杂原子掺杂碳基氧还原反应电催化剂研究进展
周宇,王宇新
(化学工程联合国家重点实验室,天津大学化工学院,天津化学化工协同创新中心,天津市膜科学与海水淡化重点实验室,天津 300072)
杂原子掺杂碳基氧还原反应电催化剂研究进展
周宇,王宇新
(化学工程联合国家重点实验室,天津大学化工学院,天津化学化工协同创新中心,天津市膜科学与海水淡化重点实验室,天津 300072)
氧还原反应(ORR)是质子交换膜燃料电池和金属空气电池等清洁能源转化技术中的关键步骤,但其反应能垒高,动力学缓慢。目前,氧还原性能最佳的催化剂仍然是已经商业化的碳载铂(Pt/C)催化剂,但其价格高、资源储量低,难以大规模应用。因此,近年来许多研究者致力于探寻低成本高性能非铂ORR催化剂,以期降低催化剂成本,推进质子交换膜燃料电池和金属空气电池的商业化进程。杂原子掺杂碳材料属于一种新型的非贵金属ORR催化剂,具有优异的电化学性能且成本低廉,显示了广阔的应用前景。以杂原子的不同作用原理为线索综述最近几年杂原子掺杂碳基ORR催化剂的研究进展,着重论述杂原子对于碳材料电子结构的影响,并讨论了杂原子掺杂碳材料催化剂面临的问题以及发展趋势。
氧还原反应;杂原子掺杂;电催化剂;电子材料;催化;电子学
Key words: oxygen reduction reaction; heteroatom doping; electrocatalysts; electronic materials; catalysis; electrochemistry
引 言
质子交换膜燃料电池、金属空气电池等新型电源技术具有能量转换效率高、清洁、可持续等突出优点,有望在新能源汽车、分散式固定式电站、便携式电子设备等重要领域广泛应用[1]。然而,由于成本高、可选用材料范围窄等因素,这些技术的大规模应用仍然面临着巨大的挑战。氧还原反应(oxygen reduction reaction, ORR)是燃料电池和金属空气电池等的关键步骤,但此反应过程复杂、能垒较高,通常需要较大的过电位驱动[2]。因此,氧还原反应通常需要高活性的电催化剂。目前,Pt催化剂的ORR催化活性最高,但是Pt易一氧化碳中毒失活,特别是其价格高昂、资源稀缺,故难以大规模应用。已经有许多研究着眼于降低Pt负载量,通过Pt颗粒尺度与晶面调控、核壳结构、合金化等方法提高Pt利用率[3]。
但是更根本的方法应该是探求高性能非铂系ORR催化剂。近年来,非铂系ORR催化剂成为新能源和催化领域的研究热点。文献中大量报道了多类非铂系ORR催化剂,包括杂原子掺杂碳材料、过渡金属氧化物、金属-氮-碳结构催化剂等。相比Pt催化剂,非铂系催化剂价格优势明显,日益受到研究者们的重视,论文发表数量显著增加(图1)。若干非铂系ORR催化剂的性能已经接近、达到甚至超过了商品化的Pt/C催化剂。关于非铂系ORR催化剂近年来的进展,已经有若干研究者做过很好的综述。Higgins等[4]综述了石墨烯相关的ORR电催化剂研究进展。Wood等[5]专门针对氮掺杂的多种纳米碳材料在ORR催化和其他领域的近年研究成果做了综述。Duan等[6]综述了不同杂原子掺杂的石墨烯基催化剂在能源领域的研究进展。Zhu等[7]总结了3D多孔纳米碳非贵金属ORR催化剂的合理设计及合成方法。Zhou等[8]按照材料几何形态进行划分,分类总结了不同维度碳材料在杂原子掺杂以及金属复合ORR催化剂研究方面的近期成就。He等[9]专门综述了非贵金属ORR催化剂的碳基体结构效应。
不同于前人的非贵金属ORR催化剂综述性文章,本文以掺杂元素的作用机理为线索和脉络,分别从电荷极化、自旋极化以及复合作用等角度综述杂原子掺杂碳基催化剂的最新研究进展。本文着重论述杂原子掺杂对碳材料电子结构的影响,并讨论杂原子掺杂碳材料催化剂面临的问题以及发展趋势。
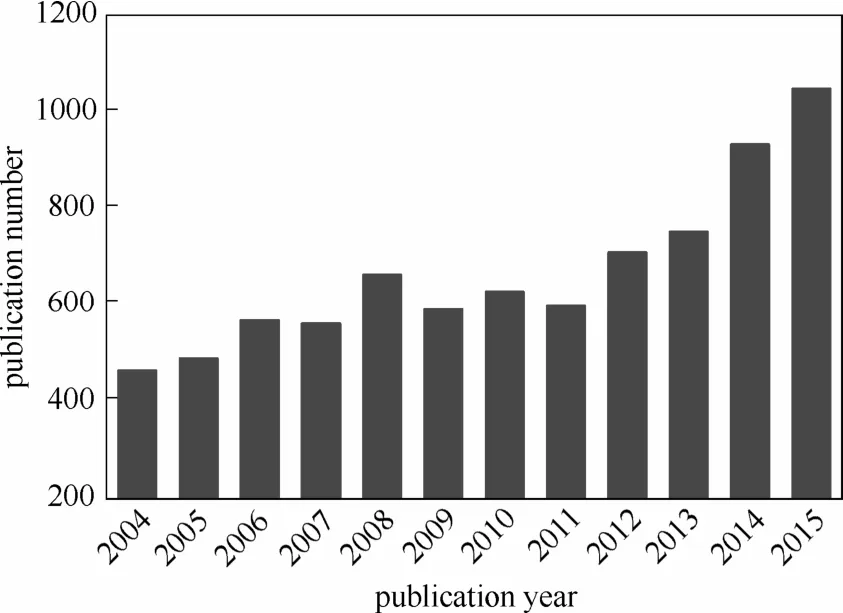
图1 非铂系ORR催化剂研究论文数量统计(数据来源:Web of Science)Fig.1 Statistic on number and year of publication of research papers on non-Pt ORR catalyst(source: Web of Science)
1 电荷极化作用
纳米碳材料形态和结构多样,包括零维的富勒烯、一维的碳纳米管、二维的石墨烯以及多种不同的三维结构纳米碳。许多纳米碳材料由于具有高比表面积、高电导率、结构可调控等许多优异的物理化学性质,加之碳资源丰富易得,在电化学能源转化领域受到了广泛的关注[1]。但是单纯碳纳米材料难于替代Pt系贵金属用于ORR催化剂,因为普通碳材料电荷分布均匀,活性位点较少,催化活性较低[10-13]。为克服此缺点,研究者们通过引入杂原子掺杂的方法改变相邻碳原子的电荷与自旋密度分布,增加活性位点,从而实现碳材料催化活性的提高[14-16]。目前,研究者普遍接受的主要有两种掺杂作用机制[10,13,17]:其一,掺杂的杂原子与碳原子电负性差异较大,对相邻碳原子产生电荷极化作用,改变电荷密度分布,形成对氧有较强吸附能力的活性位点;其二,杂原子的引入改变了sp2杂化碳原子的自旋密度,从而改变表面电子结构分布,形成活性位点。
杂原子掺杂碳材料一般以掺杂N、B、S、P、F等非金属元素的碳材料为主。B、P电负性较小,与碳原子成键时存在电子排斥作用,对相邻碳原子产生负电荷极化作用,自身带正电,成为活性位点。N、F等元素电负性相对碳原子较大,与碳原子成键时存在电子吸引作用,对相邻碳原子产生正电荷极化作用,提高了相邻碳原子的正电荷密度,有利于氧分子的吸附[图2(a)][9]。
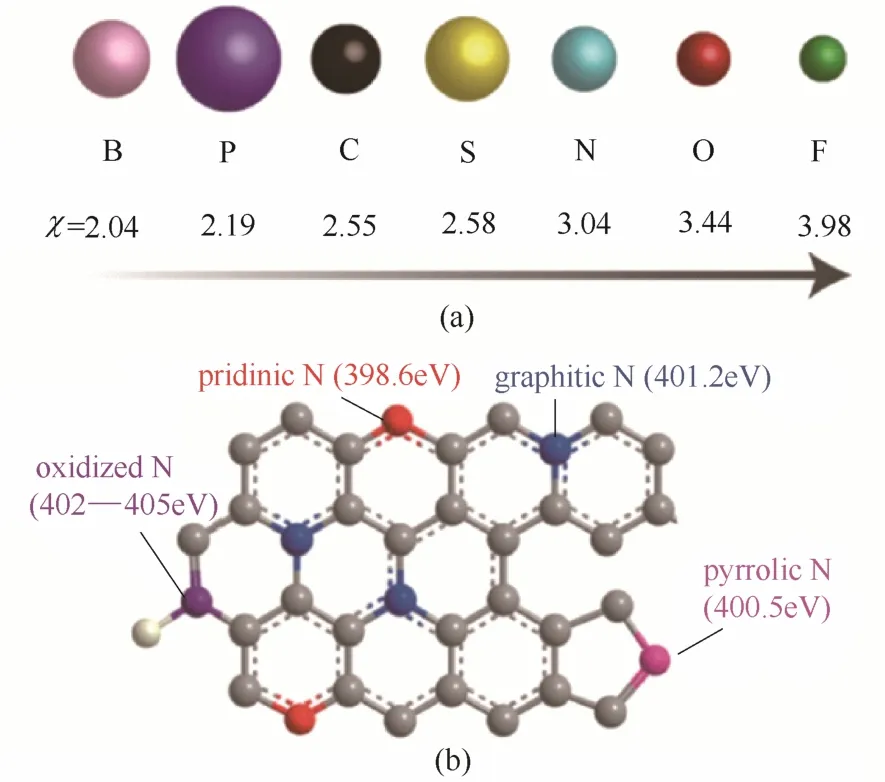
图2 原子电负性(χ)及原子半径相对大小(a)和常见N键构型(b)[9]Fig.2 Electronegativity (χ) and relative radius of various atoms (a) and schematic diagram of common N bonding configurations (b)[9]
1.1 正电荷极化
在正电荷极化作用体系中,对N掺杂碳材料的研究最早也最为广泛。2009年Gong等[18]在NH3气氛下热解酞菁铁制备了垂直定向排列N掺杂碳纳米管。所得材料不仅催化活性超过了商业Pt/C催化剂,稳定性以及抗CO中毒性能相比Pt基催化剂也有显著的提高。自此,面向ORR催化剂应用的杂原子掺杂碳纳米材料开始受到广泛关注。
N原子在碳材料中的掺杂有多种构型,不同的掺杂构型对催化性能有着不同的影响。人们发现在氮掺杂的碳材料中N原子与碳主要有4种键合类型,分别为石墨型氮(graphitic-N)、吡啶型氮(pyridinic-N)、吡咯型氮(pyrrolic-N)和氧化态氮(oxidized-N或pyridinic oxide-N)[9][图2(b)]。不同前体和制备条件得到的催化剂中N掺杂构型不同,前体的选择和制备方法对掺杂度和材料的石墨化程度也具有显著的影响,而催化活性与这些因素密切相关[19-43]。因此,到底是哪种构型的掺杂氮能够更好地提高氧还原催化活性尚没有一致的结论。
Liu等[19]采用介孔二氧化硅SBA-15作为模板、含氮芳香染料PDI作为N源和C源,合成了N掺杂有序介孔石墨阵列。研究发现合成温度对掺杂构型具有显著影响。随着石墨型氮的比例增加,催化活性以及四电子路径选择性得到了显著的提高。Sheng等[20]采用一锅法热解氧化石墨烯和三聚氰胺制备得到了N掺杂碳材料,通过调控前体中C源和N源的比例控制掺杂N含量。ORR测试结果表明催化活性随吡啶型氮含量增加显著提高,故催化活性主要取决于吡啶型氮。Chung等[33]受到肺泡的启发,采用多巴胺包覆的ZIF8沸石作为前体,随后热解,得到了内部具有互联网络的多孔碳三维结构,反应物的扩散与电子转移速度显著提高,ORR测试表明起始电位随石墨型氮含量增加向正值方向移动。Unni等[43]制备了富含吡咯型氮的石墨烯,催化活性与吡咯型氮含量呈现良好的正相关性,因此认为催化活性的提高主要是由于吡咯型氮含量的提高。
