Metformin prevents hormonal and metabolic disturbances and 1,2-dimethylhydrazine-induced colon carcinogenesis in non-diabetic rats
Viktoria V. Bekusova, Vasily M. Patsanovskii, Alexander D. Nozdrachev,, Alexandr P. Trashkov, Margarita R. Artemenko, Vladimir N. AnisimovDepartment of Physiology, St. Petersburg State University, St. Petersburg 978, Russia;I.P.Pavlov Institute of Physiology, Russian Academy of Sciences, St. Petersburg 990, Russia;Deparment of Experimental Pharmacology, I.M. Sechenov Institute of Evolutionary Physiology and Biochemistry, The Russian Academy of Sciences, St. Petersburg 9, Russia;Department of Carcinogenesis and Oncogerontology, N.N. Petrov Research Institute of Oncology, St. Petersburg 97758, Russia
Metformin prevents hormonal and metabolic disturbances and 1,2-dimethylhydrazine-induced colon carcinogenesis in non-diabetic rats
Viktoria V. Bekusova1, Vasily M. Patsanovskii2, Alexander D. Nozdrachev1,2, Alexandr P. Trashkov3, Margarita R. Artemenko3, Vladimir N. Anisimov41Department of Physiology, St. Petersburg State University, St. Petersburg 197183, Russia;2I.P.Pavlov Institute of Physiology, Russian Academy of Sciences, St. Petersburg 199034, Russia;3Deparment of Experimental Pharmacology, I.M. Sechenov Institute of Evolutionary Physiology and Biochemistry, The Russian Academy of Sciences, St. Petersburg 194223, Russia;4Department of Carcinogenesis and Oncogerontology, N.N. Petrov Research Institute of Oncology, St. Petersburg 197758, Russia
Effects of two doses of the anti-diabetic drug, metformin (MF), on hormonal and metabolic levels of serum of non-diabetic male Wistar rats with 1,2-dimethylhydrazine (DMH)-induced colon tumor adenocarcinomas were studied. Carcinogenesis in the animals was also observed. Rats with DMH-induced colon adenocarcinomas had elevated levels of serum glucose, insulin, insulinlike growth factor-1, total cholesterol, triglycerides, catalase, malonic dialdehyde, glycated hemoglobin, aspartate aminotransferase, and alanine aminotransferase and decreased hemoglobin. Treatment with two doses of MF normalized majority of these changes in DMH-treated rats, whereas the drug was ineffective in rats without DMH treatment. The only exception was the decreased triglyceride levels in MF-treated rats. A 100 mg/kg dose of MF increased DMH-induced exophytic colon carcinomas and decreased endophytic tumors compared with untreated rats. Moreover, both MF doses increased DMH-induced and highly differentiated tumors and decreased the invasiveness of colon carcinomas compared with rats provided with DMH and water. Therefore, effects of MF on metabolic homeostasis are critical for preventing colon cancer.
Colon cancer; prevention; 1,2-dimethylhydrazine; metformin; rat
Introduction
Colorectal cancer (CRC) is the third most common malignancy in humans worldwide; more than one million new cases occur annually1. Age is a leading CRC risk factor. More than 90% of CRC cases occur in people aged over 50, and approximately 75% of the cases are diagnosed in people older than 65. Furthermore, risks starting at 40 years of age increase sharply at 50 and double each decade until age 802. Hyperinsulinemia and obesity are key factors in cancer pathogenesis, including CRC3-6. The chemical carcinogen, 1, 2-dimethylhydrazine (DMH), has been widely used for induction of colon cancer. Regardless of mode of administration, DMH specifically induces tumors within thedescending colon of rats and some mouse strains, and resulting histopathologies are similar to those observed in human sporadic colon tumors7,8. Meanwhile, the antidiabetic biguanide, metformin (MF), lowers elevated insulin levels in type 2 diabetes3,9, significantly reduces various cancer risks in humans with the condition, and prevents tumor development in numerous rodent tissues and organs10,11. Furthermore, MF inhibits some chemically induced colon carcinogenesis in diabetic, obese12, and nondiabetic rats13. In this work, we showed the inhibitory effects of MF on DMH-induced colon carcinogenesis in nondiabetic rats. Such effects mainly involve the normalizing influence of MF on hormonal and metabolic homeostases.
