?☆
- Fight fir with fire Promoting physical activity and health through active video games