碳材料的石墨化程度、比表面积、掺杂构型对催化活性具有显著的影响,这些复杂因素增加了活性位点确定的困难。为了简化问题,Guo等[40]设计合成了一种具有均匀π电子结构的高度取向热解石墨HOPG作为模型催化剂,控制反应条件分别得到了以石墨型氮和吡啶型氮为主要掺杂类型的催化剂。研究发现起始电位随吡啶型氮含量增加而增加。在不同的电位下,吡啶型氮含量与电流密度均存在线性关系,与制备方法无关,电催化活性只依赖于吡啶型氮含量(图3)。二氧化碳吸附实验证明了Lewis碱位点的存在,ORR测试结果表明活性位点就是与吡啶型氮相邻的具有Lewis碱性的碳原子。
除了控制N掺杂构型外,研究者们还设法提高N掺杂程度、控制石墨化程度、增大比表面积、提高催化剂内部的传质效率以及电导率,以提高所得掺杂碳材料的催化活性。Zhu等[41]通过无溶剂球磨法制备了N含量高达31.7%的有序介孔碳。Tang等[32]选择双嵌段共聚物胶束的自发共组装作为软模板,利用多巴胺的自聚得到球形结构,随后热解产生大量介孔结构。特殊的孔结构显著提高了传质效率,使得催化活性得到明显提高,基本达到了商业Pt/C的水平。另外,微波辅助[37]、溶液等离子体处理[27]、有机缩合[22]等制备方法也显示了合成不同结构类型的N掺杂碳催化剂的潜力。
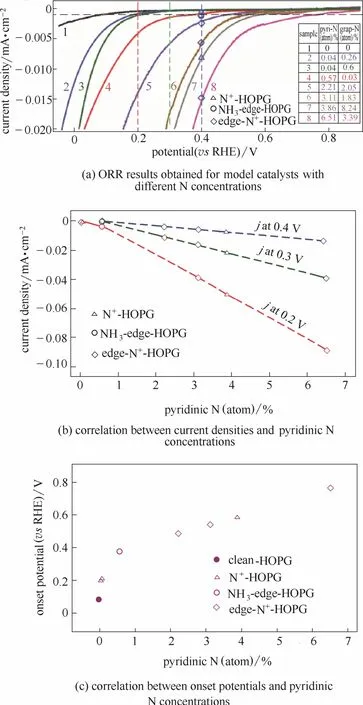
图3 N掺杂高度取向热解石墨N-HOPG模型催化剂的ORR活性[40]Fig.3 Catalytic performance of N-HOPG model catalysts in ORR[40]
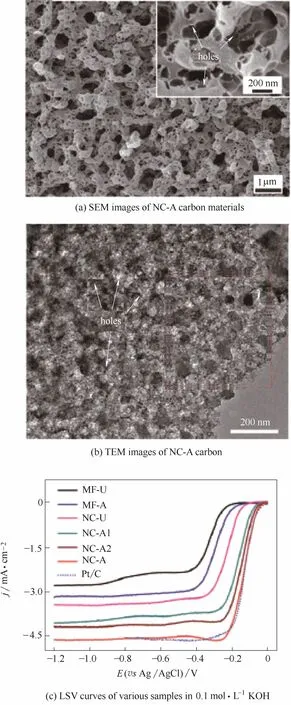
图4 NC-A碳材料的形貌及电化学性能[31]Fig.4 Morphology and electrochemical performance of NC-A carbon materials[31]
在杂元素掺杂碳材料中,往往表现出3种“矛盾”现象:第一,杂元素掺杂度提高会增加活性位点数量,但是电导率会随之下降;第二,随着电导率的增加,比表面积和孔体积下降;第三,微孔结构能够显著提高反应比表面积,但会阻碍反应物的传质,而大孔结构的作用则与之相反。因此,为获得更高的催化性能,设计和实现合理的组成和结构,平衡各“矛盾”因素的影响,成为当前掺杂碳材料催化剂研究的焦点。He等[31]选择聚多巴胺改性混合纤维素酯滤膜同时作为三维大孔模板以及内孔的生孔剂,设计了一种三维分级多孔N掺杂碳材料(NC-A)[图4(a)、(b)]。大孔的存在提高了反应物的传质效率,而大孔内部产生的介孔与微孔显著提高了催化剂的比表面积。这种精确设计的分级多孔结构降低了反应物扩散的阻力,使得更多的活性位点暴露出来,催化活性得到了显著提高[图4(c)]。Sa等[26]利用碳纳米管(CNTs)的高电导率与杂原子掺杂碳材料(HDC)的高催化活性制备得到了CNTs@HDC型催化剂(制备过程如图5所示)。在保持原有结构的基础上电导率得到显著的提高,催化活性进一步增强。
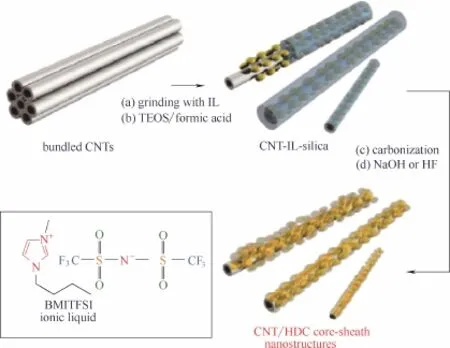
图5 CNT/HDC核壳纳米结构的制备[26]Fig.5 Synthesis of CNT/HDC core-sheath nanostructures[26]
除了N原子掺杂外,F原子掺杂也具有正电荷极化作用[44-46]。F原子半径略小于N原子、电负性大于N原子,在与C原子成键过程中电子严重地偏向F原子但并未完全脱离C原子,因此键型由共价键过渡到半离子键半共价键的形式。Sun等[44]采用价格低廉的炭黑和氟化氨作为前体,首次制备了F掺杂炭黑催化剂。测试表明,F原子的引入显著地提高了起始电位与极限电流密度,电催化性能甚至超过了商业Pt/C催化剂(图6)。另外,以F掺杂炭黑为阴极催化剂的碱性燃料电池功率密度可以达到商业Pt/C催化剂的1.6倍。如此高的催化活性归于半离子键形式的C—F键的引入产生了大量的活性位点。除此之外,采用与N掺杂合成类似的模板法,在合成过程用含F前体替代N源,也能获得具有较高催化活性的F掺杂碳材料[45-46]。
1.2 负电荷极化
B、P等原子的电负性较C原子的电负性更小,与碳原子成键过程中存在负电荷极化作用。当它们取代sp2碳晶格中的碳原子或者接枝到碳材料的基面和边缘位时,均匀分布的电荷密度被打破,掺杂原子带正电,成为吸附氧活性位点(图7)[47]。N由于含有一对孤对电子,可以与sp2碳的大π键形成π共轭体系,显著地影响碳原子的自旋密度。而B不含孤对电子,只能通过电负性的差异对碳原子产生负电荷极化作用,因此电催化活性一般低于同类型N掺杂碳材料。
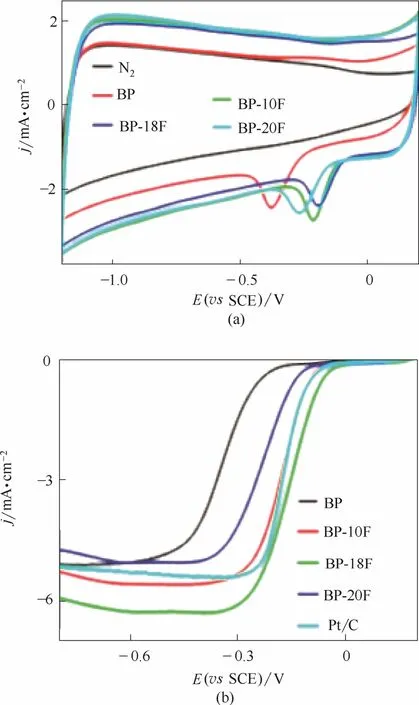
图6 F掺杂碳催化剂在0.1 mol·L-1KOH溶液中有O2或无O2条件下的循环伏安曲线(a)和不同掺杂量BP-F催化剂及商品质量分数20% Pt/C催化剂的线性扫描曲线(b)[44]Fig.6 CV curves of BP-F catalysts with or without O2in 0.1 mol·L-1KOH(a) and linear sweep curves of different BP-F catalysts and commercial 20% Pt/C catalyst(b)[44]
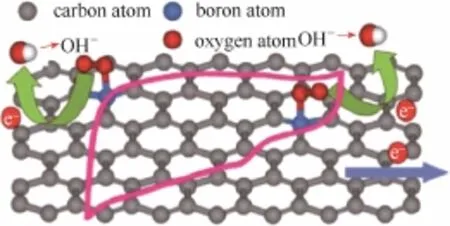
图7 硼掺杂石墨烯BG表面的ORR电子转移示意图[47]Fig.7 Illustration of electron transfer for ORR on BG surface[47]
在B掺杂碳材料中,主要有BC3、B4C、BC2O以及BCO24种活性物种[48]。DFT理论计算表明,B通过电荷极化作用自身带正电,增强了吸附氧分子的能力。同时,正离子吸引sp2共轭的π*电子填充到2pz轨道,随后直接转移到吸附态氧分子的反键轨道上。反键轨道电子的填充降低了氧分子的稳定性,大大削弱O—O键的强度,反应所需活化能大大减少(降低了过电位),促进了氧还原反应的发生[49](图8)。因此,在保证电导率的同时尽可能提高B掺杂量,可以显著地提高催化活性。Vineesh等[50]和Sheng等[47]分别合成得到了B掺杂石墨烯,所得材料催化活性均随B含量提高而提高,但是催化性能均略低于Pt催化剂。
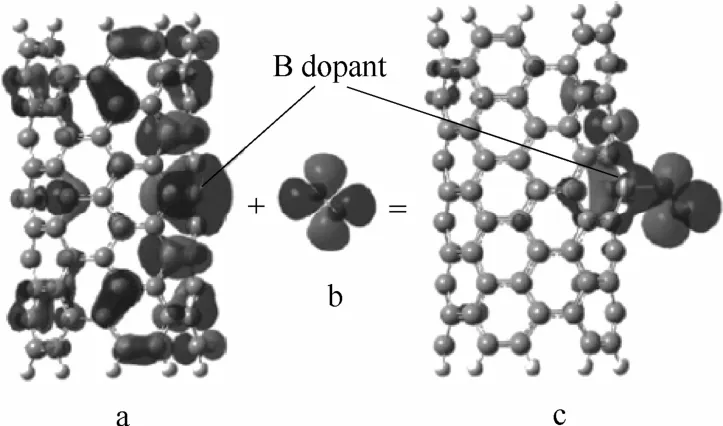
图8 硼掺杂碳纳米管BCNT上参与O2吸附的重要分子轨道[49]Fig.8 Important molecular orbitals involved in O2adsorption on BCNT[49]
与N掺杂碳材料类似,几何结构、传质速率和电子转移速率也是B掺杂碳材料催化活性的关键影响因素。Panomsuwan等[51]采用溶液等离子体处理法制备了具有联通孔结构的B掺杂碳纳米颗粒。Zhou等[52]利用二氧化碳超临界流体技术,采用一锅法制备了具有分级多孔结构的三维还原氧化石墨烯(图9)。特殊的几何结构以及较高的B掺杂量[2.9%(atom)]使得催化活性显著提高。
