Correlating oxidative stress-related factors with bone metabolic markers in older adult male patients exhibiting degenerative osteoporosis in the high-altitude hypoxic area of China: study protocol for a non-randomized controlled trial
Jian-wen Ma, De-chun Li, Zhong-guo Zhang, Yu Li, Ying-bing Wang, Zhi-qiang Cao
1 Department of Bone Traumatology, Affiliated Hospital of Qinghai University, Xining, Qinghai Province, China
2 Department of Orthopedics, Qinghai Province Reserve Brigade Hospital, Xining, Qinghai Province, China
INTRODUCTION
In patients with osteoporosis, bone loss, bone microstructure destruction, and osteosis directly increase bone fragility,particularly in older adult individuals.1,2Residents from Qinghai Province of China, which is located in a highaltitude area, are exposed to a lifelong hypoxic environment.3Hypoxia has been reported to be an important factor that affects bone formation and regulates bone growth.4-10Clinical evidence suggests that hypoxia leads to difficulties in bone healing and decreased bone mineral density,and can result in osteoporosis, which increases the risk of chronic bone injury.11-15Therefore, hypoxia is an important risk factor for osteoporosis and it is very important to effectively prevent and treat osteoporosis in high-altitude hypoxic environments.
Oxidative stress-related hypoxia-inducible factors can induce abnormal expression of various factors including vascular endothelial growth factor (VEGF), insulin-like growth factor, and endothelin.16However, it remains unclear whether these factors in fluence changes in bone metabolic markers.
This study aimed to investigate the correlation between serum levels of oxidative stress-related factors and bone metabolic markers in older adult male patients with degenerative osteoporosis who reside in a high-altitude hypoxic area.
METHODS/DESIGN
Study design
A prospective, single-center, non-randomized controlled trial.
Study setting
Affiliated Hospital of Qinghai University of China.
Study participants
One hundred and twenty older adult male patients with degenerative osteoporosis who received treatment in the Affiliated Hospital of Qinghai University of China (osteoporosis group), and 120 healthy elderly males who concurrently received physical examination (control group) are included in this study.
Diagnostic criteria for osteoporosis group
Osteoporosis is defined as bone mineral density less than 30% of peak bone mineral density, as evaluated in males from the Qinghai province of China, and osteoporosiscaused pain lasting for over 3 weeks.17
Inclusion criteria for osteoporosis group
Patients with osteoporosis presenting with all of the following conditions will be considered for inclusion in this study∶
• Male
• Loading pain
• Aged between 60–70 years
• Unbearable spontaneous low back pain
• Able to sit up, turn body over, and walk with bearable pain
• Unable to sleep because of pain
Strengths and limitations
Strengths
• Participant selection characteristics
• Measurement of bone mineral density in three regions
• Complete collection of data
Limitations
• Small number of serum oxidative stress indicators
• Bone metabolic markers examined
• A small sample size
Exclusion criteria for osteoporosis group
Patients presenting with one or more of the following criteria will be rejected for this study∶
• Presence of vertebral compression fracture on X-ray film
• Definitive diagnosis of rheumatoid arthritis, osteomalacia,hyperthyroidism, hyperparathyroidism, or other bone metabolic disease
• External fixation with gypsum for repair of fracture, or within 6 months after removal of gypsum
• Pain-inducing disease such as lumbar disc herniation,deformed vertebral arthritis, lumbar spinal stenosis,vertebral spondylolisthesis, or ankylosing spondylitis
• Unable to judge for curative effects, provide disease condition, or unable to cooperate with treatment because of mental illness, senile dementia, or severe neurosis
• No daily life self-care ability or long-term bed rest
• Continuous administration of activated vitamin D preparations, calcitonin, corticosteroid hormone, androgen,estrogen or other hormonal preparation within 3 months prior to participation in this trial
• Administration of selective estrogen receptor modulator within 6 months prior to participation in this trial
• Severe kidney or liver disease, or history of cancer
Inclusion criteria for control group
Participants presenting with both of these conditions will be considered for inclusion in this study∶
• Healthy older adult males who have resided in a highaltitude hypoxic environment for over 10 years
• Good compliance
Exclusion criteria for control group
Participants presenting with one or more of the following conditions will be rejected for this study∶
• Hyperthyroidism, hypoparathyroidism, or other bone metabolic diseases
• Diabetes mellitus
• Kidney or liver disease
Withdrawal criteria for osteoporosis and control groups
Participants presenting with one or more of the following conditions will be withdrawn from this study∶
• Unable to follow program referral, or cannot comply with any of the terms of the study protocol
• Potential risks from imaging examinations
Expected total trial duration
Based on the number of patients with osteoporosis previously admitted to our hospital, a time period of 3 years is considered adequate for participant recruitment.
