孕期不良环境所致的子代多种疾病易感及其宫内编程机制
汪 晖,焦哲潇
(1.武汉大学基础医学院药理学系,湖北武汉 430071;2.发育源性疾病湖北省重点实验室,湖北武汉 430071)
孕期不良环境所致的子代多种疾病易感及其宫内编程机制
汪 晖1,2,焦哲潇1,2
(1.武汉大学基础医学院药理学系,湖北武汉 430071;2.发育源性疾病湖北省重点实验室,湖北武汉 430071)
汪 晖,博士,二级教授,博士生导师。现任武汉大学基础医学院药理学系主任,兼湖北省发育源性疾病湖北省重点实验室主任。研究方向为外源物发育毒性。主持国家自然科学基金重点及重点国际合作项目3项、教育部科学研究重大项目等。首次在国际上系统展现了孕期外源物暴露下糖皮质激素编程胎儿疾病易感的“宫内神经内分泌代谢编程机制”,为解析国际前沿问题“健康与疾病的发育起源”,探寻胎源性疾病的早期防治策略,提供了重要的研究依据。2012年成功承办了国家基金委“孕期不良环境与胎源性疾病”学科发展战略研讨会。发表系列SCI论文62篇。
流行病学调查提示,孕期不良环境可引起子代低出生体重及其成年后多种慢性疾病的易感性增加,如代谢性疾病和神经精神性疾病等。然而,其发生机制尚未见系统的阐明。下丘脑-垂体-肾上腺(HPA)轴是机体应激相关的重要神经内分泌轴,在出生前、后的应激防御应答中发挥着重要的作用,也是宫内时期胎儿易受损伤的重要靶位。研究发现,孕期多种不良环境因素(包括外源环境和母体健康因素)可通过母体-胎盘-胎儿生物学单位,多途径地影响宫内胎儿发育,造成其出生后HPA轴发育编程改变及成年后多种慢性疾病易感。本综述结合本实验室的最新研究结果,综述了国际上有关孕期不良环境所致子代成年疾病易感的病因学和宫内编程机制的最新进展,提出宫内母源性糖皮质激素过暴露可引起子代宫内神经内分泌代谢编程改变,其核心是多器官糖皮质激素-胰岛素样生长因子1轴编程,表观遗传修饰异常参与其编程过程。
孕期不良环境;代谢综合征;下丘脑-垂体-肾上腺轴;表观遗传修饰;宫内编程
早在20世纪90年代初,英国学者David Barker基于大规模流行病学调查结果,提出低出生体重患儿成年后高血压、糖尿病的发病率增加和“成人疾病胎儿起源”假说。近10年来,国内外学者开展了大量有关孕期不良环境、胎儿出生体重与成年慢性疾病之间的相关性研究,并基于循证研究的结果,提出人类疾病起源的新概念——“健康与疾病的发育起源”(developmental origins of health and dis⁃ease,DOHaD)[1]。孕期不良环境所致子代低出生体重及成年多种慢性疾病易感的宫内起源机制假说中,至今最为认可的还是下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal,HPA)轴介导的“宫内内分泌发育编程”假说[2-3]。本文结合本实验室近10年系列研究结果,综述了国际上有关孕期不良环境所致子代成年疾病易感的病因学及宫内编程机制研究的最新进展,提出母源性糖皮质激素(glucocorticoid,GC)过暴露所致子代慢性疾病易感的“宫内神经内分泌代谢编程”机制,并指出其核心是多组织器官发育相关的“GC-胰岛素样生长因子1(GS-insulin-like growth factor 1,GC-IGF1)轴”编程,其中表观遗传修饰参与此编程过程。
1 孕期不良环境及其危害
孕期不良环境可导致多种不良妊娠结局发生,甚至会增加子代成年后多种慢性疾病易感。孕期不良环境除了先天遗传因素外,很大程度上是因为孕期宫内环境欠佳所致,宫内环境主要包括外源暴露环境和母体健康环境。
1.1 不良妊娠结局及其远期危害
孕期不良环境可引起多种不良妊娠结局,主要包括流产、死产、畸形、早产和生长迟缓等[4-6]。宫内生长迟缓(intrauterine growth retardation,IUGR)是指宫内发育时期,胎儿的正常生长态势受到阻滞,应有的生长潜能削弱,影响其特定的组织结构和功能发育。IUGR为常见的发育毒性之一,主要表现为低出生体质质量。大量流行病学调查表明,IUGR不仅可引起胎儿窘迫、新生儿窒息和围产儿死亡,其危害还将延续至出生后,导致子代出生后体格和智力发育低下,成年后多种慢性疾病的易感性增加,包括代谢性疾病和神经精神性疾病[7-10]。例如,流行病学和临床研究已经在不同国家、不同种族人群中证实了低出生体质量或IUGR与成人代谢综合征(metabolic syndrome,MS)之间的相关性[11]。传统观点认为,糖尿病的病因与成年后环境和不良生活习惯有关,但近20年来的流行病学调查显示,糖尿病与胎儿宫内发育的不良因素暴露密切相关[12]。流行病学调查还表明,低出生体质量可影响骨生长发育并持续到成年;成年骨质疏松的发生存在宫内发育编程[13-15]。本室前期研究及流行病学调查也提示,孕期不良宫内环境与子代罹患抑郁症风险增加密切相关[10,16-19]。有数据显示,肾小球硬化的发生与低出生体质量有关[20-21]。大量的动物实验也已证实,宫内不良环境所致的IUGR子代成年后多种疾病的易感性增加,包括非酒精性脂肪肝(non-alcoholic fatty liver,NAFL)[22]、糖尿病[23]和心血管疾病[24]等。
1.2 孕期不良外源环境和母体环境
孕期外源环境主要包括外源物暴露和微生物感染等。已知孕期外源物暴露是引起宫内有害环境及不良妊娠结局的最确切和危险的诱因之一。已证实能引起不良妊娠结局的外源物主要有:①环境毒物类。如有机挥发物[6]、重金属[25]、烟和空气污染物[26]等。Walfisch等[27]对约7000名孕妇的调查研究显示,40%的产妇在怀孕期间有主动或被动吸烟,且胎儿出生体质量和头围降低均与吸烟相关。吸烟还可导致胎儿出生缺陷风险增加[28]。流行病学调查显示[29],孕期暴露于三氯乙烯可导致不良妊娠结局,包括早产、低出生体质量和出生缺陷。②药物类。如地塞米松[30]、咖啡因[31]和可卡因[32]等。有学者调查指出,孕期给予倍他米松或地塞米松均会导致胎儿出生体质量降低[30]。孕期咖啡因摄入过多可引起胎儿生殖及发育毒性,如早产、自然流产、生长迟缓及先天畸形等[33]。③食品及饮料类。如酒[5]、咖啡和茶[34]等。通过对7141名孕妇的调查研究显示,孕早、晚期饮酒均可导致胎儿出生体质量降低或早产[35]。流行病学调查显示,孕期摄入咖啡和茶也可导致胎儿出生体质量降低和骨骼发育迟缓[34]。孕期HIV感染也被证实是影响胎儿正常发育的重要因素之一[36]。
孕期母体环境主要指母体的营养状况和疾病状态。产妇的饮食可以通过营养物质的量直接影响胎儿的生长发育,也可通过胎儿内分泌系统间接对其产生影响。三大营养素包括糖类、蛋白质和脂肪在孕期缺乏均可导致不良妊娠结局的发生[37-38]。孕期不良环境也可通过引起母体的生理与病理变化而影响胎儿发育。已有文献报道,孕期母体急、慢性应激均可影响子代HPA轴发育,造成其成年个体HPA轴功能异常及行为学改变[39-40]。我们前期及其他实验室研究也表明,多种外源物作为应激因子(如咖啡因、尼古丁、乙醇和地塞米松)可改变孕鼠的应激状态,使孕鼠肾上腺甾体合成功能发生变化[41-44],包括增加皮质醇和儿茶酚胺的分泌和释放[45],从而加重机体的应激状态。母体肝是外源物代谢的主要器官,其代谢功能改变对其自身药物利用的有效程度及胎儿的生长发育起着不可缺少的作用。研究发现,在健康成人咖啡因半衰期为4~5 h,在孕早期可达10 h,孕晚期延长至18 h[34];母体肝的疾病也会明显地延长咖啡因的半衰期[46]。我们前期研究也发现,烟雾暴露的孕鼠肝抗氧化功能发生改变[47]。作为IUGR发生的明确诱因,吸烟可通过减弱母体内源性抗氧化物质(如维生素C、维生素E和谷胱甘肽)的生成[48],影响机体内生物大分子的代谢和胎儿发育的内环境。
1.3 孕期不良环境所致母源性GC过暴露
已知宫内基础GC(在人为皮质醇、啮齿动物为皮质酮)的水平是调节胎儿组织形态和功能成熟的关键,但过高GC浓度的暴露可引起胎儿发育异常(如IUGR)[49]。胎盘在整个孕期承担了重要的代谢与排泄功能,是维系胎儿正常发育的重要器官。胎盘上2型11β-羟类固醇脱氢酶(11β-hydroxyster⁃oid dehydrogenase 2,11β-HSD2)可氧化灭活过多的母源性GC,保护胎儿免受母体GC干扰[50]。人群和啮齿类动物研究表明,胎盘11β-HSD2活性易受到孕期多种不良环境(如外源物、饮食、感染、低氧和应激)的影响,导致发育中胎儿接触过多的母源性GC[51-55]。临床研究和动物实验表明,母体连续使用促肾上腺皮质激素(adrenocorticotropic hormone,ACTH)和人工合成GC(如地塞米松和倍他米松)能使胎儿GC暴露增多,导致出生低体质量和多器官发育不良[56]。本室也通过系列动物实验证实,孕期咖啡因、尼古丁和乙醇暴露均可通过应激反应,升高母体GC水平并开放胎盘GC屏障,导致胎儿过暴露于母源性GC[42-43,57-58]。另外有研究报道,妊娠期母亲在营养限制、情感障碍(抑郁或焦虑)或子痫等情况下,均可导致胎儿暴露于母体高GC水平[59-60]。这些研究均提示,孕期不良环境所致的胎儿发育毒性皆伴有母源性GC过暴露,而这很可能是孕期不良环境所致子代IUGR发生的启动因素[61]。
2 HPA轴相关宫内神经内分泌代谢编程