P掺杂方式与B有所不同。P原子由于半径较大,插入sp2碳晶格较困难,通常接枝在碳基面或边缘处。XPS测试结果表明P掺杂的活性主要由P—C和P—O产生[53]。Liu等[54]通过热解甲苯和三苯基磷得到了表面富含帽状凸起的石墨片层结构,这种特殊结构的形成与P掺杂形成的缺陷位有关。由于P原子难以直接插入sp2碳晶格,在原位掺杂过程中破坏了晶体结构,产生了缺陷,这种缺陷随P掺杂含量增加愈发明显[55]。EIS测试显示,P原子的引入显著降低了反应电阻,即提高了催化活性[56]。Zhang等[57]通过非模板热解法在还原氧化石墨烯的过程中原位掺杂P,制备了P原子含量高达1.81%原子数的掺杂石墨烯。Raman光谱实验结果表明,在G峰强度基本不变的情况下,D峰的强度得到了明显增强,ID/IG比值增大,说明高含量的P掺杂在边缘产生了大量缺陷位。但大量的P掺杂导致所得碳材料的导电性较差,需要加入一定量导电炭黑才可以使高催化活性得以显现。混合了炭黑的P掺杂碳材料性能甚至超过了商业Pt/C催化剂。
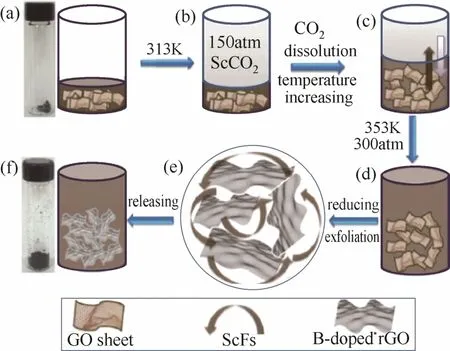
图9 硼掺杂三维还原氧化石墨烯的合成示意图[52]Fig.9 Schematic illustration for synthesis of B-3DrGO[52]
2 自旋极化作用
与N、B等元素不同,S的电负性与C非常接近(分别为2.58和2.55),因此S原子的引入对于碳晶格的电荷密度分布影响较小。但与P原子类似,S原子半径也明显大于C,故也往往接枝在碳基面边缘处。在S掺杂碳出现前,人们普遍认为杂原子掺杂主要是通过电荷极化作用提高催化活性[13,58-60],研究主要集中在电负性与碳原子差异较大的元素与碳掺杂。Yang等[61]首先制备出具有高电催化活性的S掺杂石墨烯,从而证明S催化活性不仅仅由电负性差异产生。随后,研究者们选取不同的前体,例如有机凝胶[62]、磺酸型阳离子交换树脂[63]、碳纳米管[64]、氧化石墨烯[61,65-66]以及杂环化合物[62]等,制备了各具不同结构特点的S掺杂碳材料。Jeon等[67]通过在三氧化硫气氛下球磨石墨,在S掺杂的同时通过机械作用实现石墨片层的剥离,得到了边缘选择性功能化石墨烯纳米片。Chen等[66]选择Na2S作为硫源和还原剂,在低温条件下同时实现了氧化石墨烯的还原和S元素掺杂。Yang等[65]利用氧化石墨烯对十六烷基三甲基溴化铵(CTAB)的吸附以及正硅酸乙酯(TEOS)的水解制备了氧化石墨烯-多孔二氧化硅片层,随后在NH3或H2S气氛下热解,分别制备了N掺杂石墨烯和S掺杂石墨烯(图10)。结果证明S元素主要以噻吩型S和氧化型S形式掺杂在石墨烯的边缘处。
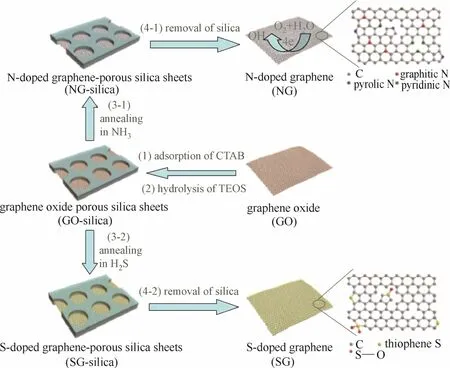
图10 N和S掺杂石墨烯合成示意图[65]Fig.10 Schematic illustration of fabrication of N-doped and S-doped graphene[65]
研究表明,掺杂S主要以表面吸附态S、锯齿形边缘S(SO2)、椅形边缘S(SO2)、S环簇等构型存在。Jeon等[68]用DFT方法模拟计算了几种掺杂S构型的电荷密度、自旋密度以及磁矩(表1)。相比初始状态的石墨烯,表面吸附态S和环簇状态S的自旋密度和电荷密度均未发生明显改变,因此可以认为这两种形式的S掺杂对于氧还原反应催化性能影响有限。而锯齿形边缘和椅形边缘共价键合的S或SO2对自旋密度和电荷密度均产生了显著影响,可以作为活性位点。轨道计算结果表明,石墨烯边缘共价键合的S原子对最高已占轨道(HOMO)与最低未占轨道(LUMO)产生了强烈的极化作用(图11),这些极化区域可以作为ORR的活性位点,对催化性能的提高起到关键作用。
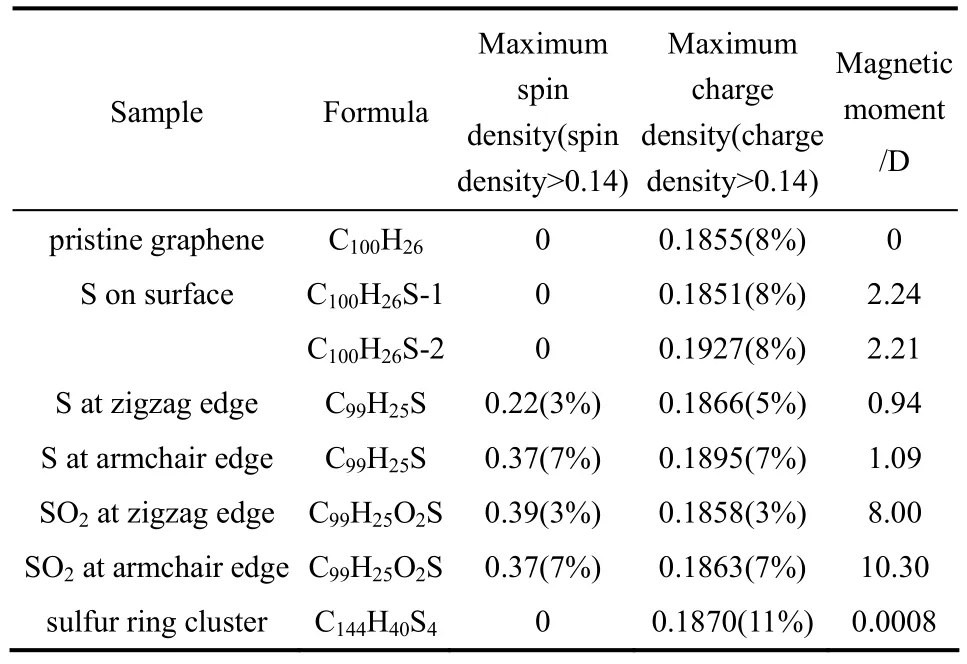
表1 不同掺杂S构型的Mulliken自旋密度、电荷密度以及磁矩计算结果[68]Table 1 Mulliken spin densities, charge densities, and magnetic moments in structures of pristine graphene, SGnP, and SOGnP[68]
除了N、B、P、S、F等元素的掺杂之外,Cl、Br、I等卤族元素[69-70]以及Se[71]、Sb[72]等元素单掺杂碳材料催化剂也已经有研究者报道。研究结果表明这些杂元素掺杂催化剂电催化活性均得到了明显的提高,然而具体作用机理还有待深入研究。
尽管若干单杂原子掺杂碳材料催化剂的性能已经接近甚至超过现行商业Pt/C催化剂,但是依然未达到理想的目标[73]。对杂原子掺杂石墨烯进行模拟计算发现单杂原子掺杂石墨烯材料均位于火山型曲线右侧,这显示单元素掺杂无法达到理想的X-G催化剂的性能[10](图12)。
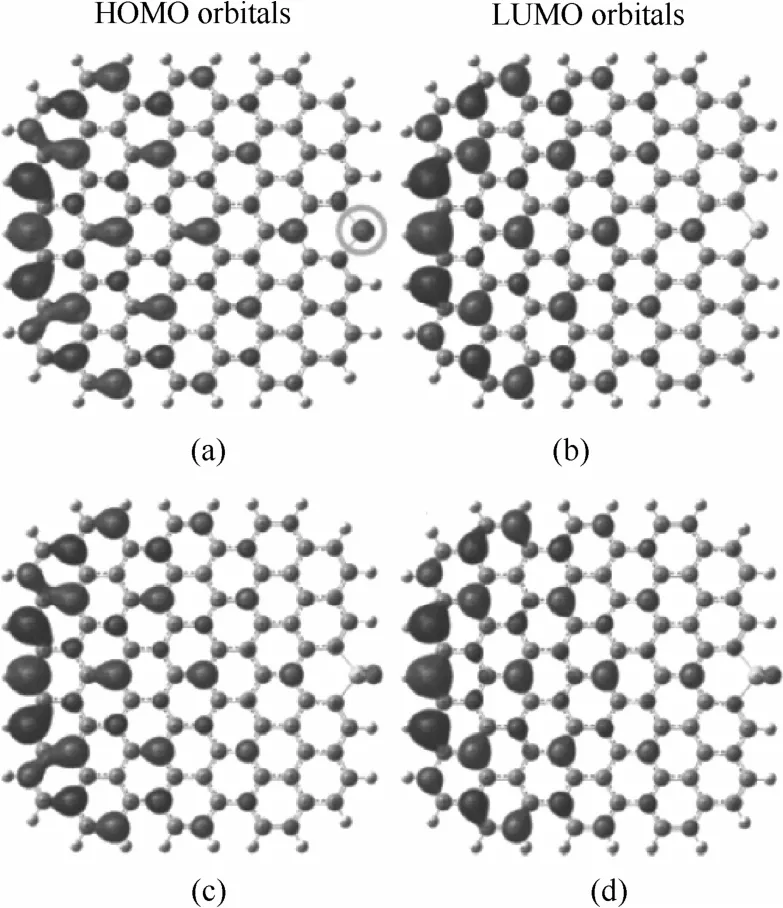
图11 HOMO轨道分布[SGnP(a)和SOGnP(c)]以及LUMO轨道分布[SGnP(b)和SOGnP(d)][68]Fig.11 HOMO and LUMO distributions[68]
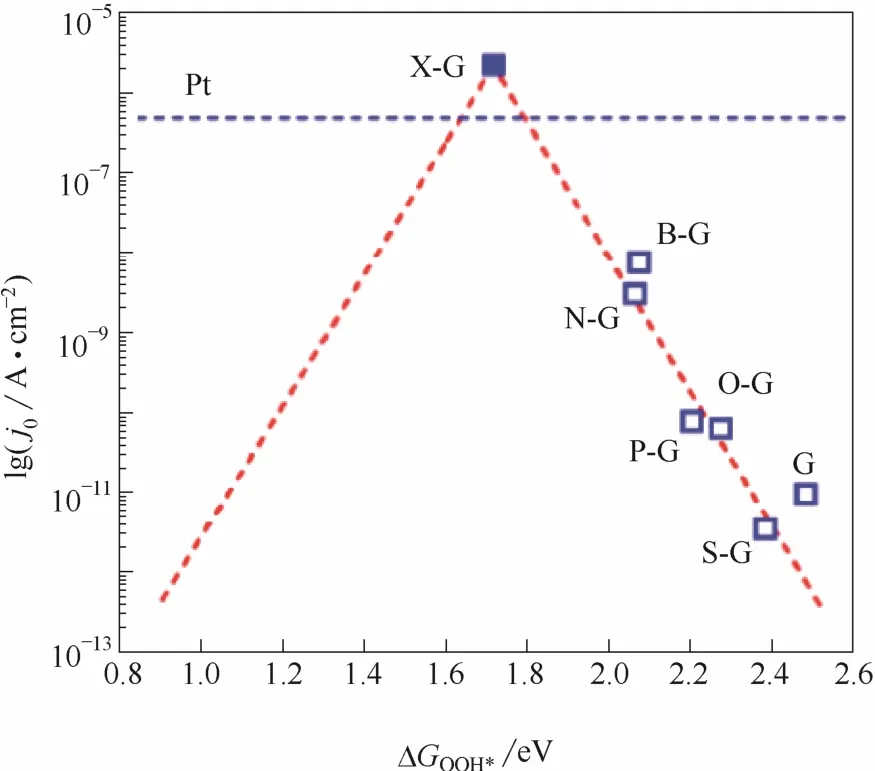
图12 掺杂石墨烯催化剂理论交换电流密度和OOH*吸附能火山型曲线关系(红色虚线)[10](蓝色方块为每种掺杂石墨烯催化剂的实验交换电流密度和DFT计算出的OOH*吸附能)Fig.12 Volcano plot betweenj0theoryand ΔGOOH*(red dashed line)[10](blue hollow squares arej0exptand DFT derived ΔGOOH*for each doped graphene catalyst)
3 复合作用
单一杂元素掺杂对碳原子的电荷密度和自旋密度改变有限,为了得到更好的催化性能,研究者们将目光转向多元素掺杂碳材料,希望通过不同组分之间的协同作用对催化剂的电子性质进行调控,获得更理想的杂原子掺杂碳材料催化剂。
3.1 杂元素共掺杂
目前,研究者们已经尝试了多种杂元素共掺杂碳材料,例如N、B共掺杂[74-77],N、S共掺杂[78-99],甚至三元掺杂[100-104]等。相比单掺杂,多掺杂可以充分利用不同元素之间的极化作用。例如N、B掺杂不仅可以利用N元素的正电荷极化作用和B元素的负电荷极化作用,而且在形成N-C-B结构时产生协同效应,显著提高活性碳位点的催化活性[74]。然而,共掺杂体系较单掺杂体系更为复杂,定向合成某种掺杂构型的碳材料也更为困难[58]。
研究者们通常采用在合成单掺杂碳材料过程中同时加入另一种原料作为前体实现共掺杂[81],或者将单掺杂材料进行后处理,通过调整反应温度以及原料配比控制掺杂元素含量。由于合成过程采用了不同的原料,反应过程相对复杂,通常需要采用合成模板[78,100],增加了额外处理步骤。因此,为了简化这一过程,同时能够合成可控形貌的多掺杂碳材料,对于前体的优化成了亟需解决的问题。一系列金属有机化合物[105-107]、生物质材料[97-98,108-113]等,由于其特殊的结构特性以及丰富的元素组成,可以作为自牺牲模板合成得到具有丰富孔结构的多元素共掺杂碳材料催化剂,受到了广泛关注。
Shao等[108]选择富含C、N、P、S的鸡蛋黄作为前体,通过简单的一步热解法实现了生物质的碳化和N、P、S 3种元素的原位掺杂。在热解过程中,利用熔融KCl的作用形成了富含松散堆积的石墨纳米片的层状结构,这种结构显著地提高了材料的比表面积(图13)。电化学测试表明,此三元掺杂碳材料的ORR催化性能已经达到了商业Pt/C催化剂的水平,而且有更高的稳定性。
3.2 杂元素、金属元素共掺杂