Material and methods
Animals
Male Wistar rats aged 2 months were bred at the Animal Laboratory of the I.P. Pavlov Institute of Physiology. Six toseven rats were kept in T3-type cages under a standard light/dark regimen (12 h light: 12 h darkness) at (22±2)°C and received standard laboratory PK-120 (Laboratorkorm, Russia)14and tap water ad libitum.
Animals were checked daily by animal care personnel and weekly by a veterinarian. The weights were measured weekly as well. The study was conducted per the regulations for ensuring humane treatment of animals under the approval of the Committee on Animal Research of N.N. Petrov Research Institute of Oncology.
Chemicals
DMH was provided by Sigma Chemical Co., St. Lois, MO, USA and kept at –20°C. MF (MF HCl, Siophor) was purchased from Berlin-Chemie, Menarini Group, Germany.
Experiment 1
A total of 58 male Wistar rats with 2-month-old were randomly subdivided into 6 groups. A total of 24 rats in groups 1–3 were not exposed to carcinogens, whereas 34 rats from groups 4–6 were administered with 5 subcutaneous DMH injections weekly at a single dose of 21 mg/kg of body weight (calculated as a base). In this regimen, carcinogens induced colon tumors in majority of rats7. DMH was ex tempore dissolved in normal saline and neutralized with sodium bicarbonate (pH 7.0). Starting from the first carcinogen injection, groups 1 and 4 were provided with 1 mL tap water via intragastric gavage, whereas groups 2 and 5 were administered daily with MF (100 mg/kg) via gavage. Groups 3 and 6 were given MF (300 mg/kg) dissolved in 1 mL tap water. This treatment was concluded 2 months after the first DMH injection. The experiment was finalized six months after the first carcinogen injection. After being sacrificed by ether vapor, rats were decapitated, and blood samples were collected in plastic vessels without anticoagulants. After letting the samples stand for 30 min at room temperature, they were centrifuged (30 min at 1200 g) and then kept at –20°C until biochemical analyses. Metabolic parameters were assessed in blood serum. Glucose concentration was electrochemically estimated via an express analyzer (i-STAT, Abbot) that uses CG8+ cartridges. Total cholesterol, triglyceride, hemoglobin, and malonic dialdehyde (MDA) concentrations, and Cu, Zn-superoxide dismutase (SOD), catalase, alaninaminotransferase (ALT), and aspartataminotransferase (AST) activities were processed using Stat Fax 3300 analyzer and commercial reagent kits following the manufacturers’ instructions. Vascular endothelial growth factor (VEGF), insulin, and insulin-like growth factor (IGF-1) concentrations were assessed using enzyme-linked immunosorbent assay using Cusabio and R&B Systems reagent kits per standard procedures. Glycated hemoglobin was estimated using high performance liquid chromatography.
Experiment 2
A total of 24 male Wistar rats with 2-month-old were randomly subdivided into three groups and exposed to DMH and the same two doses of MF, as in Experiment 1. Experiment 2 was finalized six months after the first carcinogen injection. After being sacrificed using ether vapor, rats were autopsied by longitudinally opening the intestines. Tumor position and size were recorded7. After histological processing, tissues were embedded in paraffin. Histological sections measuring 3 µm thick were stained with hematoxylin-eosin and microscopically examined; in the experimental group, examination was performed as blind process. Tumors were classified per International Agency for Research on Cancer recommendations15.
Statistical analysis
Experimental results were statistically processed following variation statistics using Statistica-10. All data were expressed as mean ± standard error (Figures 1 and 2) or confidence interval for the standard deviation (Table 1). The significance of discrepancies was defined according to Chi-square analysis between experimental and control groups (Table 1). Differences in estimated parameters among the groups were assessed using non-parametric criterion of Mann-Whitney U test (Figures 1 and 2)16. P<0.01 and 0.05 were considered as significant.