Recruitment
Assignment
A non-randomized grouping method is used. Osteoporosis and control groups (n= 120 participants/group) are designated to detect serum markers and bone mineral density.This is an open-label study, and blind grouping is not used.Study flow chart is shown in Figure 1.
Detection of serum markers
At 1 day after admission, 5 mL of peripheral venous blood will be collected in the morning and centrifuged. Supernatant is collected to harvest serum samples. Serum levels of hypoxia-inducible factor 1-alpha (HIF-1α), HIF-2α,osteocalcin, VEGF, and tartrate-resistant acid phosphatase 5b (TRACP 5b) are measured using an enzyme-linked immunosorbent assay (ELISA; USCN, Wuhan, China). ELISA reader is from Bio-Tek (EXL-200, Winooski, VT).
Outcome measures
Primary outcome measure
HIF-1α level:HIF-1α is a core regulator of hypoxia induction and repair of the intracellular oxygen environment.18-21Higher serum levels of HIF-1α indicate more severe hypoxia.
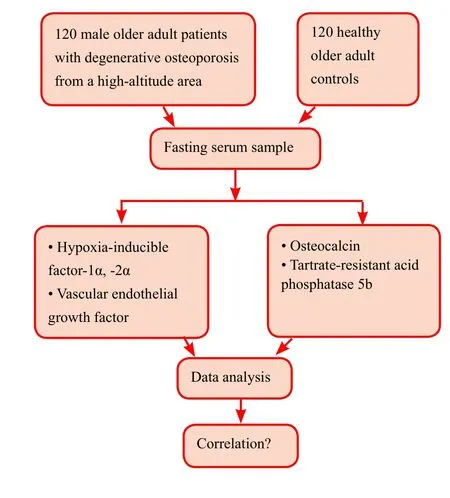
Figure 1: Study flow chart.
Secondary outcome measures
HIF-2α level:This hypoxia-inducible factor 1 subunit is used to evaluate the severity of body hypoxia.
VEGF level:Reflects the state of hypoxia and antagonizes hypoxia-induced apoptosis of vascular endothelial cells.22,23
Osteocalcin level:Changes with aging and bone metabolism rates; higher levels indicate increased bone metabolism rate, andvice versa.24,25
TRACP 5b level:Good marker of bone resorption and osteoclast activity under various physiological and pathological conditions.26,27
Correlation between serum levels of oxidative stress indicators and bone metabolic markers:A correlation analysis method is used to correlate serum levels of oxidative stress indicators (HIF-1α, HIF-2α, and VEGF) with bone metabolic markers (osteocalcin and TRACP 5b).
Audits
Initial stage
The study protocol should be approved by the Ethics Committee of the Affiliated Hospital of Qinghai University of China, and a protocol agreement should be signed. Researchers should fully understand the study protocol and clinical operation.
During the trial
During participant recruitment, regular audits are necessary to ensure included participants are eligible, the trial is being performed in strict accordance with the study protocol, and related data are complete.
Final stage
At the end of all trial procedures, a final visit is required to ensure relevant records are complete and accurate.
Statistical analysis
Statistical design, method, and analysis principle
All data are statistically analyzed using SPSS13.0 software(SPSS, Chicago, IL) and follow the intention-to-treat principle. Normally distributed measurement data are expressed as the mean ± SD. Non-normally distributed measurement data are expressed as lower quartile (q1), median, and upper quartile (q3). Two-samplet-test is used to evaluate normally distributed serum indicators and bone metabolic markers in the two groups. A Pearson correlation analysis is used.Mann-WhitneyUtest is used to evaluate non-abnormally distributed serum indicators and bone metabolic markers in the two groups. Spearman rank correlation is used to test the correlation between serum levels of oxidative stress indicators and bone metabolic markers.