除了杂原子元素共掺杂外,杂原子与金属原子共掺杂也能显著提高催化活性。Razmjooei等[114]在P掺杂石墨烯的基础上合成了Fe、P共掺杂石墨烯。由于Fe原子与P原子之间的相互作用,产生Fe-P活性物种的同时P元素掺杂量也得到显著的提高(表2)。通过Fe原子与P原子之间的协同作用,Fe、P共掺杂石墨烯催化活性相比单一P掺杂石墨烯得到了显著的提高(图14)。Guo等[115]通过一步热解铁盐处理后的茶叶制备了N、P、Fe三元掺杂碳材料(图15)。茶叶本身具有特殊的三维结构,热解后形成分级三维多孔结构。多酚物质作为配体与Fe反应形成配合物,在随后的热解过程中产生了高催化活性的Fe-N活性位点。几何结构与元素掺杂形成的协同效应显著提高了催化活性,起始电位与极限电流密度均超过了Pt/C催化剂。
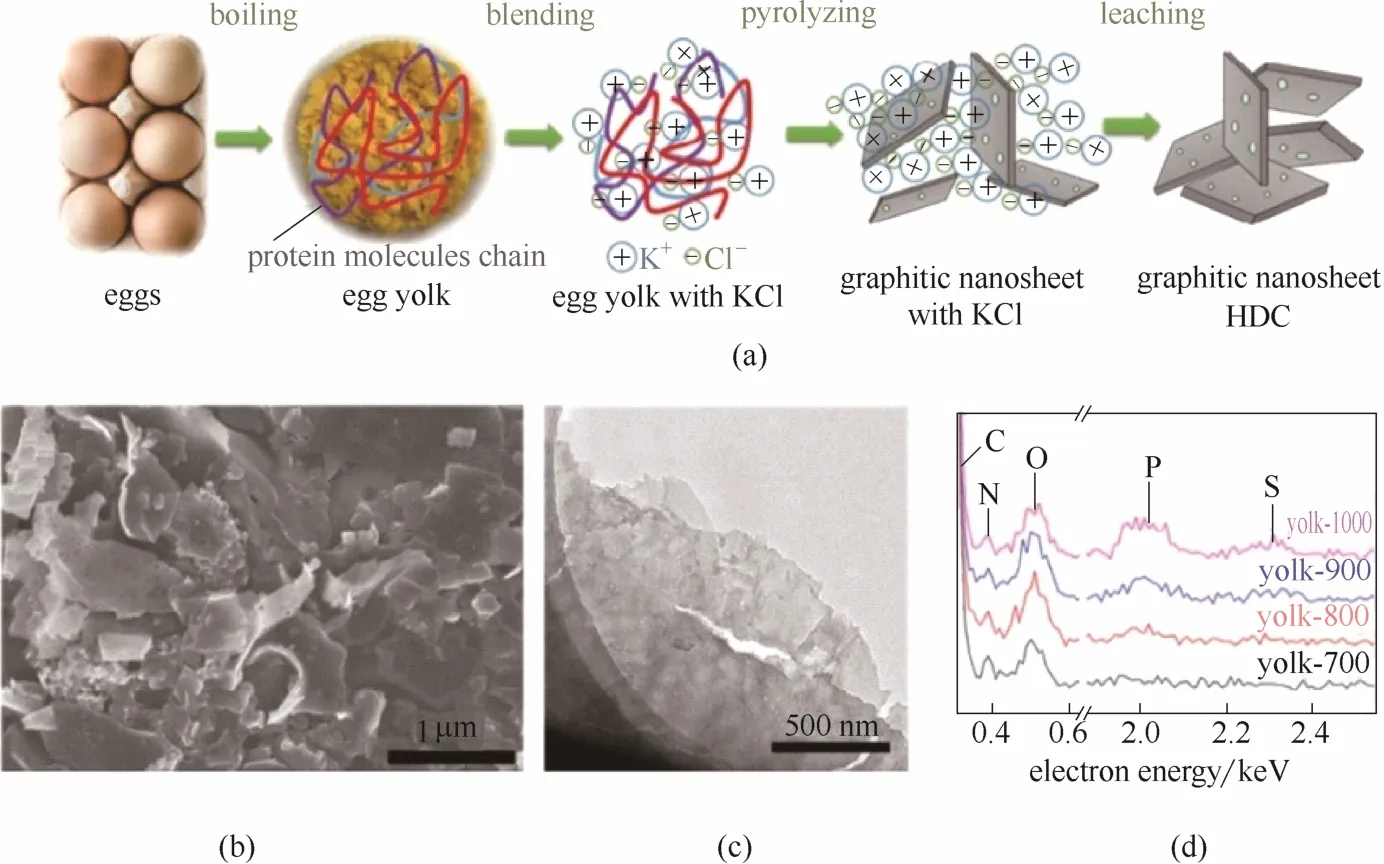
图13 热解鸡蛋黄HDC催化剂的制备路线(a), Yolk-CN-800样品的扫描电镜照片(b)和透射电镜照片(c)以及不同温度下得到的HDC催化剂EDS谱图(d)[108]Fig.13 Schematic illustration for synthesis process of yolk derived HDC catalysts (a), SEM (b) and TEM (c) images of sample Yolk-CN-800, and EDS spectra of yolk derived HDC catalysts at different temperatures(d)[108]
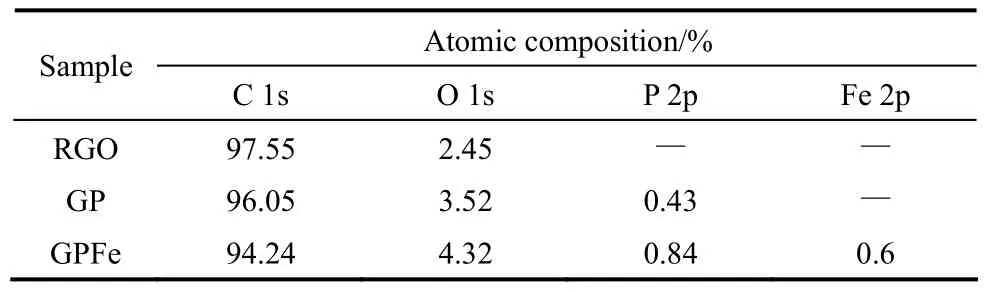
表2 不同样品表面XPS元素分析结果[114]Table 2 Surface element contents obtained from XPS analysis for pristine RGO, GP, and GPFe[114]
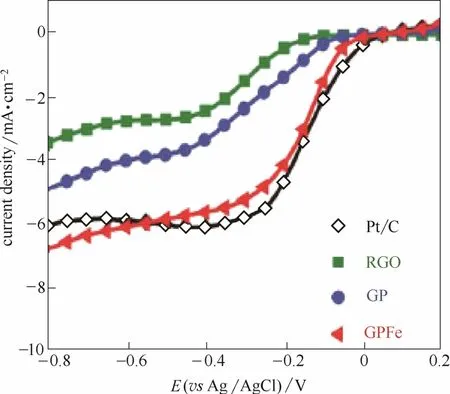
图14 P掺杂以及P、Fe共掺杂对还原氧化石墨烯ORR性能的影响[114]Fig.14 Effect of introduction of P into RGO and Fe into P-doped RGO on ORR[114]
除了金属元素与杂元素共同掺入碳结构外,在杂原子掺杂碳材料上负载金属氧化物或硫化物等的研究也有报道[116-121]。Zhu等[121]利用N掺杂碳纳米管包覆碳化铁纳米颗粒形成核壳结构。内核Fe元素的加入并未产生新的活性位点,而是通过电子效应改变外层碳材料电子分布,从而提高催化活性。Liang等[116]采用N掺杂还原氧化石墨烯负载Co3O4纳米颗粒,相比未掺杂复合材料催化活性有了显著的提高(图16)。X射线吸收近边结构(XANES)表明石墨烯中的C原子与O原子或其他原子形成了新的化学键,这种化学键很有可能是Co—O—C和Co—N—C。这种协同耦合作用改变了原子的电荷密度,增加了反应的活性位点,因而显著提高了复合材料的催化活性。
在杂元素掺杂碳材料的合成过程中难以避免微量金属元素的引入。例如Hummer法制备的氧化石墨烯中含有少量金属杂质[122],热解过程采用的催化剂[123]、前体中均可能含有金属元素[18]。这些金属通常为Fe、Ni、Co等存在孤对电子或外层空轨道的过渡金属元素,对于sp2碳的电子结构具有显著的影响,也有实验研究证明[124]合成过程中微量金属元素的引入对于杂元素掺杂碳材料的催化活性具有显著的影响,因此不能忽略微量金属元素对掺杂碳材料的作用。Guo等[125]在热解聚(3,4-亚乙基二氧噻吩)-聚苯乙烯磺酸的过程中,由于氧化剂硝酸铁的残留,得到了S、N、Fe共掺杂的分级多孔泡沫碳结构。痕量Fe元素的引入不仅增加了活性位点数量,还通过与杂原子之间的协同作用提高了位点的反应活性。
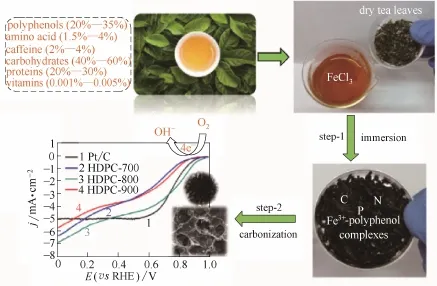
图15 原始茶叶热解制备ORR电催化剂HDPC-X过程[115]Fig.15 Schematic illustration of fabrication of HDPC-Xelectrocatalysts towards ORR from pristine tea leaves[115]
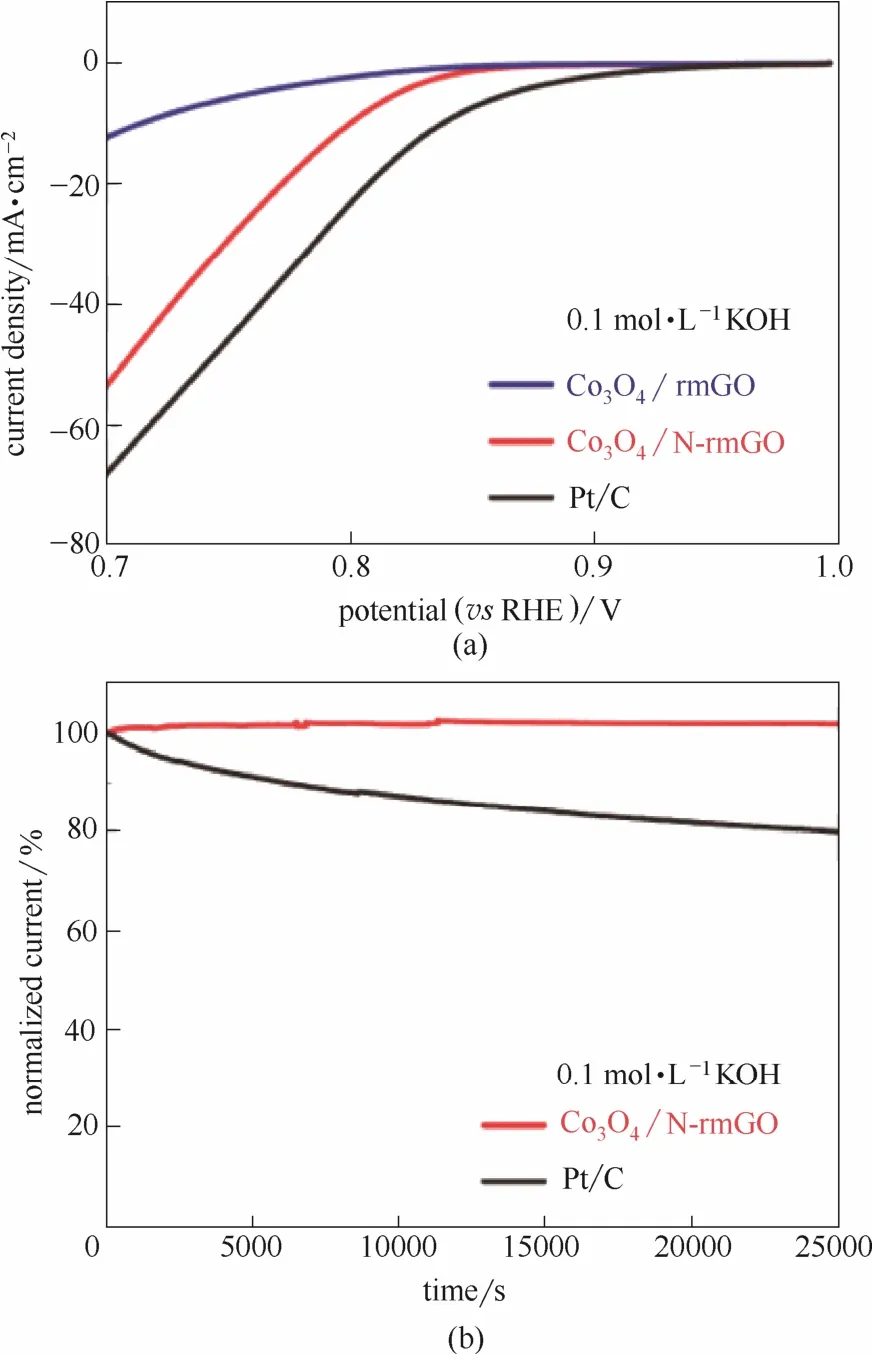
图16 催化剂的ORR性能以及稳定性[116]Fig.16 ORR performance and stability of catalysts[116]
4 催化剂在单电池中的性能
对新催化剂的研究中,多数只用薄膜电极测试催化剂的性能。但薄膜电极与燃料电池电极的结构和工作条件有一定差别,在薄膜电极上性能优异的催化剂不一定在电池中同样性能优异。为了综合评价新催化剂,对其在电池中做测试十分必要。因此,为数不多的杂原子掺杂碳基催化剂在单电池中性能的数据尤有参考价值。
Venkateswara等[126]对N掺杂碳纳米管进行了阴离子交换膜燃料电池测试。结果表明,掺杂碳材料的开路电压略低于Pt催化剂,最大功率密度约为Pt催化剂的1/2(图17)。Sa等[26]在碳纳米管的基础上引入N掺杂碳,制备得到了CNTs@HDC型催化剂。相比碳纳米管阴极,电池功率密度提高了23.3倍,开路电压接近Pt/C催化剂。
单掺杂碳材料由于活性位点相对较少,在实际电池测试中性能与Pt基催化剂仍有较大差距。研究者们通过引入多元素共掺杂的方法增加活性位点数量,通过协同效应提高材料在实际电池中的催化活性。Sun等[127]在N掺杂碳材料的基础上引入F元素,活性位点数量得到了显著提高。碱性直接甲醇燃料电池测试结果表明N、F共掺杂碳材料催化活性已经达到Pt/C催化剂的水平。Xiao等[128]采用N掺杂多孔石墨层对Fe3C纳米颗粒进行封装。内层Fe元素的电子效应改变了外层碳原子的电荷密度,吸附氧分子的能力进一步提高,氧还原反应活性得到增强。尽管酸性条件下功率密度仍低于Pt基催化剂,但是稳定性得到了显著的提高。而在碱性条件下,无论是开路电压还是功率密度,复合催化剂的性能均超过了Pt/C催化剂的水平(图18)。
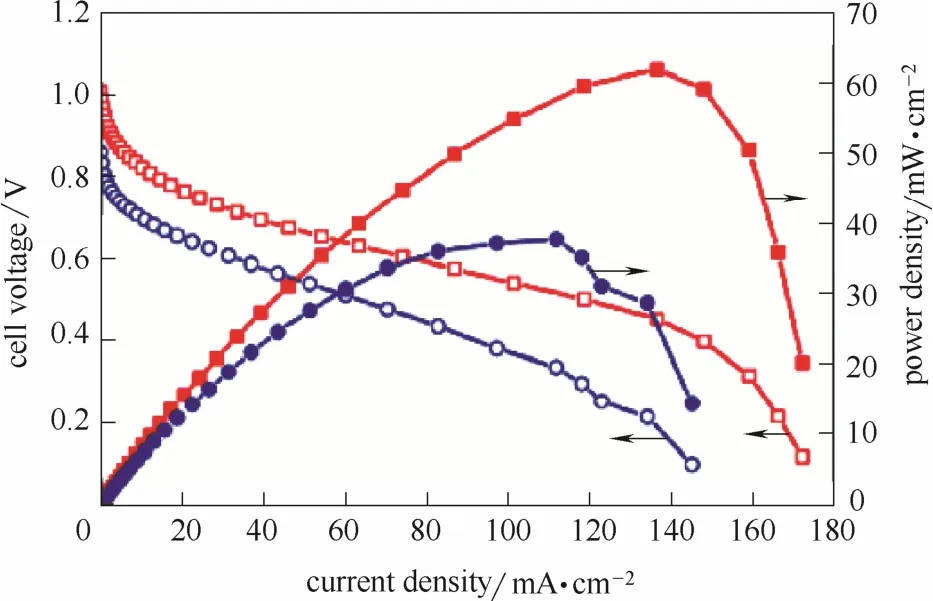
图17 以N-CNT(蓝)和Pt/C (红)为阴极的膜电极的阴离子交换膜燃料电池性能[126]Fig.17 Anion-exchange membrane fuel cell performance of MEA with N-CNT (blue) and Pt/C (red) as cathode[126]
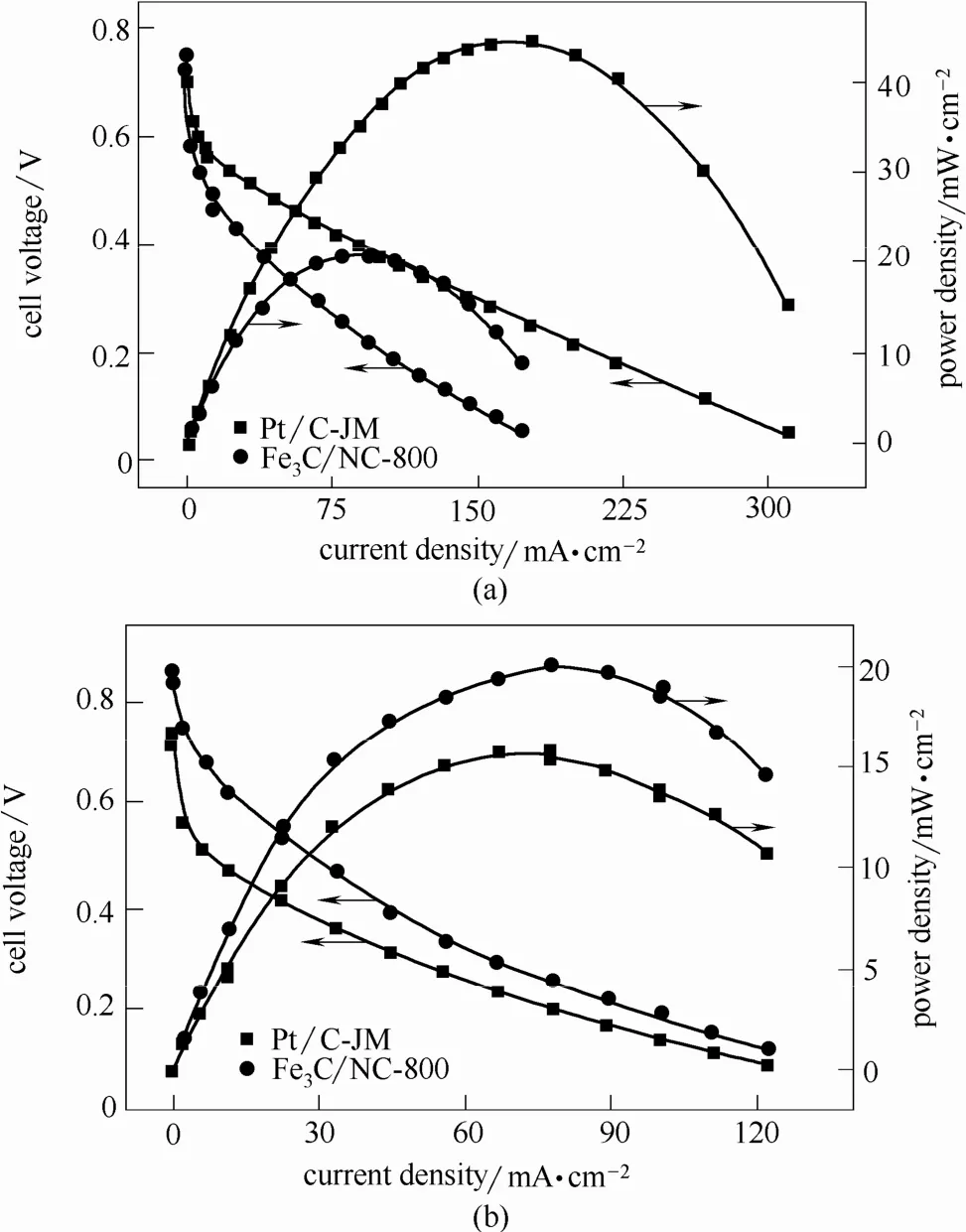
图18 Fe3C/NG-800和Pt/C (60%)催化剂作为阴极的直接甲醇燃料电池在60℃下性能测试[128]Fig.18 DMFC performance at 60℃ using Pt/C (60%) or Fe3C/NG-800 as cathode[128]
5 小结与展望
相比于现行的铂族贵金属ORR催化剂,杂原子掺杂碳基催化剂来源广泛、价格低廉,具有大规模广泛应用的潜力。近年来若干杂原子掺杂碳基催化剂的活性和稳定性已经达到甚至超过了目前应用最多的商品Pt/C催化剂。对碳分子结构中杂原子掺杂的作用机制也已经有了较明确的认识。被掺杂的杂原子主要是通过电荷极化以及自旋极化作用改变碳原子的电子结构,从而实现催化活性的提高。N、F等元素电负性远大于碳原子,在与碳原子成键时对相邻碳原子产生正电荷极化,提高了碳原子的电荷密度,成为更好吸附氧分子的位点。B、P元素电负性较小,与碳原子成键时存在电子排斥作用,对相邻碳原子产生负电荷极化作用,自身成为活性位点。S元素电负性与C接近,对于电荷密度影响较小,主要通过自旋极化作用改变碳原子的自旋密度,从而形成活性位点。理论上,只要杂原子的掺杂能够引起电荷密度或自旋密度足够的非对称分布,就能显著提高催化活性。为了获得更高性能的碳基ORR催化剂,还探索了包括金属原子在内的多种原子的共掺杂碳材料以及这些材料与金属氧化物、硫化物等的复合材料,期望通过多原子之间的协同效应形成高ORR活性位点。