Results
Effect of MF on DMF-induced hormonal and metabolic disturbances in male rats
MF treatment failed to influence weight gain in both non-(groups 2 and 3) and DMH-exposed rats (groups 5 and 6). Thus, MF did not significantly affect weight gain between non- and DMH-exposed groups (data not shown). Twomonth administration of both doses of MF to non-exposed rats significantly decreased triglyceride serum levels and failed to influence other metabolic parameters (Figure 1). Dramatic parameter disturbances were observed in DMH and water-treated rats. The animals were sacrificed six months after the first carcinogen injection. Compared withthe control group, non-treated rats had increased levels of glucose (+25.6%), insulin (+36.2%), IGF-1 (+37.1%), total cholesterol (+47.4%), and triglycerides (+106.9%) and increased activities of catalase (+35.3%), MDA (+33.3%), AST (+93.8%), ALT (+71.4%), VEGF (+65.5%), and glycated hemoglobin (+56.7%). SOD activity did not change significantly (+19.2%, P>0.05). Both MF doses alleviated carcinogenic effects. Majority of parameters were normal, and indices covered those DMH-unexposed and MF-untreated rats (Figure 1A–1M).

Figure 1 Effect of 1,2-dimethylhydrazine (DMH) and metformin on hormonal and metabolic parameters in the serum male Wistar rats. (A) glucose. (B) Insulin. (C) IGF-1. (D) Total cholesterol. (E) Triglycerides. (F) Cu, Zn-superoxide dismutase. (G) Catalase. (H) Malonic dialdehyde. (I) Hemoglobin. (J) Glycated hemoglobin. (K) Alaninaminetransferase. (L) Aspartataminetransferase. (M) VEGF. Data presented as mean±SEM, n=6–15 per group. P≤0.05. a: DMH vs. control; b: DMH+MF vs. DMH. Rats bearing DMH-induced colon adenocarcinomas have elevated serum level of glucose, insulin, IGF-1, total cholesterol, triglycerides, catalase, malonic dialdehyde, glycated hemoglobin, AST, ALT and decreased level of hemoglobin. Treatment with MF in both doses normalized majority of these changes in DMH-treated group of rats, whereas failed to modify them in rats not treated with DMH. Only exception was decreased level of triglycerides in MF-treated rats (Figure 1E, P<0.05).

Table 1 Colon tumors localization, incidence, multiplicity and size in rats exposed to 1,2-dimethylhydrazine (DMH) and metformin
Effects of MF on DMH-induced colon carcinogenesis in male rats
In Experiment 2, intestinal tumors were found in majority of DMH-exposed rats (Table 1).
In group 1 (DMH+water), all rats developed colon tumors (100%). Tumor incidences in different colon parts in group 1 varied: 63% in ascending colon, 100% in descending colon, and 25% in rectum. Moreover, 76.7% of colon tumors were observed in descending colons, 16.7% in ascending colons, and 6.7% in rectums. Maximal effect of 100 mg/kg daily MF dose was observed in the ascending colon, in which DMH-induced carcinogenesis was also completely inhibited (Table 1). The two MF doses did not affect colon carcinoma incidence in rat rectums and descending colons. Higher MF dose (300 mg/kg) was less effective in suppressing colon carcinogenesis compared with lower amounts (100 mg/kg).
Macroscopically, neoplasms are exophytic or endophytic. Microscopically, malignant intestinal tumors have different types, among which tubular adenocarcinomas are predominant. All carcinoma types are typical in DMH-induced neoplasms15. Table 1 and Figure 2 present the data on the effects of MF on DMH-induced colon tumor development.

Figure 2 Effect of metformin on some parameters of 1,2-dimethylhydrazine-induced colon carcinogenesis in male Wistar rats. Data presented as mean±SEM, n=7–9 per group. * Р<0.01; § P<0.05 vs. DMH group. Treatment with MF in dose 100 mg/kg increased relative number of induced with DMH exophytic colon carcinomas and decreased number of endophytic tumors. Both doses of MF increased relative number of DMH-induced highly differentiated tumors and decreased invasiveness of colon carcinomas as compare with group given DMH with water.