Sample size
In accordance with our experience,27we hypothesized that the HIF-1α level in osteoporosis and control groups was 12 and 8 μg/L, respectively, with a standard deviation of 2 μg/L. Takingβ= 0.2, power = 80%,α= 0.05 (two-sided),the final effective sample size ofn= 199 per group was calculated. Assuming a participant loss rate of 20%, we require 239 participants per group. At the end of expected recruitment, at least 120 participants per group are included.
Inspection level
An α = 0.05 (two-sided) will be used for inspection level.
Estimated participant loss rate
The estimated participant loss rate is no more than 20%.
Processing method for missing data
If a participant’s records are lost, the participant is rejected from this study. Corresponding numbers of new cases are supplemented.
Baseline analysis
The baseline data of included participants are shown in Table 1.
Data management
Researchers complete the case report form for each case.After the completed case report form is reviewed by aninspector, data input and management are performed. After data transfer, contents recorded in the case report form are not modified.
Table 1: Patient’s baseline data
After the database is confirmed to be correct, data are audited by a data manager, the researchers in charge, and statisticians. Finally, the database is password-protected,after which the data are not altered.
Quality control of the clinical trial
Participating researchers must undergo unified training.Unified data recording methods and evaluation criteria are used. During the clinical trial, sponsor inspectors conduct regular periodic visits to the research center to ensure strict adherence to all aspects of the research program. In addition,the original data are checked to ensure the contents of the case report forms are correct and complete.
Ethical considerations and informed consent
This clinical trial follows the relevant laws and regulations of theDeclaration of Helsinki. This manuscript is prepared and modified according to SPIRIT guidelines (Additional file 1). The researchers take the responsibility of providing the independent ethics committee with the clinical trial protocol and informed consent forms, and providing patients with related informational materials. The trial cannot be initiated until approval from ethics committee is received.This study was approved by the Ethics Committee of the Affiliated Hospital of Qinghai University of China (approval No. QHY1402G).
RESULTS
Trial status
This trial was registered with the Chinese Clinical Trial Registration (registration number∶ ChiCTR-ROC-17012848).Participant recruitment and data analysis are expected to be completed by February 2018.
Preliminary experiments
Preliminary experiments have been concluded.28Detection of serum indicators and bone metabolic markers were detected in 60 older adult male patients with degenerative osteoporosis and 58 healthy controls. In the osteoporosis group, HIF-1α levels positively correlated with VEGF and osteocalcin levels (P< 0.05), but were negatively correlated with bone mineral density and TRACP 5b level (P< 0.05).In addition, VEGF levels were negatively correlated with bone mineral density (P< 0.05).
DISCUSSION
Significance of this study
Findings from this study intend to confirm that for older adult male patients with degenerative osteoporosis residing in high-altitude areas, monitoring of bone mineral density and detection of hypoxia-inducible factor should be applied in addition to treatment of primary osteoporosis.
Limitations of this study
Patient cohort sample size is small and random grouping is not used. Serum indicators and bone metabolic markers are relatively simple indicators. All of these influence the accuracy of results. In future studies, these factors will be improved.29-31
Evidence for contribution to future studies
This study aimed to investigate the correlation between oxidative stress-related factors and bone metabolic markers in older adult male patients with degenerative osteoporosis who reside in a high-altitude hypoxic area. Findings from this study intend to confirm that for older adult patients with degenerative osteoporosis residing in high-altitude hypoxic areas, providing oxygen to improve hypoxia-induced injury along with calcium supplementation and increased exercise to increase bone mineral density can effectively prevent and treat osteoporosis.
Author contributions
Conception and design of study protocol∶ JWM and DCL; conduction of study protocol∶ ZGZ, YL, YBW and ZQC; data collection∶JWM and DCL; trial evaluation∶ YL and ZQC.
Conflicts of interest
None declared.