杂原子掺杂碳基ORR催化剂已经显示了光明的前景,但距离开发出高性能、低价格的商业化产品还有较长路程。杂原子掺杂碳基ORR催化剂发展较晚,几乎所有已经探索的领域都值得进行更深入细致的探索,例如对N单掺杂高活性位点构型的理论和实验确定,再如多掺杂复合型碳基催化剂的合理设计及高选择性合成。另外,电催化剂的性能不但受限于活性点的多寡和活性高低,还取决于电荷和反应剂的传递。因此,应发展从量子化学到多孔结构中传质的多尺度模拟计算方法,以更全面深入地认识杂原子掺杂碳基催化剂性能的影响因素并更合理地设计ORR催化剂。目前的研究中,许多杂原子掺杂碳基ORR催化剂与商品Pt/C催化剂的性能是用浸于液体电解质中的薄膜电极比较的。为更接近实际使用条件,今后新杂原子掺杂碳基催化剂性能应更多地用气体电极和电池单池检验。而且,目前的膜电极技术是基于Pt/C催化剂,因此应当研究适用于新型的碳基催化剂的膜电极制备方法。最后,杂原子掺杂碳基ORR催化剂的商品化开发条件已经基本具备,相应的开发工作有待开展,相关的技术难点有待挑战。
[1] LIU J, MOONEY H, HULL V,et al. Systems integration for global sustainability[J]. Science, 2015, 347(6225): 1258832.
[2] XIA W, MAHMOOD A, LIANG Z,et al. Earth-abundant nanomaterials for oxygen reduction[J]. Angewandte Chemie International Edition, 2016, 55(8): 2650-2676.
[3] NIE Y, LI L, WEI Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction[J]. Chemical Society Reviews, 2015, 44(8): 2168-2201.
[4] HIGGINS D, ZAMANI P, YU A,et al. The application of graphene and its composites in oxygen reduction electrocatalysis: a perspective and review of recent progress[J]. Energy & Environmental Science, 2016, 9(2): 357-390.
[5] WOOD K N, O'HAYRE R, PYLYPENKO S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications[J]. Energy & Environmental Science, 2014, 7(4): 1212-1249.
[6] DUAN J, CHEN S, JARONIEC M,et al. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes[J]. ACS Catalysis, 2015, 5(9): 5207-5234.
[7] ZHU C, LI H, FU S,et al. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures[J]. Chem. Soc. Rev., 2016, 45(3): 517-531.
[8] ZHOU M, WANG H L, GUO S. Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials.[J]. Chemical Society Reviews, 2016, 45(5): 1273-1307.
[9] HE W, WANG Y, JIANG C,et al. Structural effects of a carbon matrix in non-precious metal O2-reduction electrocatalysts[J]. Chemical Society Reviews, 2016, 45(9): 2396-2409.
[10] JIAO Y, ZHENG Y, JARONIEC M,et al. Origin of the electrocatalytic oxygen reduction activity of graphene-based catalysts: a roadmap to achieve the best performance[J]. Journal of the American Chemical Society, 2014, 136(11): 4394-4403.
[11] ZHAO Y, YANG L, CHEN S,et al. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes[J]. Journal of the American Chemical Society, 2013, 135(4): 1201-1204.
[12] WINTHER-JENSEN B, WINTHER-JENSEN O, FORSYTH M,et al. High rates of oxygen reduction over a vapor phase-polymerized PEDOT electrode[J]. Science, 2008, 321(5889): 671-674.
[13] ZHANG L, XIA Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells[J]. Journal of Physical Chemistry C, 2011, 115(22): 11170-11176.
[14] DAI L, XUE Y, QU L,et al. Metal-free catalysts for oxygen reduction reaction[J]. Chemical Reviews, 2015, 115(11): 4823-4892.
[15] ZHU C, DU D, EYCHMULER A,et al. Engineering ordered and nonordered porous noble metal nanostructures: synthesis, assembly, and their applications in electrochemistry[J]. Chemical Reviews, 2015, 115(16): 8896-8943.
[16] 聂瑶, 丁炜, 魏子栋. 质子交换膜燃料电池非铂电催化剂研究进展[J]. 化工学报, 2015, 66(9): 3305-3318. NIE Y, DING W, WEI Z D. Recent advancements of Pt-free catalysts for polymer electrolyte membrane fuel cells[J]. CIESC Journal, 2015, 66(9): 3305-3318.