Morphological analysis showed that tumors with exophytic growth patterns developed more frequently in the group treated with 100 mg/kg MF compared with DMH + water group. Opposite results were observed with endophytic colon tumors (Figure 2A). The group that was treated with lower MF dose had less invasive (Figure 2B) and more differentiated tumors (Figure 2C) compared with the DMH + water group. Figure 3 shows microphotographs of the observed colon adenocarcinoma types. Tumor size distribution analysis showed that in descending colons of DMH + water and DMH + MF groups, 300 small tumors (<10 mm2) appeared less frequently compared with the MF group with 100 mg/kg dose (26%, 26%, and 50%, correspondingly). Thus, these data indicate the inhibitory effects of MF on DMH-induced colon carcinogenesis.
Discussion
The DMH-induced carcinogenesis in epithelial cells includes the following: formation of most active metabolites (methylazoxymethanol or methyldiazohydrate) in the liver; binding of metabolites to glucuronic acid; delivery of conjugates to intestines via blood flow; release of active metabolites through enzymatic activity of intestinal flora (ßglucuronidase); formation of carbonium ion (CH3+); specific methylation of macromolecules, which in enterocytes, are mainly DNA at O6position of guanine; miscoding effects7. These events result in mutation and activation of Ki-ras oncogene and inactivation of p5317. Moreover, reports presented the significant role of free radicals in DMH-induced colon carcinogenesis18,19. The present study confirmed the effects of DMH on oxidative stress parameters. Furthermore, we showed the normalizing effect of MF on MDA levels in DMH-treated rats (Figure 1). Bordini et al.20observed similar effects of MF in azoxymethane (AOM)-exposed mice. Shortly after starting DMH treatment, exposed organisms experienced significant disturbances in their neuroendocrine and immune systems and lipid and carbohydrate metabolisms. DMH treatment was followed by an increase in sensitivity threshold of the hypothalamus to inhibition by estrogen21, decrease in hypothalamic biogenic amine content22, and disturbances in diurnal rhythms at biogenic amine levels in hypothalamic nuclei of rats23. Antidiabetic biguanide treatment alleviated immunodepression in rodents exposed to DMH24and AOM25. We observed increased levels of glucose, insulin, IGF-1, total cholesterol, triglycerides, MDA, glycated hemoglobin, and VEGF in serum of rats with DMH-induced colon tumors compared with the group without DMH (Figure 1). These findingsagree with available data12,13,26,27. Furthermore, we observed the normalizing effects of both MF doses on these parameters in DMH-exposed rats (Figure 1). Notably, AST and ALT levels were not different in rats treated with higher and lower MF doses, thereby suggesting the non-toxicity of MF on liver functions.

Figure 3 Microphotographs of 1,2-dimethylhydrazine-induced colon adenocarcinomas. (A) Highly differentiated adenocarcinoma. (B) Moderately differentiated adenocarcinoma. (C) Low differentiated adenocarcinoma (H&E stainning, 70×).
Table 2 summarizes the data on inhibitory effects of antidiabetic biguanides on colon carcinogenesis. In most studies, anti-diabetic biguanides inhibited AOM- or DMH-induced colon carcinogenesis in mice and rats. Detailed analysis of experimental results are provided elsewhere28,29. MF treatment was followed by decreased levels of proliferation indices, which were evaluated with 5-bromodesoxyuridine, proliferating cell nuclear antigen indices, phosphorylated mechanistic target of rapamycin (mTOR), S6 kinase, and S6 proteins, as revealed by Western blot analysis, in the colonic mucosa of AOM-treated mice30. The authors believe that MF suppresses colonic epithelial proliferation by inhibiting the mTOR pathway through 5’ adenosine monophosphateactivated protein kinase activation. However, MF did not affect the level of O6-Methylguanin in the colon or liver of AOM-treated mice. Results showed that MF did not affect the AOM alkylation capacity and carcinogenicity. Therefore, the normalizing effect of MF on neuroendocrine and hormonal metabolic shifts, which occurred in rodents during colon carcinogenesis, are critical in colon cancer prevention.