虽然大量的流行病学证据提示,孕期不良环境可引起子代IUGR及成年多种慢性疾病的易感,然而有关宫内胎儿发育的研究报道甚少,也缺乏系统的理论体系去解析这一普遍现象。已知宫内环境对生命发育过程具有持久、决定性的影响。“宫内编程(intrauterine programming)”是指宫内发育时期遭受的损伤导致组织结构与功能永久改变的过程[62]。目前最为认可的机制是“宫内内分泌发育编程”假说[63]。我们基于孕期外源物暴露和摄食限制所致的大鼠IUGR模型,进一步提出“宫内神经内分泌代谢编程”机制,并指出其核心可能是“GC-IGF1轴”编程。
2.1 HPA轴低基础活性和高应激敏感性编程
已知HPA轴是机体应激反应的重要神经内分泌轴,也是宫内时期易受损伤的重要靶位[64]。越来越多的学者认识到,HPA轴编程改变是介导MS胎儿起源的最可能机制[65-66],并与成年多种慢性疾病的易感性增加有关[2-3,67]。已有研究表明,孕期不良环境可通过影响胎儿HPA轴及其高位调节中枢(如海马)发育,导致子代出生后HPA轴功能编程改变,主要表现为HPA轴低基础活性和高应激敏感性编程[68]。
2.1.1 HPA轴低基础活性编程
临床研究和动物实验证实,不良宫内环境易使胎儿HPA轴发育编程改变,主要表现为出生后HPA轴低基础活性变化[40,69]。肾上腺作为HPA轴的终末效应器官,其分泌的GC对维持妊娠、促进胎儿生长和神经系统发育有着重要意义。胎肾上腺也可通过自身的甾体合成功能来调节其宫内内环境稳态和发育成熟[70],因此宫内时期肾上腺的正常发育及基础GC水平是胎儿成熟的关键[71]。本室研究发现,胎儿发育时期肾上腺已具备合成多种甾体激素的能力[72];孕期外源物(如尼古丁、地塞米松、乙醇和咖啡因)暴露下胎肾上腺功能发育明显受损,其甾体合成酶包括甾体合成急性调节蛋白(ste⁃roidogenic acute regulatory protein,StAR)和胆固醇侧链裂解酶(P450 cholesterol side chain cleavage,P450scc)的表达均降低[41-43,57];进一步发现,孕期咖啡因和尼古丁暴露通过调节多个甾体合成酶系统的转录激活因子类固醇生成转录因子(steroidogenic factor 1,SF1)启动子区甲基化和组蛋白乙酰化修饰,抑制SF1及甾体合成酶系统表达,从而减少胎肾上腺GC的合成[73-74]。我们还发现,孕期咖啡因、尼古丁和乙醇暴露所致的胎鼠肾上腺甾体合成功能降低具有宫内编程效应,还能延续到出生后甚至成年,表现为低基础活性[19,75-76],其发生机制主要与宫内母源性高GC所致胎肾上腺“GC-IGF1轴”编程有关[75-76]。
2.1.2 HPA轴高应激敏感性编程
应激反应的敏感性改变是HPA轴功能异常的主要表现之一。越来越多的研究提示,孕期不良环境可编程性地改变子代HPA轴应激敏感性[77]。然而,其宫内发生机制并不清楚。已知HPA轴的上游活性主要表现在下丘脑的室旁核(paraventricular nucleus,PVN)区神经内分泌小细胞(parvocellu⁃lar neuroendocrine,PNC)活性上。当机体受到应激刺激时,下丘脑PNC分泌促肾上腺皮质激素释放激素(corticotropin-releasing hormone,CRH)和精氨酸加压素(arginine vasopressin,AVP),以启动机体的应激反应。CRH和AVP迅速刺激垂体的ACTH分泌,并进一步促使肾上腺分泌GC。本室前期研究发现[44,78-79],孕期多种外源物(如咖啡因、尼古丁和乙醇)暴露的成年子代ACTH和皮质酮在基础状态下降低,但给予慢性应激后却显著高于对照组,即应激后血ACTH和CORT变化率增加。提示,这些子代存在HPA轴高应激敏感性。有趣的是,在孕期乙醇暴露所致的子代HPA轴宫内编程过程中,无论是在宫内HPA轴功能发育抑制状态下,还是在出生后HPA轴低基础活性和高应激敏感性下,下丘脑PVN区均存在局部兴奋性潜能增加,表现为兴奋性递质谷氨酸转运体—囊泡谷氨酸转运体(glutamate transporter,VGluT)表达升高,而抑制性递质谷氨酸脱羧酶(glutamic acid decarboxyl⁃ase,GAD)表达降低,致使VGluT/GAD表达比增加[80]。提示,这些外源物孕期暴露可永久性改变子代下丘脑PVN的调定点与敏感度,引起子代HPA轴高应激敏感性变化。
已知下丘脑PVN区的活性和功能状态主要受海马和杏仁核的调控。其中,海马不仅可抑制HPA轴的应激反应,还可促进应激状态下亢进的HPA轴恢复到基础水平[81]。海马中GC受体(GC receptor,GR),盐皮质激素受体(mineralocorticoid receptor,MR)均有大量分布。MR对GC的亲和力是GR对GC亲和力的10倍以上[82],当机体内GC水平较低时,海马局部GC几乎全部与MR结合,以调控HPA轴的基础活性,使GR处于空载状态;而当机体内GC水平升高,使得MR处于饱和状态时,GC与海马GR结合,进一步通过Glu-GABA突触联系介导途径,抑制PVN区的CRH神经元,从而抑制HPA轴的过度活化[83]。已知海马GR是对GC最易感和易损的神经靶位,过量的GC可通过过度活化海马GR介导局部神经元的损伤改变[84]。此外,MR和GR的表达失衡也参与介导了HPA轴的功能紊乱。已有研究证实,海马区域MR/GR表达比的降低,可使海马在应激状态下对HPA轴的负反馈调节能力减弱,这可能是HPA轴高应激敏感性发生的主要原因[85]。综上,孕期不良环境所致的宫内母源性高GC可过度活化海马GR,引起神经元功能发育异常,进而影响海马对HPA轴的负反馈调控作用,致使下丘脑局部的兴奋性潜能增加;这些改变可延续到出生后,表现为慢性应激下出现的HPA轴高敏感性。其中,海马结构损伤和调控功能的编程异常可能是介导子代HPA轴高应激敏感性的重要机制[80]。
2.2 GC-IGF1轴宫内编程
2.2.1 胎儿发育与GC-IGF1轴编程
IGF1信号通路是机体内分泌调节系统的核心,参与调控宫内时期各组织和器官的分化、发育及代谢等过程[86-87]。IGF1与其受体IGF1R结合后,一方面磷酸化MEK/ERK,启动MAPK调节细胞增殖和凋亡,另一方面磷酸化PI3K/Akt,通过固醇调节元件结合蛋白-1c(sterol regulatory element binding protein-1c,SREBP1c)等转录因子,调节细胞糖、脂代谢功能。研究发现,从着床开始,几乎所有胚胎组织即可检测到IGF1的表达[88]。宫内时期,IGF1主要来自于肝,途经血液调控全身组织和器官发育,而组织器官局部的IGF1自分泌或旁分泌调控机制则是在胚胎发育中、晚期才初步建立,并在出生后逐步完善。已证实,肝IGF1或IGF1R敲除可显著降低胎儿体质量和体长[89-90]。因此,宫内时期肝IGF1水平决定了胎儿的出生体质量、器官结构与功能发育状况[86-88]。出生后,肝IGF1表达持续升高,到青春期达到高峰,之后维持一定水平,到老年期逐渐下降,这与机体的发育和成熟相吻合[88,91]。研究表明,胚胎发育时期IGF1是诱导干细胞(包括胚胎干细胞和间充质干细胞)富集和功能分化的重要因子,在器官发生和结构分化中起着重要作用[92-94]。因此,IUGR患儿的低IGF1状态可能诱导器官发生、发育障碍(如干细胞储备不足),而青春期肝IGF1表达过度激活又是IUGR子代出生后在营养条件好的情况下出现追赶性生长的主要因素,而追赶性生长又可进一步加重组织器官功能异常及糖脂代谢紊乱(如干细胞池耗竭)[95-98]。
至今,宫内时期肝组织IGF1表达调控机制尚未阐明。已发现,GC是调节胎儿生长发育的关键因子,广泛应用于早产儿的“催熟”[99-100]。大量研究发现,孕期不良环境所致的IUGR胎儿存在母源性GC过暴露、肝低IGF1表达的现象,且伴随以肝为核心的全身性糖、脂代谢紊乱[22,79,98,101-104]。大量研究表明,GC可抑制多种组织或细胞内IGF1表达,并引起机体糖脂代谢功能增强、全身脂肪分布异常[105-106]。我们发现,孕期咖啡因或乙醇暴露的胎鼠和哺乳期仔鼠,血皮质酮水平显著升高的同时,血和多种组织(如肝、肾上腺)的IGF1水平显著降低;断奶后直至成年的仔鼠血皮质酮水平逐步降低的同时,血和多种组织(如肝、肾上腺)IGF1水平却逐步升高;断奶后如给予高脂饮食,引起子代血皮质酮水平进一步降低的同时,血和组织(肝、肾上腺)IGF1水平进一步升高。这种血GC和多组织IGF1水平之间的良好负相关,提示宫内母源性高GC编程了胎儿多组织IGF1及其下游信号通路的功能变化(即所谓“GC-IGF1轴”编程)[22,75-76,98]。
基于上述,我们在国际上首次提出孕期不良环境所致子代IUGR及成年代谢性疾病易感的“GCIGF1轴”编程机制(图1):即孕期不良环境所致的母源性高GC暴露可通过负向调节IGF1表达及下游信号通路,诱导多组织器官的结构与功能发育呈GC依赖性编程改变,主要表现为宫内IUGR发生和出生后体质量追赶性生长,并因此造成各脏器功能发育异常及全身糖脂代谢功能紊乱。
2.2.2 GC-IGF1轴编程介导多组织器官发育
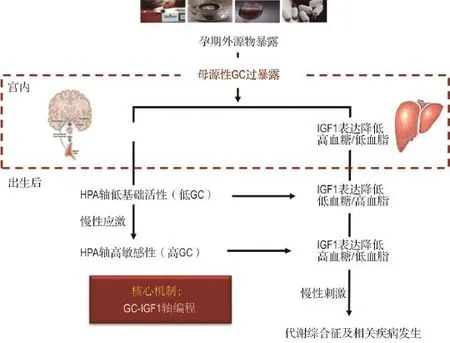
图1 孕期外源物暴露所致的子代宫内糖皮质激素-胰岛素样生长因子1(GC-IGF1)轴编程机制.HPA:下丘脑-垂体-肾上腺;GC:糖皮质激素.