[17] ZHAO Z, LI M, ZHANG L,et al. Design principles for heteroatom-doped carbon nanomaterials as highly efficient catalysts for fuel cells and metal-air batteries[J]. Advanced Materials, 2015, 27(43): 6834-6840.
[18] GONG K, DU F, XIA Z,et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction[J]. Science, 2009, 323(5915): 760-764.
[19] LIU R, WU D, FENG X,et al. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction[J]. Angewandte Chemie, 2010, 122(14): 2619-2623.
[20] SHENG Z H, SHAO L, CHEN J J,et al. Catalyst-free synthesis of nitrogen-doped grapheneviathermal annealing graphite oxide with melamine and its excellent electrocatalysis[J]. ACS Nano, 2011, 5(6): 4350-4358.
[21] GAO S, FAN H, CHEN Y,et al. One stone, two birds: gastrodia elata-derived heteroatom-doped carbon materials for efficient oxygen reduction electrocatalyst and as fluorescent decorative materials[J]. Nano Energy, 2013, 2(6): 1261-1270.
[22] ZUO Z, LI W, MANTHIRAM A. N-heterocycles tethered graphene as efficient metal-free catalysts for an oxygen reduction reaction in fuel cells[J]. Journal of Materials Chemistry A, 2013, 1(35): 10166-10172.
[23] PARK M, LEE T, KIM B. Covalent functionalization based heteroatom doped graphene nanosheet as a metal-free electrocatalyst for oxygen reduction reaction[J]. Nanoscale, 2013, 5(24): 12255-12260.
[24] PALANISELVAM T, VALAPPIL M O, ILLATHVALAPPIL R,et al. Nanoporous graphene by quantum dots removal from graphene and its conversion to a potential oxygen reduction electrocatalystvianitrogen doping[J]. Energy & Environmental Science, 2014, 7(3): 1059-1067.
[25] ZHANG P, SUN F, XIANG Z,et al. ZIF-derivedin situnitrogen-doped porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction[J]. Energy & Environmental Science, 2014, 7(1): 442-450.
[26] SA Y J, PARK C, JEONG H Y,et al. Carbon nanotubes/heteroatom-doped carbon core-sheath nanostructures as highly active, metal-free oxygen reduction electrocatalysts for alkaline fuel cells[J]. Angewandte Chemie International Edition, 2014, 53(16): 4102-4106.
[27] PANOMSUWAN G, CHIBA S, KANEKO Y,et al.In situsolution plasma synthesis of nitrogen-doped carbon nanoparticles as metal-free electrocatalysts for the oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2014, 2(43): 18677-18686.
[28] HU C, WANG L, ZHAO Y,et al. Designing nitrogen-enriched echinus-like carbon capsules for highly efficient oxygen reductionreaction and lithium ion storage[J]. Nanoscale, 2014, 6(14): 8002-8009.
[29] ZHANG L, SU Z, JIANG F,et al. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions[J]. Nanoscale, 2014, 6(12): 6590-6602.
[30] CHEN L, CUI X, WANG Y,et al. One-step hydrothermal synthesis of nitrogen-doped carbon nanotubes as an efficient electrocatalyst for oxygen reduction reactions[J]. Chemistry—An Asian Journal, 2014, 9(10): 2915-2920.
[31] HE W, JIANG C, WANG J,et al. High-rate oxygen electroreduction over graphitic-N species exposed on 3D hierarchically porous nitrogen-doped carbons[J]. Angewandte Chemie International Edition, 2014, 53(36): 9503-9507.
[32] TANG J, LIU J, LI C,et al. Synthesis of nitrogen-doped mesoporous carbon spheres with extra-large pores through assembly of diblock copolymer micelles[J]. Angewandte Chemie International Edition, 2015, 54(2): 588-593.