Zaafar et al.32studied the effects of MF on cancer development in diabetic and non- diabetic mice. In diabetic mice, MF treatment alone increased the number of surviving mice compared with the diabetic DMH group. Moreover, MF significantly reduced histopathological scores in diabetic mice colons. Serum VEGF levels in non-diabetic DMH group were not significantly higher than those of non-diabetic saline groups. In non-diabetic mice, MF reduced the serum concentration of VEGF compared with those in the nondiabetic/DMH control. In our study, we observed a tendency toward an increased VEGF level in DMH-treated rats compared with control animals, which were not exposed to carcinogens, whereas MF did not influence this parameter. Statistical analysis showed a significantly reduced histopathologic score for colon mucosa of MF-treated diabetic mice compared with DMH control, whereas in nondiabetic animals, the drug failed to improve the scores. This study highlighted the high susceptibility of diabetic rodents to carcinogenic effects of DMH. Inhibitory effects of MF on DMH-induced colon carcinogenesis were observed in Sprague Dawley rats with type 2 diabetes; the condition was induced with small dose of streptozotocin combined with a high-fat diet12. MF treatment was followed by decreases in ACF number, colonic tissue proliferation, and colon tumor incidence, multiplicity, and size. These results and other studies confirmed the role of diabetes as risk factor for cancer and established a connection between glucose levels and development of micro- and macro-vascular complications37,38. Our data agree with the other studies, showing that MF is effective in cancer prevention in both diabetic and non-diabetic animals that are exposed to DMH or AOM (Table 2)10,11. Moreover, clinical trials demonstrated the decrease in colon cancer risk of type 2 diabetes and non-diabetic patients39-51. Meta-analysis of 37 studiescomprising more than 1.5 million participants showed that risk of colon cancer mortality was reduced by 23% in MF users compared with non-users46. Thus, most epidemiological data and clinical trial results present sufficient evidence of MF efficacy in CRC prevention and treatment in humans. Furthermore, low daily MF dose (500 mg) was effective in reducing cancer risk in type 2 diabetes patients52. Our findings on efficacy of lower MF dose in rats agree with clinical data. Given the practical significance of these observations, future investigations are needed to elucidate the advantage of using lower doses of the drug.