已有大量研究证实,孕期不良环境可导致IUGR个体多组织结构与功能发育异[72,100,107-108]。“节俭表型”是指由于胎儿时期个体对不良宫内环境的调节与适应,引起机体的组织结构、功能和代谢等改变,以适应于不良环境的表型变化[109]。该假说认为,胎儿在发育过程中,当遇到不利生长环境时,为了维持其生存和发育,胎儿会改变自身的新陈代谢过程,将有限的能量进行重新分配,限制次要器官(如骨、肾、性腺)的能量消耗,以确保重要器官(如脑和肝)的发育,即胎儿变得“节俭”,这种改变会持续很长时间,甚至是永久性的[110]。如果出生后营养供应高于出生前的预期,子代将出现早期的生长加速和后期的脂肪堆积。子代宫内的不良生长加之出生后早期快速的追赶性生长,使得多脏器发育毒性及其相关疾病的风险增大[111]。
肾上腺和肝是GC-IGF1轴相关的两个重要器官。肾上腺作为HPA轴的终末器官,也是GC合成和分泌的主要器官。本室研究发现,孕期乙醇或咖啡因暴露下的肾上腺甾体合成功能降低,与母源性高GC激活肾上腺组织局部GC活化系统(包括11β-HSD,GR和C/EBPa)而抑制IGF1及其下游信号通路功能有关,即存在肾上腺“GC-IGF1轴编程”改变;这种“GC-IGF1轴编程”也可导致出生时母体GC撤离后的肾上腺GC活化系统灭活而增强IGF1信号通路功能,致使肾上腺功能(如GC合成功能)逐渐增强甚至超过正常,后者可增加MS及其相关代谢性疾病的易感性[75-76]。肝是机体最大、最重要的代谢器官之一,IGF1、胰岛素、瘦素和脂联素等代谢性激素均可与肝细胞膜上相关受体结合,通过各级信号传导机制而调控糖脂代谢功能[91,112]。肝是胎儿血循环中IGF1的主要来源[113]。宫内时期生长激素-IGF1轴还未建立,肝IGF1水平并非由生长激素所调控[114],可能主要受血GC的调控,GC可抑制肝IGF1的表达及分泌[115]。我们前期研究发现,孕期外源物暴露可导致IUGR胎鼠肝脏GC-IGF1轴发生改变,表现为血GC升高而血IGF1降低,可能与高GC抑制肝IGF1的表达及分泌有关;出生后脱离了母源性高GC环境的抑制,子代IGF1呈现逆转性升高[22,98],表现为肝IGF1表达及分泌的升高。因此,肝GC-IGF1轴的建立对胎儿的生长、发育及成熟具有关键作用,也是IUGR子代出现体质量追赶性生长的主要原因。
3 宫内神经内分泌代谢编程与表观遗传修饰
表观遗传是指DNA序列不发生变化,但基因表达却发生了可遗传的改变[116]。表观遗传修饰主要形式有DNA甲基化、组蛋白修饰和非编码RNA。表观遗传修饰存在于正常胚胎或胎儿发育过程中[117],且对外源环境因素(如外源物)敏感[118]。异常的表观遗传修饰可引起脂肪肝、高血压和糖尿病等疾病[102,119-121]。研究发现,环境与遗传互作相关疾病(如IUGR和MS)存在表观遗传修饰异常[122]。
3.1 宫内神经内分泌代谢编程及其多代遗传效应
研究已证明,宫内不良环境可致子代HPA轴功能发育受损,且可持续至下一代[123-124]。流行病学研究显示,在1944-1945年荷兰大饥荒期间出生的女性生育子代较正常女性子代身材矮小、健康状况欠佳,而男性生育的子代发生肥胖和慢性代谢性疾病的概率增大[8-9]。在动物模型上也观察到了孕期不良因素致子代糖代谢异常的可遗传现象。如孕期营养受限或高脂饮食会影响大鼠子代(F1代)的胰岛素敏感性和胰岛β细胞功能。即便子代出生后不良因素已去除,该影响仍会遗传至第二代(F2代),表现为β细胞数量减少、糖耐量降低等“糖尿病样反应”[125]。跨代遗传过程中存在亲源性和性别差异现象,可能与印记基因(如Igf-2)的表遗传修饰改变有关[126-127]。近期有研究发现,小鼠妊娠期糖尿病可导致F1和F2代糖代谢异常和Igf-2高甲基化,同时雄性F1代精子中Igf-2的表达减少[127];高GC作用下,GR与GC结合并被激活,激活的GC/GR复合体可与DNA甲基转移酶(DNA methyltransfer⁃ases,DNMT)启动子区的糖皮质激素反应元件结合,上调DNMT的表达[128]。提示,GC通过与GR结合,过度激活DNMT而引起IGF-2异常甲基化。我们的研究也发现[101],孕期咖啡因暴露所致的宫内神经内分泌代谢编程改变可持续至F2代,且F2代仍然对MS及相关代谢性疾病易感性较强,具体表现为慢性应激下的血糖升高和血甘油三酯(triglyceride,TG)降低,且TCH/HDL-C和LDL-C/HDL-C的比值升高。
3.2 宫内神经内分泌代谢编程的表观遗传机制
由于表现遗传的不稳定性表观遗传机制对宫内不良外源环境(如外源物)是非常敏感的[129]。已证实,孕期外源物暴露可改变胎儿表观遗传修饰模式,影响其生长发育[130]。尽管宫内不良环境暴露所致表观遗传修饰改变多有证实,但其潜在的发生机制仍尚未阐明,且是近年来的研究热点,尤其是HPA轴功能发育相关基因的表遗传修饰改变[131-132]。研究发现,孕期高水平GC暴露可致雄性子代器官表遗传改变[133],GC过暴露编程的靶点可能是胎海马的GR和MR[134]。我们的前期研究也发现,咖啡因可导致海马11β-HSD2的表遗传修饰及表达改变,从而增强GC所致的海马GR高表达变化[57]。我们通过整体和细胞实验证实,孕期外源物暴露(咖啡因、尼古丁)可通过下调胎肾上腺甾体合成酶系统(包括StAR/P450scc,3β-HSD,P450c21,P450c11)而抑制甾体合成功能,其发生机制与转录因子SF-1启动子区的表遗传修饰异常(组蛋白去乙酰化和(或)DNA甲基化)及表达降低有关[73-74]。Lillycrop等[135]发现,IUGR大鼠肝GR和过氧化体增殖物激活型受体α(peroxisome proliferator acti⁃vated receptor α,PPARα)启动子区也存在低甲基化,同时GR及其下游基因磷酸烯醇式丙酮酸羧激酶(phosphoenolpyruvate carboxkinase,PEPCK)、PPARα的表达升高,由此可增加IUGR子代成年后高血压和肝胰岛素抵抗的风险性。有学者发现,7周龄的IUGR大鼠出现了约1400个位点的甲基化改变,且主要集中于调控胰腺β细胞分化、胰岛素分泌、细胞凋亡等基因附近,提示DNA甲基化参与了糖尿病易感基因的表达调控,并增加了IUGR患者糖尿病的发病风险[136]。动物实验也发现,IUGR大鼠肝H3K9,H3K14和H3K18的乙酰化水平明显升高,这些差异出生后仍持续存在[137]。Wolfe等[138]认为,组蛋白去乙酰化酶可能也参与了IUGR大鼠的肝脂肪积聚,其肝组蛋白去乙酰化酶表达出生后持续降低,下游转录因子表达也相应改变,由此可能与肝脂肪变性有关。
4 胎源性疾病的发生机制
至今,孕期不良环境所致的子代成年后多种慢性疾病易感及其发生机制尚缺乏完整、系统的理论体系。越来越多的文献提示,GC参与的宫内编程改变可能增加了包括MS在内的一系列成年慢性疾病的易感性[2,70]。
4.1 胎源性疾病的“两种编程”和“两次打击”机制
近10年来,我们在孕期外源物(如咖啡因、尼古丁、乙醇、地塞米松)暴露所致的大鼠IUGR模型上,基于胎源性NAFL、糖尿病、肾小球硬化、骨质疏松症、骨关节炎等疾病宫内发生机制的研究进展,提出孕期外源物暴露所致成年子代慢性疾病发生的“两种编程”和“两次打击”机制(图2)。我们认为,孕期外源物暴露所致的母源性高GC水平作为“第一次打击”,可引起子代多种组织或器官功能的“两种编程”改变:“第一种编程”是各组织或器官功能的特征性改变(功能增强或降低),这种变化可从宫内一直延续到出生后甚至成年,与子代“节俭表型”编程有关;“第二种编程”为多种胎组织脏器的“GCIGF1轴”编程改变,这种编程可引起子代宫内低IGF1而出生后高IGF1水平改变,导致IUGR及出生后营养过剩状况下的追赶性生长,是IUGR个体面对不同生活环境而出现的整体适应性和代偿性变化。这两种编程相互作用,导致机体多组织或器官发育异常及成年后多种慢性疾病易感。而出生后的环境因素变化或不良生活习惯(如高脂饮食、慢性应激或过度运动)作为“第二次打击”,可加速或加重子代慢性疾病的发生。
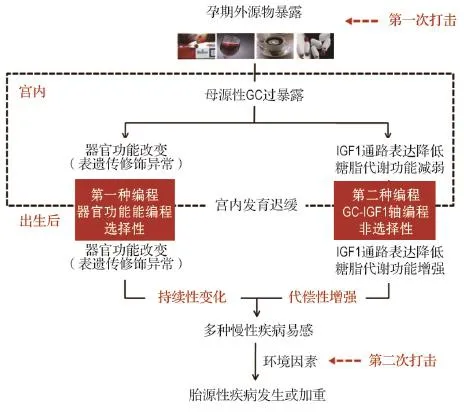
图2 孕期外源物暴露所致子代慢性疾病发生的“两种编程”和“两次打击”机制.