Research ethics
The study protocol was approved by the Ethics Committee of Affiliated Hospital of Qinghai University of China (approval No. QHY1402G).The study followed international and national regulations in accordance with the Declaration of Helsinki and relevant ethical principles.
Declaration of participant consent
The authors certify that they will obtain participant consent forms.In the form, participants will give their consent for their images and other clinical information to be reported in the journal. Participants understand that their names and initials will not be published, and while due efforts will be made to conceal their identity, anonymity cannot be guaranteed.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Additional file
Additional file 1∶ SPIRIT checklist.
1. Force UPST. Screening for osteoporosis∶ us preventive ser vices task force recommendation statement.Ann Int Med.2011;154∶356.
2. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis∶ now and the future.Lancet377∶1276-1287.
3. Jiang C, Chen J, Liu F, et al. Chronic mountain sickness in chi nese han males who migrated to the qinghai-tibetan plateau∶application and evaluation of diagnostic criteria for chronic mountain sickness.BMC Public Health. 2014;14∶701.
4. Maes C,Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease.Nat Rev Rheumatol. 2012;8∶358-366.
5. Terrizzi AR, Fernandez-Solari J, Lee CM, Conti MI, Martínez MP. Deleterious effect of chronic continuous hypoxia on oral health.Arch Oral Biol. 2016;72∶1-7.
6. Li Q, Liu R, Zhao J, Lu Q. N-methyl pyrrolidone (NMP) amelio rates the hypoxia-reduced osteoblast differentiation via inhibiting the NF-κB signaling.J Toxicol Sci. 2016;41∶701-709.
7. Huang J, Peng J, Cao G, et al. Hypoxia-induced MicroRNA-429 Promotes Differentiation of MC3T3-E1 osteoblastic cells by mediating ZFPM2 expression.Cell Physiol Biochem.2016;39∶1177-1186.
8. Kim JH, Yoon SM, Song SU, et al. Hypoxia suppresses sponta neous mineralization and osteogenic differentiation of mesenchymal stem cells via IGFBP3 up-regulation.Int J Mol Sci.2016;17∶E1389.
9. Wang G, Wang J, Sun D, et al. Short-term hypoxia accelerates bone loss in ovariectomized rats by suppressing osteoblastogenesis but enhancing osteoclastogenesis.Med Sci Monit.2016;22∶2962-2971.
10. Gu Q, Gu Y, Shi Q, Yang H. Hypoxia promotes osteogenesis of human placental-derived mesenchymal stem cells.Tohoku J Exp Med. 2016;239∶287-296.
11. Peng J, Hui K, Hao C, et al. Low bone turnover and reduced an giogenesis in streptozotocin-induced osteoporotic mice.Connect Tissue Res. 2016;57∶277-289.
12. Tournis S, Dede AD, Savvidis C, Triantafyllopoulos IK, Kat tamis A, Papaioannou N. Effects of teriparatide retreatment in a patient with β-thalassemia major.Transfusion. 2016;55∶2905-2910.
13. Li W, Wang K, Liu Z, Ding W. HIF-1α change in serum and cal lus during fracture healing in ovariectomized mice.Int J Clin Exp Pathol. 2015;8∶117-126.
14. Liu X, Tu Y, Zhang L, Qi J, Ma T, Deng L. Prolyl hydroxy lase inhibitors protect from the bone loss in ovariectomy rats by increasing bone vascularity.Cell Biochem Biophys.2014;69∶141-149.
15. Miller RG, Segal JB, Ashar BH, et al. High prevalence and cor relates of low bone mineral density in young adults with sickle cell disease.Am J Hematol. 2006; 81∶236-241.
16. Wagegg M, Gaber T, Lohanatha FL, et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner.PLoS One. 2012;7∶e46483.
17. Li DC, Liu ZH, Shi SH, Li YY. A retrospective literature study of osteoporosis incidence based on -2.5SD criteria in mainland China. 2015;22∶1-8.
18. Li S, Li J, Dai W, et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death.Br J Cancer.2017. doi∶ 10.1038/bjc.2017.323.
19. Guo X, Chen Y, Fang W, Yang W, Shi L, Zhu R. Metastasis as sociated protein 1 correlates with Hypoxia inducible-factor 1 alpha expression and lymphangiogenesis in esophageal cancer.Thorac Cancer. 2013;4∶312-317.