[33] CHUNG D Y, LEE K J, YU S H,et al. Alveoli-inspired facile transport structure of N-doped porous carbon for electrochemical energy applications[J]. Advanced Energy Materials, 2015, 5(3): 1401309.
[34] ITO Y, CHRISTODOULOU C, NARDI M V,et al. Tuning the magnetic properties of carbon by nitrogen doping of its graphene domains[J]. Journal of the American Chemical Society, 2015, 137(24): 7678-7685.
[35] SUN J, WANG L, SONG R,et al. Enhancing pyridinic nitrogen level in graphene to promote electrocatalytic activity for oxygen reduction reaction[J]. Nanotechnology, 2016, 27(5): 55404-55410.
[36] LIANG H, WU Z, CHEN L,et al. Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: an efficient metal-free oxygen reduction electrocatalyst for zinc-air battery[J]. Nano Energy, 2015, 11: 366-376.
[37] PATEL M, FENG W, SAVARAM K,et al. Microwave enabled one-pot, one-step fabrication and nitrogen doping of holey graphene oxide for catalytic applications[J]. Small, 2015, 11(27): 3358-3368.
[38] LI J, ZHANG Y, ZHANG X,et al. Direct transformation from graphitic C3N4to nitrogen-doped graphene: an efficient metal-free electrocatalyst for oxygen reduction reaction[J]. ACS Applied Materials & Interfaces, 2015, 7(35): 19626-19634.
[39] WAN K, YU Z P, LIANG Z X. Polyaniline-derived ordered mesoporous carbon as an efficient electrocatalyst for oxygen reduction reaction[J]. Catalysts, 2015, 5(3): 1034-1045.
[40] GUO D, SHIBUYA R, AKIBA C,et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts[J]. Science, 2016, 351(6271): 361-365.
[41] ZHU J, YANG J, MIAO R,et al. Nitrogen-enriched, ordered mesoporous carbons for potential electrochemical energy storage[J]. Journal of Materials Chemistry A, 2016, 4(6): 2286-2292.
[42] HE Y, HAN X, DU Y,et al. Bifunctional nitrogen-doped microporous carbon microspheres derived from poly(o-methylaniline) for oxygen reduction and supercapacitors[J]. ACS Applied Materials & Interfaces, 2015, 8(6): 3601-3608.
[43] UNNI S M, DEVULAPALLY S, KARJULE N,et al. Graphene enriched with pyrrolic coordination of the doped nitrogen as an efficient metal-free electrocatalyst for oxygen reduction[J]. Journal of Materials Chemistry, 2012, 22(44): 23506-23513.
[44] SUN X, ZHANG Y, SONG P,et al. Fluorine-doped carbon blacks: highly efficient metal-free electrocatalysts for oxygen reduction reaction[J]. ACS Catalysis, 2013, 3(8): 1726-1729.
[45] WANG H, KONG A. Mesoporous fluorine-doped carbon as efficient cathode material for oxygen reduction reaction[J]. Materials Letters, 2014, 136(1): 384-387.
[46] PANOMSUWAN G, SAITO N, ISHIZAKI T. Simple one-step synthesis of fluorine-doped carbon nanoparticles as potential alternative metal-free electrocatalysts for oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2015, 3(18): 9972-9981.
[47] SHENG Z, GAO H, BAO W,et al. Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells[J]. Journal of Materials Chemistry, 2012, 22(2): 390-395.
[48] AGNOLI S, FAVARO M. Doping graphene with boron: a review of synthesis methods, physicochemical characterization, and emerging applications[J]. Journal of Materials Chemistry A, 2016, 4(14): 5002-5025.
[49] YANG L, JIANG S, ZHAO Y,et al. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2011, 50(31): 7132-7135.
[50] VINEESH T V, KUMAR M P, TAKAHASHI C,et al. Bifunctional electrocatalytic activity of boron-doped graphene derived from boron carbide[J]. Advanced Energy Materials, 2015, 5(17) : 1500658.
[51] PANOMSUWAN G, SAITO N, ISHIZAKI T. Electrocatalytic oxygen reduction activity of boron-doped carbon nanoparticles synthesizedviasolution plasma process[J]. Electrochemistry Communications, 2015, 59: 81-85.
[52] ZHOU Y, YEN C H, FU S,et al. One-pot synthesis of B-doped three-dimensional reduced graphene oxideviasupercritical fluid for oxygen reduction reaction[J]. Green Chemistry, 2015, 17(6): 3552-3560.
[53] LIU Z, PENG F, WANG H,et al. Novel phosphorus-doped multiwalled nanotubes with high electrocatalytic activity for O2reduction in alkaline medium[J]. Catalysis Communications, 2011, 16(1): 35-38.
[54] LIU Z W, PENG F, WANG H J,et al. Phosphorus-doped graphite layers with high electrocatalytic activity for the O2reduction in an alkaline medium[J]. Angewandte Chemie, 2011, 123(14): 3315-3319.
[55] YANG D, BHATTACHARJYA D, INAMDAR S,et al. Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media[J]. Journal of the American Chemical Society, 2012, 134(39): 16127-16130.
[56] LIU Y, LI K, LIU Y,et al. The high-performance and mechanism of P-doped activated carbon as a catalyst for air-cathode microbial fuel cells[J]. Journal of Materials Chemistry A, 2015, 3(42): 21149-21158.
[57] ZHANG C, MAHMOOD N, YIN H,et al. Synthesis of phosphorusdoped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries[J]. Advanced Materials, 2013, 25(35): 4932-4937.
[58] PARAKNOWITSCH J P, THOMAS A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications[J]. Energy & Environmental Science, 2013, 6(10): 2839-2855.
[59] ZHU C, DONG S. Recent progress in graphene-based nanomaterials as advanced electrocatalysts towards oxygen reduction reaction[J]. Nanoscale, 2013, 5(5): 1753-1767.
[60] YANG Z, NIE H, CHEN X,et al. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction[J]. Journal of Power Sources, 2013, 236(15): 238-249.
[61] YANG Z, YAO Z, LI G,et al. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction[J]. ACS Nano, 2011, 6(1): 205-211.
[62] KICINSKI W, DZIURA A. Heteroatom-doped carbon gels from phenols and heterocyclic aldehydes: sulfur-doped carbon xerogels[J]. Carbon, 2014, 75: 56-67.
[63] ZHANG Y, CHU M, YANG L,et al. Synthesis and oxygen reduction properties of three-dimensional sulfur-doped graphene networks[J]. Chemical Communications, 2014, 50(48): 6382-6385.
[64] LI W, YANG D, CHEN H,et al. Sulfur-doped carbon nanotubes as catalysts for the oxygen reduction reaction in alkaline medium[J]. Electrochimica Acta, 2015, 165(20): 191-197.
[65] YANG S, ZHI L, TANG K,et al. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions[J]. Advanced Functional Materials, 2012, 22(17): 3634-3640.
[66] CHEN Y, LI J, MEI T,et al. Low-temperature and one-pot synthesis of sulfurized graphene nanosheetsvia in situdoping and their superior electrocatalytic activity for oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2014, 2(48): 20714-20722.
[67] JEON I Y, CHOI H J, JUNG S M,et al. Large-scale production of edge-selectively functionalized graphene nanoplateletsviaball milling and their use as metal-free electrocatalysts for oxygen reduction reaction[J]. Journal of the American Chemical Society, 2013, 135(4): 1386-1393.
[68] JEON I Y, ZHANG S, ZHANG L,et al. Edge-selectively sulfurized graphene nanoplatelets as efficient metal-free electrocatalysts for oxygen reduction reaction: the electron spin effect[J]. Advanced Materials, 2013, 25(42): 6138-6145.
[69] YAO Z, NIE H, YANG Z,et al.Catalyst-free synthesis of iodine-doped grapheneviaa facile thermal annealing process and its use for electrocatalytic oxygen reduction in an alkaline medium[J]. Chemical Communications, 2012, 48(7): 1027-1029.
[70] JEON I, CHOI H, CHOI M,et al. Facile, scalable synthesis of edge-halogenated graphene nanoplatelets as efficient metal-free eletrocatalysts for oxygen reduction reaction[J]. Scientific Reports, 2013, 3: 1810-1810.
[71] JIN Z, NIE H, YANG Z,et al.Metal-free selenium doped carbon nanotube/graphene networks as a synergistically improved cathode catalyst for oxygen reduction reaction[J]. Nanoscale, 2012, 4(20): 6455-6460.
[72] JEON I Y, CHOI M, CHOI H J,et al. Antimony-doped graphene nanoplatelets[J]. Nature Communications, 2015, 6: 7123-7123.
[73] DEBE M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486(7401): 43-51.
[74] ZHAO Y, YANG L, CHEN S,et al. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes?[J]. Journal of the American Chemical Society, 2013, 135(4): 1201-1204.
[75] ZHANG Y, ZHUANG X, SU Y,et al. Polyaniline nanosheet derived B/N co-doped carbon nanosheets as efficient metal-free catalysts for oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2014, 2(21): 7742-7746.
[76] ZHONG S, ZHOU L, WU L,et al. Nitrogen- and boron-co-doped core shell carbon nanoparticles as efficient metal-free catalysts for oxygen reduction reactions in microbial fuel cells[J]. Journal of Power Sources, 2014, 272(25): 344-350.
[77] YOU B, KANG F, YIN P,et al. Hydrogel-derived heteroatom-doped porous carbon networks for supercapacitor and electrocatalytic oxygen reduction[J]. Carbon, 2016, 103: 9-15.
[78] LI Y, ZHANG H, WANG Y,et al. A self-sponsored doping approach for controllable synthesis of S and N co-doped trimodal-porous structured graphitic carbon electrocatalysts[J]. Energy & Environmental Science, 2014, 7(11): 3720-3726.
[79] QU K, ZHENG Y, DAI S,et al. Graphene oxide-polydopamine derived N, S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution[J]. Nano Energy, 2016, 19: 373-381.
[80] HIGGINS D C, HOQUE M A, HASSAN F,et al. Oxygen reduction on graphene-carbon nanotube composites doped sequentially with nitrogen and sulfur[J]. ACS Catalysis, 2014, 4(8): 2734-2740.
[81] WOHLGEMUTH S, WHITE R J, WILLINGER M,et al. A one-pot hydrothermal synthesis of sulfur and nitrogen doped carbon aerogels with enhanced electrocatalytic activity in the oxygen reduction reaction[J]. Green Chemistry, 2012, 14(5): 1515-1523.
[82] WOHLGEMUTH S, VILELA F, TITIRICI M,et al. A one-pot hydrothermal synthesis of tunable dual heteroatom-doped carbon microspheres[J]. Green Chemistry, 2012, 14(3): 741-749.
[83] ZHU J, LI K, XIAO M,et al. Significantly enhanced oxygen reduction reaction performance of N-doped carbon by heterogeneous sulfur incorporation: synergistic effect between the two dopants in metal-free catalysts[J]. Journal of Materials Chemistry A, 2016, 4(19): 7422-7429.
[84] LIU Z, FU X, WEI X,et al. Facile and scalable synthesis of coal tar-derived, nitrogen and sulfur-codoped carbon nanotubes with superior activity for O2reduction by employing an evocating agent[J]. Journal of Materials Chemistry A, 2015, 3(45): 22723-22729.
[85] SAMANTARA A K, SAHU S C, GHOSH A,et al. Sandwiched graphene with nitrogen, sulphur co-doped CQDs: an efficient metal-free material for energy storage and conversion applications[J]. Journal of Materials Chemistry A, 2015, 3(33): 16961-16970.
[86] LIU Z, NIE H, YANG Z,et al. Sulfur-nitrogen co-doped three-dimensional carbon foams with hierarchical pore structures as efficient metal-free electrocatalysts for oxygen reduction reactions[J]. Nanoscale, 2013, 5(8): 3283-3288.