4.2 胎源性脂肪肝和骨关节炎及其发生机制
IUGR是胎儿面对宫内不良环境产生即刻适应反应的一个典型例子。IUGR患儿除了围生期并发症(如死亡)的发病率升高外,出生后多种慢性疾病(如代谢性疾病)的易感性升高,其发生机制与孕期不良环境所致子代宫内神经内分泌代谢编程改变有关[2-3]。
NAFL是一类肝组织学改变与酒精性肝病类似但无过量饮酒史的临床胎儿病理综合征。临床和动物实验证据提示,NAFL的发生与低出生体质量有关,NAFL存在发育起源[108,139]。Rueda-Clausen等[108]发现,高脂饮食下的IUGR大鼠,其代谢紊乱及脂代谢异常的易感性增加,不仅表现为腹内脂肪增多、脂肪细胞体积增大、血浆TG含量上升,而且肝TG积聚。TG的积聚和肝脂代谢的紊乱都会导致脂肪肝的易感性增加,甚至表明已经进入了单纯脂肪肝的阶段。研究表明,肝脂质从头合成在NAFL发生中起关键作用[140],并可能介导了胎源性NAFL的发生[107]。其过程主要受到脂质生成转录因子—SREBP1和脂质合成关键酶—脂肪酸合酶的调控[141]。此外,肝线粒体氧化及脂质输出障碍也参与了NAFL的进展[142-143]。我们在孕期外源物暴露和摄食限制[144]所致子代胎源性NAFL发生模型上,提出其“两种编程”和“两次打击”机制[22,98,145]:“第一种编程”为母源性高GC所致的子代胎肝细胞脂质从头合成功能增加,具体表现为脂质合成酶FASN、ACCa及其转录激活因子SREBP1c表达增加,而脂质输出酶MTTP表达降低,导致子代肝脂质合成功能增强并延续至成年;“第二种编程”为母源性高GC诱导子代“GC-IGF1轴编程”相关的糖、脂代谢功能变化,引起宫内低IGF1水平而出生后高IGF1水平改变,由此增强子代出生后全身性糖脂代谢功能,加速糖脂代谢紊乱及NAFL易感。出生后的高脂饮食作为“第二次打击”诱导或加重了NAFL的发生。
骨关节炎(osteoarthritis,OA)是一种以关节软骨退行性变为主要病理特征的慢性关节疾病。流行病学资料显示,低出生体重者成年后发生OA的比例明显较高[146-147]。提示,OA存在胎儿起源[148]。文献结果提示,关节软骨组织主要形成于胚胎时期,宫内关节软骨发育异常可能是成年OA易感的重要原因之一[149];软骨质量的高低与OA发生具有明显相关性[150-151]。本室研究证实,孕期外源物(如咖啡因、尼古丁和乙醇)暴露可引起胎鼠软骨IGF1信号通路及基质合成减少,这些功能变化可一直延续到出生后甚至成年,造成OA易感[102,152-153];进一步发现,IUGR子代出生后在高脂饮食下出现血胆固醇水平升高,导致关节软骨局部胆固醇蓄积,从而进一步降低关节软骨质量并诱发OA[154-156]。综上,孕期咖啡因暴露可引起子代OA易感性增加,并存在“两种编程”和“二次打击”机制:“第一种编程”为宫内高GC所致胎关节软骨IGF1低功能编程,导致软骨发育不良;“第二种编程”为肝“GC-IGF1轴编程”所致的成年子代高胆固醇血症,后者可增加软骨细胞局部的胆固醇蓄积。综上,这“两种编程”构成了对关节软骨的“第一次打击”,导致关节软骨质量低下及OA易感性增加,在成年“第二次打击”(如高脂饮食和过度运动)下,诱导或加重OA发生。
5 结语
综上所述,孕期不良环境可对子代产生深远的影响,包括出生后HPA轴低基础活性与高应激敏感性变化、成年后多种慢性疾病易感性增加。其发生机制可能主要与宫内神经内分泌代谢编程所致的重要基因/器官表观遗传修饰及功能发育异常有关。然而,胎儿宫内及出生后不同时期HPA轴功能变化的特点、具体机制、性别差异和跨代遗传的关键点均尚未完全阐明。随着孕期不良环境与胎源性疾病的研究深入,转化医学也在不断地推动胎源性疾病基础研究成果向临床实践或应用的转化[157]。胎源性疾病的转化研究是为了阐明疾病的胎儿起源机制,并寻找各种生物标志物;基于胎肾上腺功能改变的发育毒性早期评价系统,开展孕期有害环境因子评估;基于神经内分泌代谢编程改变的孕前和孕期风险评估技术,开展出生缺陷一级、二级预防;基于神经内分泌代谢编程改变的早期诊断技术,实现代谢性疾病早期防治;寻找引起胎盘GC屏障开放的因素,做到合理规避。另一方面,我们也可利用出生后早期的发育可塑性,给予药物干预逆转宫内不良环境编程(如出生后早期给予瘦素干预[158]);出生后要合理饮食,保证生长发育所需营养,但又避免追赶性生长。
[1]Uauy R,Kain J,Corvalan C.How can the devel⁃opmental origins of health and disease(DO⁃HaD)hypothesis contribute to improving health in developing countries?[J].Am J Clin Nutr,2011,94(6 Suppl):1759S-1764S.
[2]Reynolds RM.Corticosteroid-mediated program⁃ming and the pathogenesis of obesity and diabe⁃tes[J].J Steroid Biochem Mol Biol,2010,122(1-3):3-9.
[3]Zhang C,Xu D,Luo H,Lu J,Liu L,Ping J,et al. Prenatal xenobiotic exposure and intrauterine hypothalamus-pituitary-adrenal axis program⁃ming alteration[J].Toxicology,2014,325:74-84.
[4]RossnerP Jr, Tabashidze N, DostalM,Novakova Z,Chvatalova I,Spatova M,et al. Genetic,biochemical,and environmental fac⁃tors associated with pregnancy outcomes in new⁃borns from the czech republic[J].Environ Health Perspect,2011,119(2):265-271.
[5]Nykjaer C,Alwan NA,Greenwood DC,Simpson N A,Hay A W,White K L,et al.Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes:evidence from a British cohort[J]. J Epidemiol Community Health,2014,68(6):542-549.
[6]Longnecker MP,Klebanoff MA,Zhou H,Brock JW. Association between maternal serum concentra⁃tion of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth[J].Lan⁃cet,2001,358(9276):110-114.
[7]Nomura Y,Wickramaratne PJ,Pilowsky DJ,Newcorn JH,Bruder-Costello B,Davey C,et al. Low birth weight and risk of affective disorders and selected medical illness in offspring at high and low risk for depression[J].Compr Psychiatry,2007,48(5):470-478.
[8]Veenendaal MV,Painter RC,De Rooij SR,Bossuyt PM,Van Der Post JA,Gluckman PD,et al.Transgenerational effects of prenatal expo⁃sure to the 1944-45 Dutch famine[J].BJOG,2013,120(5):548-553.
[9]Painter RC,Osmond C,Gluckman P,Hanson M,PhillipsDI,Roseboom TJ.Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life[J]. BJOG,2008,115(10):1243-1249.
[10]O′connor MJ,Shah B,Whaley S,Cronin P,Gunderson B,Graham J.Psychiatric illness in a clinical sample of children with prenatal alcohol exposure[J].Am J Drug Alcohol Abuse,2002,28(4):743-754.