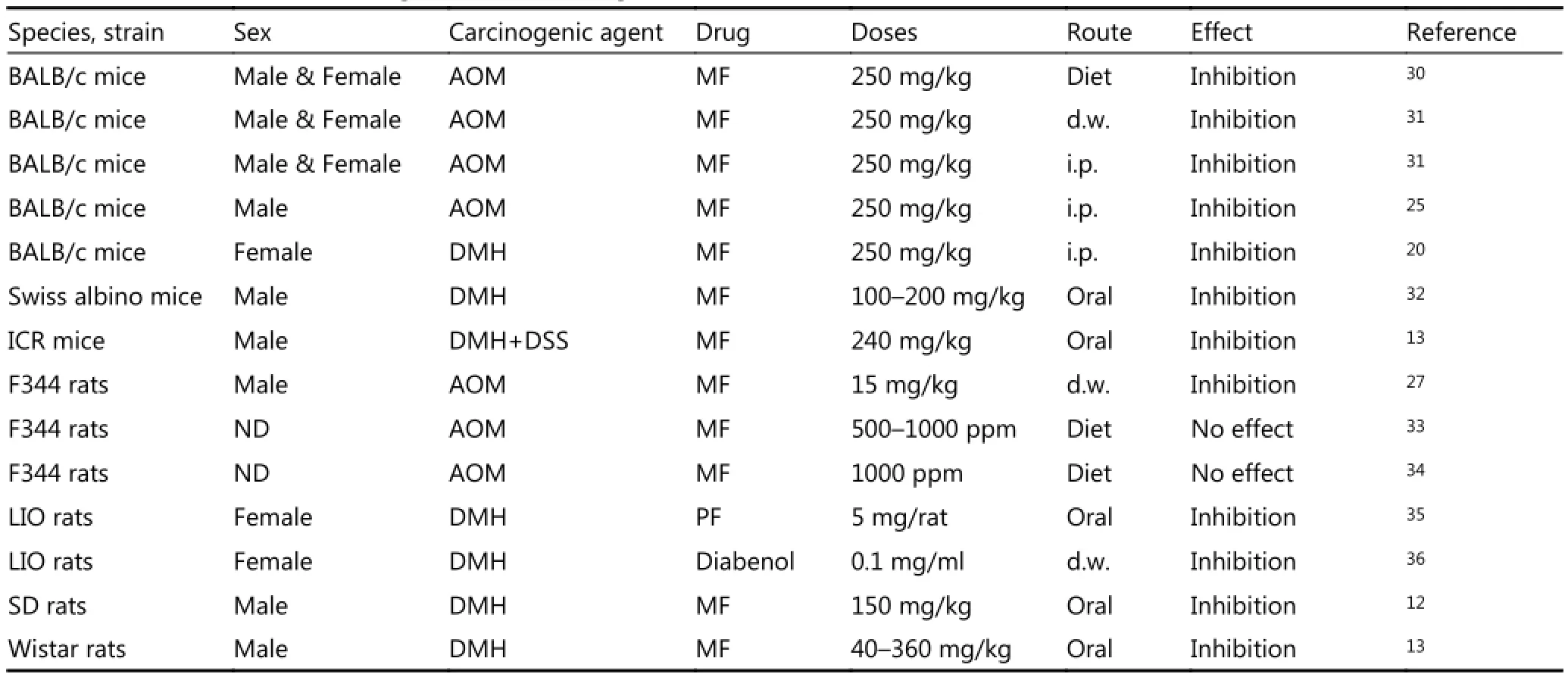
Table 2 Effect of anti-diabetic drugs on colon carcinogenesis in rodents
Acknowledgments
This work was supported in part by a grant from the Russian Foundation for Basic Research (Grant No. 14-04-01653). Authors are very thankful to Dr. A.V. Panchenko and Dr. M.A. Zabezhinski for help and valuable advice during the study.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Globocan 2013, Cancer Fact Sheets for Colorectal Cancer. http://globocan.iarc.fr/old/FactSheets/cancers/colorectal-new.asp.
2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62: 10–29.
3.Dilman VM. Development, Aging and Disease: A New Rationale for an Intervention. Chur: Harwood Academic Publishers; 1994.
4.Facchini F, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001; 86: 3574–8.
5.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002; 11: 385–91.
6.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010; 17: 351–60.
7.Pozharisski KM, Likhachev AJ, Klimashevski VF, Shaposhnikov JD. Experimental intestinal cancer research with special reference to human pathology. Adv Cancer Res. 1979; 30: 165–237.
8.Rogers AE, Nauss KM. Rodent models for carcinoma of the colon. Dig Dis Sci. 1985; 30: 87S-102S.
9.Shaw RJ, Lamia KA, Vasques D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005; 310: 1642–6.
10.Anisimov VN. Metformin for cancer and aging prevention: is it a time to make the long story short? Oncotarget. 2015; 6: 39398–407.
11.Anisimov VN. Metformin for prevention and treatment of colon cancer: a reappraisal of experimental and clinical data. Current Drug Target. 2016; 17: 439–46.
12.Jia YL, Ma ZY, Liu XF, Zhou WJ, He S, Xu X, et al. Metformin prevents DMH-induced colorectal cancer in diabetic rats by reversing the Warburg effect. Cancer Med. 2015; 4: 1730–41.
13.Li W, Wang QL, Liu X, Dong SH, Li HX, Li CY, et al. Combined use of vitamin D3 and metformin exhibits synergistic chemopreventive effects on colorectal neoplasia in rats and mice. Cancer Prev Res. 2015; 8: 139–48.