20. Shrestha P, Davis DA, Veeranna RP, Carey RF, Viollet C, Yar choan R. Hypoxia-inducible factor-1 alpha as a therapeutic target for primary effusion lymphoma.PLoS Pathog. 2017. doi∶10.1371/journal.ppat.1006628.
21. Kling L, Benck U, Breedijk A, et al. Changes in CD73, CD39 and CD26 expression on T-lymphocytes of ANCA-associated vasculitis patients suggest impairment in adenosine generation and turn-over.Sci Rep. 2017. doi∶ 10.1038/s41598-017-12011-4.
22. Hall B, Zebrowska A, Kaminski T, Stanula A, Robins A. Ef fects of hypoxia during continuous and intermittent exercise on glycaemic control and selected markers of vascular function in type 1 diabetes.Exp Clin Endocrinol Diabetes. 2017. doi∶10.1055/s-0043-110482.
23. Wang L, Jin Z, Wang J, Chen S, Dai L, Lin D. Detrimental effect of Hypoxia-inducible factor-1α-induced autophagy on multiterritory perforator flap survival in rats.Sci Rep. 2017. doi∶10.1038/s41598-017-12034-x.
24. Bentkowski W, Kuźniewski M, Fedak D, et al. Undercarboxylat ed osteocalcin (Glu-OC) bone metabolism and vascular calcification in hemodialyzed patients.Przegl Lek.2013;70∶703-706.
25. Kim KJ, Kim KM, Park KH, et al. Aortic calcification and bone metabolism∶ the relationship between aortic calcification,BMD, vertebral fracture, 25-hydroxyvitamin D, and osteocal cin.Calcif Tissue Int. 2012;91∶370-378.
26. Wang R, Zhang S, Jiang Z, Tian J, Wang T, Song S. Bone metabolism markers∶ Indicators of loading dose intravenous ibandronate treatment for bone metastases from breast cancer.Clin Exp Pharmacol Physiol. 2017. doi∶ 10.1111/1440-1681.12673.
27. Yuan TL, Chen J, Tong YL, et al. Serum heme oxygenase-1 and BMP-7 are potential biomarkers for bone metabolism in patients with rheumatoid arthritis and ankylosing spondylitis.Biomed Res Int. 2016. doi∶ 10.1155/2016/7870925.
28. Li DC. The correlation among serum hypoxia-inducible factor-1α, hypoxia-inducible factor 2α, vascular endothelial growth factor, and bone mineral density and bone metabolism in senior men with osteoporosis in Qinghai-Tibetan high plateau.Zhongguo Guzhi Shusong Zazhi. 2015;21∶1328-1332.
29. Zhang H, Lei C, Zhang TY. Transcutaneous electrical acupoint stimulation with different acupoint combinations on opioid consumption in patients undergoing off-pump coronary artery bypass grafting∶ study protocol for a randomized double-blind controlled trial.Clin Transl Degener Dis. 2016;1∶17-24.
30. Wang Y. Chinese medicine packet plus wax therapy for periar thritis of the shoulder∶ study protocol for a multi-center randomized controlled trial.Clin Transl Degener Dis. 2016;1∶25-31.
31. Ma N, Peng J, Guo QY. Local administration of enriched mono nuclear cells, platelets and zoledronic acid for preventing collapse of the femoral head in the early stage of osteonecrosis∶study protocol for a prospective randomized parallel-controlled clinical trial.Clin Transl Degener Dis. 2016;1∶32-37.
 Clinical Trials in Degenerative Diseases2017年3期
Clinical Trials in Degenerative Diseases2017年3期
- Clinical Trials in Degenerative Diseases的其它文章
- An update on clinical trials targeting human tauopathies
- Change in left ventricular global longitudinal peak strain for early diagnosis of high-risk coronary atherosclerotic heart disease in older adult patients: study protocol for a singlecenter diagnostic trial
- Effect of a long-term modified Tai Chi-based intervention in attenuating bone mineral density in postmenopausal women in southeast China: study protocol for a randomized controlled trial