[11]Gupta M,Gupta R,Pareek A,Bhatia R,Kaul V. Low birth weight and insulin resistance in mid and late childhood[J].Indian Pediatr,2007,44(3):177-184.
[12] Iliadou A,Cnattingius S,Lichtenstein P.Low birth weight and type 2 diabetes:a study on 11 162 Swedish twins[J].Int J Epidemiol,2004,33(5):948-953.
[13]Dennison EM,Syddall HE,Sayer AA,Gilbody HJ,Cooper C.Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade:the Hertfordshire cohort study[J].Pediatr Res,2005,57(4):582-586.
[14]Sliwa E,Dobrowolski P,Piersiak T.Bone devel⁃opment of suckling piglets after prenatal,neona⁃tal or perinatal treatment with dexamethasone[J].J Anim Physiol Anim Nutr(Berl),2010,94(3):293-306.
[15]Yin J,Dwyer T,Riley M,Cochrane J,Jones G. The association between maternal diet during pregnancy and bone mass of the children at age 16[J].Eur J Clin Nutr,2010,64(2):131-137.
[16]O′reilly EJ,Mirzaei F,Forman MR,Ascherio A. Diethylstilbestrol exposure in utero and depres⁃sion in women[J].Am J Epidemiol,2010,171(8):876-882.
[17]Fujimoto T,Kubo K,Aou S.Prenatal exposure to bisphenol A impairs sexual differentiation of ex⁃ploratory behavior and increases depression-like behavior in rats[J].Brain Res,2006,1068(1):49-55.
[18]Hellemans KG,Verma P,Yoon E,Yu WK,Young AH,Weinberg J.Prenatal alcohol exposure and chronic mild stress differentially alter depres⁃sive-and anxiety-like behaviors in male and female offspring[J].Alcohol Clin Exp Res,2010,34(4):633-645.
[19]Liu L,Liu F,Kou H,Zhang BJ,Xu D,Chen B,et al.Prenatal nicotine exposure induced a hypo⁃thalamic-pituitary-adrenal axis-associated neuro⁃endocrine metabolic programmed alteration in in⁃trauterine growth retardation offspring rats[J]. Toxicol Lett,2012,214(3):307-313.
[20]Ikezumi Y,Suzuki T,Karasawa T,Yamada T,Hasegawa H,Nishimura H,et al.Low birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulo⁃sclerosis[J].Am J Nephrol,2013,38(2):149-157.
[21]Wei Z,Song L,Wei J,Chen T,Chen J,Lin Y,et al.Maternal exposure to di-(2-ethylhexyl)phthalate alters kidney development through the renin-angiotensin system in offspring[J].Toxicol Lett,2012,212(2):212-221.
[22]Wang L,Shen L,Ping J,Zhang L,Liu Z,Wu Y,et al.Intrauterine metabolic programming altera⁃tion increased susceptibility to non-alcoholic adult fatty liver disease in prenatal caffeine-exposed rat offspring[J].Toxicol Lett,2014,224(3):311-318.
[23]De Blasio MJ,Dodic M,Jefferies AJ,Moritz KM,Wintour EM,Owens JA.Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring[J]. Am J Physiol Endocrinol Metab,2007,293(1):E75-E82.
[24]Riviere G,Michaud A,Breton C,Vancamp G,Laborie C,Enache M,et al.Angiotensin-convert⁃ing enzyme 2(ACE2)and ACE activities display tissue-specific sensitivity to undernutrition-pro⁃grammed hypertension in the adult rat[J].Hyper⁃tension,2005,46(5):1169-1174.
[25]Jelliffe-Pawlowski LL,Miles SQ,Courtney JG,Materna B,Charlton V.Effect of magnitude and timing of maternal pregnancy blood lead(Pb)levels on birth outcomes[J].J Perinatol,2006,26(3):154-162.
[26] Shah PS,Balkhair T,Knowledge Synthesis Group on Determinants of Preterm/LBW births. Air pollution and birth outcomes:a systematic review[J].Environ Int,2011,37(2):498-516.
[27]Walfisch A,Nikolovski S,Talevska B,Hallak M. Fetal growth restriction and maternal smoking in the macedonian roma population:a causality dilemma[J].Arch Gynecol Obstet,2013,287(6):1131-1136.
[28]Yang W,Zeng L,Cheng Y,Chen Z,Wang X,Li X,et al.The effects of periconceptional risk factor exposure and micronutrient supplementa⁃tion on birth defects in Shaanxi Province in Western China[J].PLoS One,2012,7(12):e53429.
[29]Forand SP,Lewis-Michl EL,Gomez MI.Adverse birth outcomes and maternal exposure to trichlo⁃roethylene and tetrachloroethylene through soil vapor intrusion in New York State[J].Environ Health Perspect,2012,120(4):616-621.
[30]Bar-Lev MR, Maayan-Metzger A, Matok I,Heyman Z,Sivan E,Kuint J.Short-term outcomes in low birth weight infants following antenatal ex⁃posure to betamethasone versus dexamethasone[J].Obstet Gynecol,2004,104(3):484-488.
[31]Dobson NR,Patel RM,Smith PB,Kuehn DR,Clark J,Vyas-Read S,et al.Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants[J].J Pediatr,2014,164(5):992-998 e993.
[32]Singer LT,Salvator A,Arendt R,Minnes S,Farkas K,Kliegman R.Effects of cocaine/polydrug exposure and maternal psychological distress on infant birth outcomes[J].Neurotoxicol Teratol,2002,24(2):127-135.
[33]Brent RL,Christian MS,Diener RM.Evaluation of the reproductive and developmental risks of caffeine[J].Birth Defects Res B Dev Reprod Tox⁃icol,2011,92(2):152-187.
[34]Bakker R,Steegers EA,Obradov A,Raat H,Hofman A,Jaddoe VW.Maternal caffeine intake from coffee and tea,fetal growth,and the risks of adverse birth outcomes:the Generation R study[J].Am J Clin Nutr,2010,91(6):1691-1698.
[35]Jaddoe VW,Bakker R,Hofman A,Mackenbach JP,Moll HA,Steegers E A,et al.Moderate alco⁃hol consumption during pregnancy and the risk of low birth weight and preterm birth.The genera⁃tion R study[J].Ann Epidemiol,2007,17(10):834-840.
[36]Lopez M,Palacio M,Gonce A,Hernandez S,Barranco FJ,Garcia L,et al.Risk of intrauterine growth restriction among HIV-infected pregnant women:a cohort study[J].Eur J Clin Microbiol Infect Dis,2015,34(2)223-230.
[37]Diderholm B.Perinatal energy metabolism with reference to IUGR&SGA:studies in pregnant women&newborn infants[J].Indian J Med Res,2009,130(5):612-617.
[38]Enke U,Seyfarth L,Schleussner E,Markert UR. Impact of PUFA on early immune and fetal devel⁃opment[J].Br J Nutr,2008,100(6):1158-1168.
[39]Emack J,Matthews SG.Effects of chronic maternal stress on hypothalamo-pituitary-adrenal(HPA)function and behavior:no reversal by environ⁃mental enrichment[J].Horm Behav,2011,60(5):589-598.
[40]Glover V,O′connor T G,O′donnell K.Prenatal stress and the programming of the HPA axis[J]. Neurosci Biobehav Rev,2010,35(1):17-22.
[41]Xu D,Liang G,Yan YE,He WW,Liu YS, Chen LB,et al.Nicotine-induced over-exposure to maternal glucocorticoid and activated glucocorticoid metabolism causes hypothalamic-pituitaryadrenal axis-associated neuroendocrine metabolic alterations in fetal rats[J].Toxicol Lett,2012,209(3):282-290.
[42]Xu D,Chen M,Pan XL,Xia LP,Wang H.Dexa⁃methasone induces fetal developmental toxicity through affecting the placental glucocorticoid barrier and depressing fetal adrenal function[J].Environ Toxicol Pharmacol,2011,32(3):356-363.
[43]Liang G,Chen M,Pan XL,Zheng J,Wang H. Ethanol-induced inhibition of fetal hypothalamicpituitary-adrenal axis due to prenatal overexpo⁃sure to maternal glucocorticoid in mice[J].Exp Toxicol Pathol,2011,63(7-8):607-611.
[44]Xu D,Wu Y,Liu F,Liu YS,Shen L,Lei YY,et al. A hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in offspring rats of IUGR induced by prenatal caf⁃feine ingestion[J].ToxicolApplPharmacol,2012,264(3):395-403.
[45]Yeomans MR,Mobini S,Chambers L.Additive effects of flavour-caffeine and flavour-flavour pair⁃ings on liking for the smell and flavour of a novel drink[J].Physiol Behav,2007,92(5):831-839.
[46]Thorn CF,Aklillu E,Mcdonagh EM,Klein TE,Altman RB.PharmGKB summary:caffeine path⁃way[J].Pharmacogenet Genomics,2012,22(5):389-395.
[47]Li Y,Wang H.In utero exposure to tobacco and alcohol modifies neurobehavioral development in mice offspring:consideration a role of oxidative stress[J].Pharmacol Res,2004,49(5):467-473.
[48]Dietrich M,Block G,Norkus EP,Hudes M,Traber MG,Cross CE,et al.Smoking and expo⁃sure to environmental tobacco smoke decrease some plasma antioxidants and increase gammatocopherol in vivo after adjustment for dietary anti⁃oxidant intakes[J].Am J Clin Nutr,2003,77(1):160-166.
[49] Fowden AL,Li J,Forhead AJ.Glucocorticoids and the preparation for life after birth:are there long-term consequences of the life insurance?[J].Proc Nutr Soc,1998,57(1):113-122.