[87] AKHTER T, ISLAM M M, FAISAL S N,et al. Self-assembled N/S codoped flexible graphene paper for high performance energy storage and oxygen reduction reaction[J]. ACS Applied Materials & Interfaces, 2016, 8(3): 2078-2087.
[88] ZHANG H, LIU X, HE G,et al. Bioinspired synthesis of nitrogen/sulfur co-doped graphene as an efficient electrocatalyst for oxygen reduction reaction[J]. Journal of Power Sources, 2015, 279(1): 252-258.
[89] YOU J, AHMED M S, HAN H S,et al. New approach of nitrogen and sulfur-doped graphene synthesis using dipyrrolemethane and their electrocatalytic activity for oxygen reduction in alkaline media[J]. Journal of Power Sources, 2015, 275(1): 73-79.
[90] HAN C, BO X, ZHANG Y,et al. One-pot synthesis of nitrogen and sulfur co-doped onion-like mesoporous carbon vesicle as an efficient metal-free catalyst for oxygen reduction reaction in alkaline solution[J]. Journal of Power Sources, 2014, 272(25): 267-276.
[91] CUI Z, WANG S, ZHANG Y,et al. A simple and green pathway toward nitrogen and sulfur dual doped hierarchically porous carbons from ionic liquids for oxygen reduction[J]. Journal of Power Sources, 2014, 259(1): 138-144.
[92] CHEN J, ZHANG H, LIU P,et al. Thiourea sole doping reagent approach for controllable N, S co-doping of pre-synthesized large-sized carbon nanospheres as electrocatalyst for oxygen reduction reaction[J]. Carbon, 2015, 92: 339-347.
[93] CHANG Y, HONG F, LIU J,et al. Nitrogen/sulfur dual-doped mesoporous carbon with controllable morphology as a catalyst support for the methanol oxidation reaction[J]. Carbon, 2015, 87: 424-433.
[94] SUN D, BAN R, ZHANG P,et al. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties[J]. Carbon, 2013, 64: 424-434.
[95] SU Y, ZHANG Y, ZHUANG X,et al. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction[J]. Carbon, 2013, 62: 296-301.
[96] PAN F, DUAN Y, ZHANG X,et al. A facile synthesis of nitrogen/sulfur co-doped graphene for the oxygen reduction reaction[J]. ChemCatChem, 2016, 8(1): 163-170.
[97] SONG M Y, PARK H Y, YANG D S,et al. Seaweed-derived heteroatom-doped highly porous carbon as an electrocatalyst for the oxygen reduction reaction[J]. ChemSusChem, 2014, 7(6): 1755-1763.
[98] ZHOU L, FU P, CAI X,et al. Naturally derived carbon nanofibers as sustainable electrocatalysts for microbial energy harvesting: a new application of spider silk[J]. Applied Catalysis B: Environmental, 2016, 188(5): 31-38.
[99] ZHU J, LI K, XIAO M,et al. Significantly enhanced oxygen reduction reaction performance of N-doped carbon by heterogeneous sulfur incorporation: synergistic effect between the two dopants in metal-free catalysts[J]. Journal of Materials Chemistry A, 2016, 4(19): 7422-7429.
[100] YANG S, PENG L, HUANG P,et al. Nitrogen, phosphorus, and sulfur co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes[J]. Angewandte Chemie International Edition, 2016, 55(12): 4016-4020.
[101] HASEGAWA G, DEGUCHI T, KANAMORI K,et al. High-level doping of nitrogen, phosphorus, and sulfur into activated carbon monoliths and their electrochemical capacitances[J]. Chemistry of Materials, 2015, 27(13): 4703-4712.
[102] ZHOU L, FU P, WANG Y,et al. Microbe-engaged synthesis of carbon dot-decorated reduced graphene oxide as high-performance oxygen reduction catalysts[J]. Journal of Materials Chemistry A, 2016, 4(19): 7222-7229.
[103] ZHAO S, LIU J, LI C,et al. Tunable ternary (N, P, B)-doped porous nanocarbons and their catalytic properties for oxygen reduction reaction[J]. ACS Applied Materials & Interfaces, 2014, 6(24): 22297-22304.
[104] RAZMJOOEI F, SINGH K P, SONG M Y,et al. Enhanced electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: a comprehensive study[J]. Carbon, 2014, 78: 257-267.
[105] CHEN Y Z, WANG C, WU Z Y,et al. From bimetallic metal-organic framework to porous carbon: high surface area and multicomponent active dopants for excellent electrocatalysis[J]. Advanced Materials, 2015, 27(34): 5010-5016.
[106] LI J, LI S, TANG Y,et al. Heteroatoms ternary-doped porous carbons derived from MOFs as metal-free electrocatalysts for oxygen reduction reaction[J]. Scientific Reports, 2014, 4: 5130-5130.
[107] ZHANG W, WU Z, JIANG H,et al. Nanowire-directed templating synthesis of metal-organic framework nanofibers and their derived porous doped carbon nanofibers for enhanced electrocatalysis[J]. Journal of the American Chemical Society, 2014, 136(41): 14385-14388.
[108] SHAO Z, ZHANG W, AN D,et al. Pyrolyzed egg yolk as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions[J]. RSC Advances, 2015, 5(118): 97508-97511.
[109] ZHANG P, GONG Y, WEI Z,et al. Updating biomass into functional carbon material in ionothermal manner[J]. ACS Applied Materials & Interfaces, 2014, 6(15): 12515-12522.
[110] ZHANG S, CAI Y, HE H,et al. Heteroatom doped graphdiyne as efficient metal-free electrocatalyst for oxygen reduction reaction in alkaline medium[J]. Journal of Materials Chemistry A, 2016, 4(13): 4738-4744.
[111] WU Z, LIANG H, LI C,et al. Dyeing bacterial cellulose pellicles for energetic heteroatom doped carbon nanofiber aerogels[J]. Nano Research, 2014, 7(12): 1861-1872.
[112] ZHU H, YIN J, WANG X,et al. Microorganism-derived heteroatom-doped carbon materials for oxygen reduction and supercapacitors[J]. Advanced Functional Materials, 2013, 23(10): 1305-1312.
[113] HUANG X, ZOU X, MENG Y,et al. Yeast cells-derived hollow core/shell heteroatom-doped carbon microparticles for sustainable electrocatalysis[J]. ACS Applied Materials & Interfaces, 2015, 7(3): 1978-1986.
[114] RAZMJOOEI F, SINGH K P, BAE E J,et al. A new class of electroactive Fe- and P-functionalized graphene for oxygen reduction[J]. Journal of Materials Chemistry A, 2015, 3(20): 11031-11039.
[115] GUO Z, XIAO Z, REN G,et al. Natural tea-leaf-derived, ternary-doped 3D porous carbon as a high-performance electrocatalyst for the oxygen reduction reaction[J]. Nano Research, 2016, 9(5): 1244-1255.
[116] LIANG Y, LI Y, WANG H,et al. Co3O4nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction[J]. Nature Materials, 2011, 10(10): 780-786.
[117] DOU S, TAO L, HUO J,et al. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis[J]. Energy & Environmental Science, 2016, 9(4): 1320-1326.
[118] DUAN J, CHEN S, CHAMBERS B A,et al. 3D WS2nanolayers@ heteroatom-doped graphene films as hydrogen evolution catalyst electrodes[J]. Advanced Materials, 2015, 27(28): 4234-4241.
[119] ZHANG C, ANTONIETTI M, FELLINGER T P. Blood ties: Co3O4decorated blood derived carbon as a superior bifunctionalelectrocatalyst[J]. Advanced Functional Materials, 2014, 24(48): 7655-7665.
[120] CHEN K, HUANG X, WAN C,et al. Efficient oxygen reduction catalysts formed of cobalt phosphide nanoparticle decorated heteroatom-doped mesoporous carbon nanotubes[J]. Chemical Communications, 2015, 51(37): 7891-7894.
[121] ZHU J, XIAO M, LIU C,et al. Growth mechanism and active site probing of Fe3C@N-doped carbon nanotubes/C catalysts: guidance for building highly efficient oxygen reduction electrocatalysts[J]. Journal of Materials Chemistry A, 2015, 3(43): 21451-21459.
[122] BATZILL M. The surface science of graphene: metal interfaces, CVD synthesis, nanoribbons, chemical modifications, and defects[J]. Surface Science Reports, 2012, 67(3): 83-115.
[123] WEI D, LIU Y, WANG Y,et al. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties[J]. Nano Letters, 2009, 9(5): 1752-1758.
[124] WANG L, AMBROSI A, PUMERA M. “Metal-free” catalytic oxygen reduction reaction on heteroatom-doped graphene is caused by trace metal impurities[J]. Angewandte Chemie International Edition, 2013, 52(51): 13818-13821.
[125] GUO Z, JIANG C, TENG C,et al. Sulfur, trace nitrogen and iron codoped hierarchically porous carbon foams as synergistic catalysts for oxygen reduction reaction[J]. ACS Applied Materials & Interfaces, 2014, 6(23): 21454-21460.
[126] VENKATESWARA RAO C, ISHIKAWA Y. Activity, selectivity, and anion-exchange membrane fuel cell performance of virtually metal-free nitrogen-doped carbon nanotube electrodes for oxygen reduction reaction[J]. Journal of Physical Chemistry C, 2012, 116(6): 4340-4346.
[127] SUN X, SONG P, ZHANG Y,et al. A class of high performance metal-free oxygen reduction electrocatalysts based on cheap carbon blacks[J]. Scientific Reports, 2013, 3: 2505.
[128] XIAO M, ZHU J, FENG L,et al. Meso/Macroporous nitrogen-doped carbon architectures with iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions[J]. Advanced Materials, 2015, 27(15): 2521-2527.
Recent progress on electrocatalysts towards oxygen reduction reaction based on heteroatoms-doped carbon
ZHOU Yu, WANG Yuxin
(State Key Laboratory of Chemical Engineering,Co-Innovation Center of Chemical Science & Engineering,Tianjin Key Laboratory of Membrane Science & Desalination Technology,School of Chemical Engineering,Tianjin University,Tianjin300072,China)
Electrochemical oxygen reduction reaction (ORR) is key to clean and sustainable energy technologies including proton exchange membrane fuel cells and metal-air batteries. However, the high-activation barriers in ORR often makes it the bottleneck of energy conversion processes, and thus high performance ORR electrocatalysts are desired. At present the best commercially available ORR catalyst is based on the precious metal Pt. But it suffers from resource scarcity and unsatisfactory operational stability, thus hindering a widespread and large-scale application of the clean and sustainable technologies. To tackle this problem, extensive efforts have been made in the last decade or so to search after efficient non-precious metal ORR catalysts. Among these, many heteroatoms-doped carbon (HDC) materials appear to be very promising, owing to their easy availability, low cost and excellent electrochemical performance. In this review article, recent advances in this active area are summarized, with the content being categorized according to the different underlying mechanisms of doped heteroatoms. Theoretical and experimental findings regarding HDC materials in ORR catalysis are reviewed, emphasizing the influence of heteroatom doping on the electronic structure of carbon materials. The problems facing HDC based ORR catalysts and further researches required are also discussed.
Prof. WANG Yuxin, yxwang@tju.edu.cn
O 643.36;TM 911.46
:A
:0438—1157(2017)02—0519—16
10.11949/j.issn.0438-1157.20161060
2016-07-26收到初稿,2016-09-07收到修改稿。
联系人:王宇新。
:周宇(1993—),男,博士研究生。
国家自然科学基金项目(21120102039)。
Received date: 2016-07-26.
Foundation item: supported by the National Natural Science Foundation of China(21120102039).