[50] Chapman K,Holmes M,Seckl J.11beta-hydroxys⁃teroid dehydrogenases:intracellular gate-keepers of tissue glucocorticoid action[J].Physiol Rev,2013,93(3):1139-1206.
[51]Lesage J,Blondeau B,Grino M,BréantB,Dupouy JP.Maternal undernutrition during late gestation induces fetal overexposure to glucocor⁃ticoids and intrauterine growth retardation,and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat[J].Endocrinology,2001,142(5):1692-1702.
[52]Reynolds RM.Glucocorticoid excess and the developmental origins of disease:two decades of testing the hypothesis-2012 Curt Richter Award Winner[J].Psychoneuroendocrinology,2013,38(1):1-11.
[53]Morrison JL,Botting KJ,Soo PS,Mcgillick EV,Hiscock J,Zhang S,et al.Antenatal steroids and the IUGR fetus:are exposure and physiologi⁃cal effects on the lung and cardiovascular system the same as in normally grown fetuses?[J].J Pregnancy,2012,2012:839656.
[54]Lesage J,Blondeau B,Grino M,Breant B,Dupouy J P.Maternal undernutrition during late gestation induces fetal overexposure to glucocor⁃ticoids and intrauterine growth retardation,and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat[J].Endocrinology,2001,142(5):1692-1702.
[55] Langley-Evans SC,Phillips GJ,Benediktsson R,Gardner DS,Edwards CR,Jackson AA,et al. Protein intake in pregnancy,placental glucocorti⁃coid metabolism and the programming of hyper⁃tension in the rat[J].Placenta,1996,17(2-3):169-172.
[56]Newnham JP,Moss TJ,Nitsos I,Sloboda DM. Antenatal corticosteroids:the good,the bad and the unknown[J].Curr Opin Obstet Gynecol,2002,14(6):607-612.
[57] Xu D,Zhang B,Liang G,Ping J,Kou H,Li X,et al.Caffeine-induced activated glucocorticoid metabolism in the hippocampus causes hypotha⁃lamic-pituitary-adrenal axis inhibition in fetal rats[J].PLoS One,2012,7(9):e44497.
[58]Chen M,Wang T,Liao ZX,Pan XL,Feng YH,Wang H.Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat[J].Exp Toxicol Pathol,2007,59(3-4):245-251.
[59]Li C,Mcdonald TJ,Wu G,Nijland MJ,Nathan⁃ielsz PW.Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive pep⁃tide balance[J].J Endocrinol,2013,217(3):275-282.
[60]Li C,Shu ZJ,Lee S,Gupta MB,Jansson T,Nathanielsz PW,et al.Effects of maternal nutrient restriction,intrauterine growth restriction,and glucocorticoid exposure on phosphoenolpyruvate carboxykinase-1 expression in fetal baboon hepa⁃tocytes in vitro[J].J Med Primatol,2013,42(4):211-219.
[61]Moisiadis VG,Matthews SG.Glucocorticoids and fetal programming part 2:Mechanisms[J].Nat Rev Endocrinol,2014,10(7):403-411.
[62]Meaney MJ,Szyf M,Seckl JR.Epigenetic mech⁃anisms of perinatal programming of hypothalamicpituitary-adrenal function and health[J].Trends Mol Med,2007,13(7):269-277.
[63]Xita N,Tsatsoulis A.Fetal origins of the metabolic syndrome[J].Ann NY Acad Sci,2010,1205:148-155.
[64]Xiong F,Zhang L.Role of the hypothalamic-pitu⁃itary-adrenal axis in developmental programming of health and disease[J].Front Neuroendocri⁃nol,2013,34(1):27-46.
[65]Cohen M,Brown DR,Myers MM.Cardiorespiratory measures before and after feeding challenge in term infants are related to birth weight[J].Acta Paediatr,2009,98(7):1183-1188.
[66]Schutter DJ.The cerebello-hypothalamic-pituitaryadrenal axis dysregulation hypothesis in depres⁃sive disorder[J].Med Hypotheses,2012,79(6):779-783.
[67]Kanaka-Gantenbein C.Fetal origins of adult diabetes[J].Ann NY Acad Sci,2010,1205:99-105.
[68]Weinstock M.The potential influence of maternal stress hormones on development and mental health of the offspring[J].Brain Behav Immun,2005,19(4):296-308.
[69]Davis EP,Waffarn F,Sandman CA.Prenatal treatment with glucocorticoids sensitizes the hpa axis response to stress among full-term infants[J].Dev Psychobiol,2011,53(2):175-183.
[70]Harris A, Seckl J.Glucocorticoids,prenatal stress and the programming of disease[J].Horm Behav,2011,59(3):279-289.
[71]Fowden AL,Forhead AJ.Endocrine mechanisms of intrauterine programming[J].Reproduction,2004,127(5):515-526.
[72] Ong K.Adrenal function of low-birthweight chil⁃dren[J].Endocr Dev,2005,8:34-53.
[73]Yan YE,Liu L,Wang JF,Liu F,Li XH,Qin HQ,et al.Prenatal nicotinic exposure suppresses fetal adrenal steroidogenesis via steroidogenic factor 1(SF-1)deacetylation[J].Toxicol Appl Pharmacol,2014,277(3):231-241.
[74]Ping J,Wang JF,Liu L,Yan YE,Liu F,Lei YY,et al.Prenatal caffeine ingestion induces aber⁃rant DNA methylation and histone acetylation of steroidogenic factor 1 and inhibits fetal adrenal ste⁃roidogenesis[J].Toxicology,2014,321:53-61.
[75]Huang H,He Z,Zhu C,Liu L,Kou H,Shen L,et al.Prenatal ethanol exposure-induced adrenal developmental abnormality of male offspring rats and its possible intrauterine programming mecha⁃nisms[J].Toxicol Appl Pharmacol,2015,288(1):84-94.
[76]He Z,Zhu C,Huang H,Liu L,Wang L,Chen L,et al.Prenatal caffeine exposure-induced adrenal developmental abnormality in male offspring rats and its possible intrauterine programming mecha⁃nisms[J].Toxicol Res,2016,5(2):388-398.
[77]Moisiadis VG,Matthews SG.Glucocorticoids and fetal programming part 1:Outcomes[J].Nat Rev Endocrinol,2014,10(7):391-402.
[78]Xia LP,Shen L,Kou H,Zhang BJ,Zhang L,Wu Y,et al.Prenatal ethanol exposure enhanc⁃es the susceptibility to metabolic syndrome in off⁃spring rats by HPA axis-associated neuroendo⁃crine metabolic programming[J].Toxicol Lett,2014,226(1):98-105.
[79]Xu D,Xia LP,Shen L,Lei YY,Liu L,Zhang L,et al.Prenatal nicotine exposure enhances the susceptibility to metabolic syndrome in adult off⁃spring rats fed high-fat diet via alteration of HPA axis-associated neuroendocrine metabolic pro⁃gramming[J].Acta Pharmacol Sin,2013,34(12):1526-1534.
[80]Lu J,Wen Y,Li Z,Zhang C,Zhong W,Zhang L,et al.Prenatal ethanol exposure induces an intra⁃uterine programming of enhanced sensitivity of the hypothalamic-pituitary-adrenal axis in female offspring rats fed with post-weaning high-fat diet[J].Toxicol Res,2015,4(5):1238-1249.
[81]Tasker JG,Herman JP.Mechanisms of rapid glu⁃cocorticoid feedback inhibition of the hypothalam⁃ic-pituitary-adrenal axis[J].Stress,2011,14(4):398-406.
[82]De Kloet ER,Reul JM,Sutanto W.Corticoste⁃roids and the brain[J].J Steroid Biochem Mol Biol,1990,37(3):387-394.
[83]Matthews SG.Early programming of the hypothal⁃amo-pituitary-adrenal axis[J].Trends Endocrinol Metab,2002,13(9):373-380.
[84]De Quervain DJ, Aerni A, Schelling G,Roozendaal B.Glucocorticoids and the regulation of memory in health and disease[J].Front Neuro⁃endocrinol,2009,30(3):358-370.
[85]DU Z,Han F,Shi YX.Expressions of hippocampalmineralocorticoid receptor(MR) and glucocorticoid receptor(GR)in the single-prolonged stress rats[J].Acta Histochem Cytochem,2008,41(4):89-95.
[86]Netchine I,Azzi S,Houang M,Seurin D,Perin L,Ricort JM,et al.Partial primary deficiency of insu⁃lin-like growth factor(IGF)-I activity associated with IGF1 mutation demonstrates its critical role in growth and brain development[J].J Clin Endocri⁃nol Metab,2009,94(10):3913-3921.
[87]Roberts CT,Owens JA,Sferruzzi-Perri AN.Dis⁃tinct actions of insulin-like growth factors(IGFs)on placental development and fetal growth:les⁃sons from mice and guinea pigs[J].Placenta,2008,29(Suppl A):S42-S47.
[88]AgrogiannisGD, SifakisS, PatsourisES,Konstantinidou AE.Insulin-like growth factors in embryonic and fetal growth and skeletal develop⁃ment(Review)[J].Mol Med Rep,2014,10(2):579-584.
[89]Walenkamp MJ,Losekoot M,Wit JM.Molecular IGF-1 and IGF-1 receptor defects:from genetics to clinical management[J].Endocr Dev,2013,24:128-137.
[90]Wallborn T,Wuller S,Klammt J,Kruis T,Kratzsch J,Schmidt G,et al.A heterozygous mu⁃tation of the insulin-like growth factor-I receptor causes retention of the nascent protein in the en⁃doplasmic reticulum and results in intrauterine and postnatal growth retardation[J].J Clin Endo⁃crinol Metab,2010,95(5):2316-2324.
[91]Netchine I,Azzi S,Le Bouc Y,Savage MO. IGF1 molecular anomalies demonstrate its critical role in fetal,postnatal growth and brain develop⁃ment[J].Best Pract Res Clin Endocrinol Metab,2011,25(1):181-190.
[92]Puri G,Kumar K,Singh R,Singh RK,Yasotha T,Ranjan R,et al.Effects of growth factors on establishmentand propagation ofembryonic stem cells from very early stage IVF embryos and their characterization in buffalo[J].Int J StemCells,2012,5(2):96-103.
[93]Magner NL,Jung Y,Wu J,Nolta JA,Zern MA,Zhou P.Insulin and IGFs enhance hepatocyte differentiation from human embryonic stem cells via the PI3K/AKT pathway[J].Stem Cells,2013,31(10):2095-2103.
[94]Piecewicz SM,Pandey A,Roy B,Xiang SH,Zetter BR,Sengupta S.Insulin-like growth factors promote vasculogenesis in embryonic stem cells[J].PLoS One,2012,7(2):e32191.
[95]Jungheim ES,Louden ED,Chi MM,Frolova AI,Riley JK,Moley KH.Preimplantation exposure of mouse embryos to palmitic acid results in fetal growth restriction followed by catch-up growth in the offspring[J].Biol Reprod,2011,85(4):678-683.
[96]Kamei H,Ding Y,Kajimura S,Wells M,Chiang P,Duan C.Role of IGF signaling in catch-up growth and accelerated temporal development in zebraf⁃ish embryos in response to oxygen availability[J]. Development,2011,138(4):777-786.
[97]Tosh DN,Fu Q,Callaway CW,Mcknight RA,Mcmillen IC,Ross MG,et al.Epigenetics of pro⁃grammed obesity:alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs.delayed postnatal catch-up growth[J]. Am J Physiol Gastrointest Liver Physiol,2010,299(5):G1023-G1029.
[98]Shen L,Liu Z,Gong J,Zhang L,Wang L,Magdalou J,et al.Prenatal ethanol exposure pro⁃grams an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats[J].Toxicol Appl Pharmacol,2013,274(2):263-273.
[99]Lunghi L,Pavan B,Biondi C,Paolillo R,Valerio A,Vesce F,et al.Use of glucocorticoids in pregnancy[J].Curr Pharm Des,2010,16(32):3616-3637.
[100]Marciniak B,Patro-Malysza J,Poniedzialek-Cza⁃jkowska E,Kimber-Trojnar Z,Leszczynska-Gorzelak B,Oleszczuk J.Glucocorticoids in pregnancy[J].Curr Pharm Biotechnol,2011,12(5):750-757.
[101]Luo H,Deng Z,Liu L,Shen L,Kou H,He Z,et al. Prenatal caffeine ingestion induces transgenera⁃tionalneuroendocrine metabolic programming alteration in second generation rats[J].Toxicol Appl Pharmacol,2013,274(3):383-392.
[102]Tan Y,Liu J,Deng Y,Cao H,Xu D,Cu F,et al. Caffeine-induced fetal rat over-exposure to mater⁃nal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation[J]. Toxicol Lett,2012,214(3):279-287.
[103]Gardebjer EM,Cuffe JS,Pantaleon M,Wlodek ME,Moritz KM.Periconceptional alcohol consumption causesfetalgrowth restriction and increases glycogen accumulation in the late gestation rat placenta[J].Placenta,2014,35(1):50-57.
[104]Hyatt MA,Gardner DS,Sebert S,Wilson V,Davidson N,Nigmatullina Y,et al.Suboptimal maternal nutrition,during early fetal liver develop⁃ment,promotes lipid accumulation in the liver of obese offspring[J].Reproduction,2011,141(1):119-126.
[105]Inder WJ,Jang C,Obeyesekere VR,Alford FP. Dexamethasone administration inhibits skeletal muscle expression of the androgen receptor and IGF-1-implications for steroid-induced myopathy[J]. Clin Endocrinol(Oxf),2010,73(1):126-132.
[106]Robson H,Siebler T,Shalet SM,Williams GR. Interactions between GH,IGF-I,glucocorticoids,and thyroid hormones during skeletal growth[J]. Pediatr Res,2002,52(2):137-147.
[107]Yamada M,Wolfe D,Han G,French SW,Ross MG,Desai M.Early onset of fatty liver in growth-restricted rat fetuses and newborns[J]. Congenit Anom(Kyoto),2011,51(4):167-173.
[108]Rueda-Clausen CF,Dolinsky VW,Morton JS,Proctor SD,Dyck JR,Davidge ST.Hypoxia-in⁃duced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced meta⁃bolic syndrome[J].Diabetes,2011,60(2):507-516.
[109]Hales CN, Barker DJ.The thrifty phenotype hypothesis[J].Br Med Bull,2001,60:5-20.
[110]Vaag AA,Grunnet LG,Arora GP,Brons C.The thrifty phenotype hypothesis revisited[J].Diabeto⁃logia,2012,55(8):2085-2088.
[111]Jaquet D,Gaboriau A,Czernichow P,Levy-Marchal C.Insulin resistance early in adulthood in subjects born with intrauterine growth retarda⁃tion[J].J Clin Endocrinol Metab,2000,85(4):1401-1406.
[112]Zabeau L,Defeau D,Van Der Heyden J,Iseren⁃tant H,Vandekerckhove J,Tavernier J.Functional analysis of leptin receptor activation using a Janus kinase/signal transducer and activator of transcription complementation assay[J].Mol Endocrinol,2004,18(1):150-161.
[113]Agrogiannis GD, Sifakis S, Patsouris ES,Konstantinidou AE.Insulin-like growth factors in embryonic and fetal growth and skeletal development(Review)[J].Mol Med Rep,2014,10(2):579-584.
[114]Woods KA,Camacho-Hubner C,Savage MO,Clark AJ.Intrauterine growth retardation and post⁃natal growth failure associated with deletion of the insulin-like growth factor I gene[J].N Engl J Med,1996,335(18):1363-1367.
[115]Hyatt MA,Budge H,Walker D,Stephenson T,Symonds ME.Ontogeny and nutritional program⁃ming of the hepatic growth hormone-insulin-like growth factor-prolactin axis in the sheep[J].En⁃docrinology,2007,148(10):4754-4760.
[116]Waterland RA,Jirtle RL.Transposable elements:targets for early nutritional effects on epigenetic gene regulation[J].Mol Cell Biol,2003,23(15):5293-5300.
[117]Hajkova P,Erhardt S,Lane N,Haaf T,El-Maarri O,Reik W,et al.Epigenetic reprogramming in mouse primordial germ cells[J].Mech Dev,2002,117(1-2):15-23.
[118]Cooney CA,Dave AA,Wolff GL.Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring[J].J Nutr,2002,132(8 Suppl):2393S-2400S.
[119]Schwitzgebel VM,Somm E,Klee P.Modeling intrauterine growth retardation in rodents:Impact on pancreas development and glucose homeostasis[J].Mol Cell Endocrinol,2009,304(1-2):78-83.
[120]Ferrari P,Krozowski Z.Role of the 11beta-hy⁃droxysteroid dehydrogenase type 2 in blood pres⁃sure regulation[J].Kidney Int,2000,57(4):1374-1381.
[121]Baserga M,Kaur R,Hale MA,Bares A,Yu X,Callaway CW,et al.Fetal growth restriction alters transcription factor binding and epigenetic mechanisms of renal 11beta-hydroxysteroid dehy⁃drogenase type 2 in a sex-specific manner[J]. Am J Physiol Regul Integr Comp Physiol,2010,299(1):R334-R342.
[122]Fu Q,Mcknight RA,Yu X,Callaway CW,Lane R H.Growth retardation alters the epigenetic characteristics of hepatic dual specificity phospha⁃tase 5[J].FASEB J,2006,20(12):2127-2129.
[123]Long NM,Ford SP,Nathanielsz PW.Multigener⁃ational effects of fetal dexamethasone exposure on the hypothalamic-pituitary-adrenal axis of firstand second-generation female offspring[J].Am J Obstet Gynecol,2013,208(3):217 e211-218.
[124]Iqbal M,Moisiadis VG,Kostaki A,Matthews SG. Transgenerational effects of prenatal synthetic glucocorticoids on hypothalamic-pituitary-adrenal function[J].Endocrinology,2012,153(7):3295-3307.
[125]Gniuli D,Calcagno A,Caristo M E,Mancuso A,Macchi V,Mingrone G,et al.Effects of high-fat diet exposure during fetal life on type 2 diabetes development in the progeny[J].J Lipid Res,2008,49(9):1936-1945.
[126]Govorko D,Bekdash RA,Zhang C,Sarkar DK. Male germline transmits fetal alcohol adverse ef⁃fect on hypothalamic proopiomelanocortin gene across generations[J].Biol Psychiatry,2012,72(5):378-388.
[127]Ding GL,Wang FF,Shu J,Tian S,Jiang Y,Zhang D,et al.Transgenerational glucose intoler⁃ance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia[J].Diabetes,2012,61(5):1133-1142.
[128]Rouleau J,Tanigawa G,Szyf M.The mouse DNA methyltransferase 5'-region.A unique housekeeping gene promoter[J].J Biol Chem,1992,267(11):7368-7377.
[129]Dolinoy DC,Jirtle RL.Environmental epigenom⁃ics in human health and disease[J].Environ Mol Mutagen,2008,49(1):4-8.
[130]Alworth LC,Howdeshell KL,Ruhlen RL,Day JK,Lubahn DB,Huang TH,et al.Uterine responsive⁃nessto estradioland DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice:effects of low versus high doses[J].Toxicol Appl Pharmacol,2002,183(1):10-22.
[131]Grossniklaus U,Kelly B,Ferguson-Smith AC,Pembrey M, LindquistS.Transgenerational epigenetic inheritance:how important is it?[J]. Nat Rev Genet,2013,14(3):228-235.
[132]Chen M,Zhang L.Epigenetic mechanisms in developmental programming of adult disease[J]. Drug Discov Today,2011,16(23-24):1007-1018.
[133]Crudo A, Suderman M, MoisiadisVG,Petropoulos S,Kostaki A,Hallett M,et al.Gluco⁃corticoid programming of the fetal male hippocam⁃pal epigenome[J].Endocrinology,2013,154(3):1168-1180.
[134]Crudo A,Petropoulos S,Moisiadis VG,Iqbal M,Kostaki A,Machnes Z,et al.Prenatal syntheticglucocorticoid treatment changes DNA methyla⁃tion states in male organ systems:multigenerational effects[J].Endocrinology,2012,153(7):3269-3283.
[135]Lillycrop KA,Slater-Jefferies JL,Hanson MA,Godfrey KM,Jackson AA,Burdge GC.Induction of altered epigenetic regulation of the hepatic glu⁃cocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expres⁃sion is involved in impaired DNA methylation and changes in histone modifications[J].Br J Nutr,2007,97(6):1064-1073.
[136]Thompson RF,Fazzari MJ,Niu H,Barzilai N,Simmons RA,Greally JM.Experimental intrauterine growth restriction inducesalterationsin DNA methylation and gene expression in pancreatic islets of rats[J].J Biol Chem,2010,285(20):15111-15118.
[137]Fu Q,Mcknight RA,Yu X,Wang L,Callaway CW,LaneRH.Uteroplacentalinsufficiencyinduces site-specific changes in histone H3 covalent modi⁃fications and affects DNA-histone H3 positioning in day 0 IUGR rat liver[J].Physiol Genomics,2004,20(1):108-116.
[138]Wolfe D,Gong M,Han G,Magee TR,Ross MG,Desai M.Nutrient sensor-mediated programmed nonalcoholic fatty liver disease in low birthweight offspring[J].Am J Obstet Gynecol,2012,207(4):308e301-308e306.
[139]Nobili V,Marcellini M,Marchesini G,Vanni E,Manco M,Villani A,et al.Intrauterine growth retardation,insulin resistance,and nonalcoholic fatty liver disease in children[J].Diabetes Care,2007,30(10):2638-2640.
[140]DonnellyKL,SmithCI,SchwarzenbergSJ,Jessurun J,Boldt M D,Parks E J.Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease[J].J Clin Invest,2005,115(5):1343-1351.
[141]Horton JD,Shah NA,Warrington JA,Anderson NN,Park SW,Brown MS,et al.Combined anal⁃ysis of oligonucleotide microarray data from trans⁃genic and knockout mice identifies direct SREBP target genes[J].Proc Natl Acad Sci USA,2003,100(21):12027-12032.
[142]Rector RS,Thyfault JP,Uptergrove GM,Morris EM,Naples SP,Borengasser SJ,et al.Mito⁃chondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model[J].J Hepatol,2010,52(5):727-736.
[143]Tep S,Mihaila R,Freeman A,Pickering V,Huynh F,Tadin-Strapps M,et al.Rescue of Mtp siRNA-induced hepatic steatosis by DGAT2 siRNA silencing[J].J Lipid Res,2012,53(5):859-867.
[144]Zhang L,Shen L,Xu D,Wang L,Guo Y,Liu Z,et al.Increased susceptibility of prenatal food restricted offspring to high-fat diet-induced nonal⁃coholic fatty liver disease is intrauterine programmed[J].Reprod Toxicol,2016,65:236-247.
[145]Xu D,Bai J,Zhang L,Shen L,Wang L,Liu Z,et al.Prenatal nicotine exposure-induced intra⁃uterine programming alteration increases the sus⁃ceptibility of high-fat diet-induced non-alcoholic simple fatty liver in female adult offspring rats[J]. Toxicol Res,2014,4(1):112-120.
[146]Sayer AA,Poole J,Cox V,Kuh D,Hardy R,Wadsworth M,et al.Weight from birth to 53 years:a longitudinal study of the influence on clin⁃icalhand osteoarthritis[J].Arthritis Rheum,2003,48(4):1030-1033.
[147]Jordan KM,Syddall H,Dennison EM,Cooper C,Arden NK.Birthweight,vitamin D receptor gene polymorphism,and risk of lumbar spine osteoar⁃thritis[J].J Rheumatol,2005,32(4):678-683.
[148]Aigner T,Richter W.OA in 2011:Age-related OA-a concept emerging from infancy?[J].Nat Rev Rheumatol,2012,8(2):70-72.
[149]Pitsillides AA,Beier F.Cartilage biology in osteo⁃arthritis-lessons from developmental biology[J]. Nat Rev Rheumatol,2011,7(11):654-663.
[150]Dahlberg L.Cartilage quality,overweight and os⁃teoarthritis:a case for new behaviour?[J].Ann Rheum Dis,2012,71(1):1-3.
[151]Cubukcu D,Ardic F,Karabulut N,Topuz O. Hylan G-F 20 efficacy on articular cartilage quality in patients with knee osteoarthritis:clinical and MRI assessment[J].Clin Rheumatol,2005,24(4):336-341.
[152]Ni Q,Tan Y,Zhang X,Luo H,Deng Y,Magdalou J,et al.Prenatal ethanol exposure increases osteoarthritis susceptibility in female rat offspring by programming a low-functioning IGF-1 signaling pathway[J].Sci Rep,2015,5:14711.
[153]Tie K,Zhang X,Tan Y,Deng Y,Li J,Ni Q,et al. Intrauterine low-functional programming of IGF1by prenatalnicotine exposure mediates the susceptibility to osteoarthritis in female adult rat offspring[J].FASEB J,2016,30(2):785-797.
[154]Luo H,Li J,Cao H,Tan Y,Magdalou J,Chen L,et al.Prenatal caffeine exposure induces a poor quality of articular cartilage in male adult offspring rats via cholesterol accumulation in cartilage[J]. Sci Rep,2015,5:17746.
[155]Ni Q,Wang L,Wu Y,Shen L,Qin J,Liu Y,et al. Prenatal ethanol exposure induces the osteoarthritislike phenotype in female adult offspring rats with a post-weaning high-fat diet and its intrauterine pro⁃gramming mechanisms of cholesterol metabolism[J].Toxicol Lett,2015,238(2):117-125.
[156]Tie K,Tan Y,Deng Y,Li J,Ni Q,Magdalou J,et al.Prenatal nicotine exposure induces poor ar⁃ticular cartilage quality in female adult offspring fed a high-fat diet and the intrauterine program⁃ming mechanisms[J].Reprod Toxicol,2016,60:11-20.
[157]Hochberg Z,Feil R,Constancia M,Fraga M,Junien C,Carel JC,et al.Child health,develop⁃mental plasticity,and epigenetic programming[J].Endocr Rev,2011,32(2):159-224.
[158]Vickers MH,Gluckman PD,Coveny AH,Hofman PL,Cutfield WS,Gertler A,et al.Neonatal leptin treatment reverses developmental programming[J]. Endocrinology,2005,146(10):4211-4216.
Prenatal adverse environment increased offspring susceptibility to multiple chronic diseases and intrauterine programming mechanisms
WANG Hui1,2,JIAO Zhe-xiao1,2
(1.Department of Pharmacology,Basic Medical School of Wuhan University,Wuhan 430071,China; 2.Hubei Provincial Key Laboratory of Developmentally Originated Disease,Wuhan 430071,China)
Epidemiological studies reveal that prenatal adverse environment could cause lower birthweight in offspring and increase the susceptibility to multiple chronic diseases(e.g.metabolic and neuropsychiatric diseases etc.)after maturity.However,the underlying mechanism remains unclarified.The hypothalamic-pituitary-adrenal(HPA)axis is a key neuroendocrine axis playing pivotal roles in systemic stress responses before and after birth.It is also an important but vulnerable fetal targeting organ.Previous studies showed that many environmental insults during pregnancy,including external environment and maternal health condition,could affect fetal development in multi-ways via maternalplacental-fetal unit,which leads to the intrauterine programming alteration of HPA axis and the in⁃creased susceptibility to chronic diseases in adulthood.This article reviews the latest global advances in the etiology of increased susceptibility to adult diseases induced by compromised prenatal environ⁃ment and the associated intrauterine programming mechanisms by incorporating our recent research findings,and proposes that the fetal over-exposure to maternal glucocorticoids(GC)could bring about the intrauterine neuroendocrine metabolic programming alteration in offspring:the core is the program⁃ming of GC-insulin-like growth factor 1 axis in multiple organs,and the abnormal epigenetic modification is involved in this programming.
prenatal adverse environment;metabolic syndrome;hypothalamic-pituitary-adrenal axis,epigenetic modification;intrauterine programming
WANG Hui,Tel:13627232557,E-mail:wanghui19@whu.edu.cn
R99
A
1000-3002-(2017)01-0012-16
10.3867/j.issn.1000-3002.2017.01.002
2016-12-25接受日期:2017-01-20)
(本文编辑:乔 虹)
国家自然科学基金(30830112);国家自然科学基金(81430089);国家自然科学基金(81220108026)
汪 晖,Tel:13627232557,E-mail:wanghui19@ whu.edu.cn
Foundation item:The project supported by National Natural Science Foundation of China(30830112);National Natural Science Foundation of China(81430089);and National Natural Science Foundation of China(81220108026)