14.Anisimov VN, Popovich IG, Zabezhinski MA. Methods of testing pharmacological drugs effects on aging and life span in mice. In: Tollefsbol TO, editor. Biological Aging: Methods and Protocols. 2nd ed. New York: Humana Press; 2013. p.145–60.
15.Turusov VS. Pathology of Tumours in Laboratory Animals, Vol. 1: Tumours of the Rat. 2nd ed. Lyon: International Agency for Research on Cancer; 1990.
16.Goubler EV. Calculation Methods in Analysis and Identification of Pathological Processes. Moscow: Meditsina; 1978.
17.Zusman I. The role of p53 tumor-associated protein in colon cancer detection and prevention (Review). Int J Oncol. 1997; 10: 1241–9.
18.Salim AS. The permissive role of oxygen-derived free radicals in the development of colonic cancer in the rat. A new theory for carcinogenesis. Int J Cancer. 1993; 53: 1031–5.
19.Arutiunian AV, Prokopenko VM, Burmistrov SO, Oparina TI, Frolova EV, Zabezhinskĭ MA, et al. Free-radical processes in blood serum, liver and large bowel during 1,2-dimethylhydrazine-induced carcinogenesis in rats. Vopr Onkol. 1997; 43: 618–22.
20.Bordini HP, Kremer JL, Fagundes TR, Melo GP, Conchon-Costa I, da Silva SS, et al. Protective effect of metformin in an aberrant crypt foci model induced by 1, 2-dimethylhydrazine: Modulation of oxidative stress and inflammatory process. Mol Carcinog. 2016. DOI: 10.1002/mc.22545.
21.Pozharisski K, Anisimov V. On the role of endocrine system in development of experimental colon tumors in rats. Pathol Physiol. 1975; 1: 47–50.
22.Anisimov VN, Pozdeev VK, Dmitrievskaia A, Gracheva GM, Il’in AP. Effect of enterotropic carcinogen 1, 2-dimethylhydrazine on the level of biogenic amines in the hypothalamus of rats. Bull Eksp Biol Med. 1976; 82: 1359–61.
23.Arutjunyan AV, Kerkeshko GO, Anisimov VN, Stepanov MG, Prokopenko VM, Pozdeyev NV, et al. Disturbances of diurnal rhythms of biogenic amines contents in hypothalamic nuclei as an evidence of neurotropic effects of enterotropic carcinogen 1, 2-dimethylhydrazine. Neuro Endocrinol Lett. 2001; 22: 229–37.
24.Dil’man VM, Sofronov BN, Anisimov VN, Nazarov PG, L’vovich EG. Phenformin elimination of the immunodepression caused by 1, 2-dimethylhydrazine in rats. Vopr Onkol. 1977; 23: 50–4.
25.Chung EJ, Do EJ, Kim SY, Cho EA, Kim DH, Pak S, et al. Combination of metformin and VSL#3 additively suppresses western-style diet induced colon cancer in mice. Eur J Pharmacol.2017; 794: 1–7.
26.Dubina MV, Petrishchev NN, Anisimov VN. Microvascular endothelium dysfunction in rats bearing 1, 2-dimethylhydrazineinduced colon tumors. Cancer Lett. 1999; 144: 125–9.
27.Shimomoto T, Luo Y, Ohmori H, Chihara Y, Fujii K, Sasahira T, et al. Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane-injected Fischer 344 rats fed with a high-linoleic acid and high-glucose diet. J Gastroenterol. 2012; 47: 1073–83.
28.Anisimov VN. Do metformin a real anticarcinogen? A critical reappraisal of experimental data. Ann Trans Med. 2014; 2: 60.
29.Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY). 2010; 2: 760-74.
30.Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, Suzuki K, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010; 49: 662–71.
31.Abdulkareem HA, Hussein AG, Mahmood AS. Efficacy of intraperitoneal administration of metformin and 5-fluoruracil in prevention of induced-colorectal aberrant crypt foci in mice. Int J Pharmacy Pharmaceut Sci. 2014; 6: 305–8.
32.Zaafar DK, Zaitone SA, Moustafa YM. Role of metformin in suppressing 1, 2-dimethylhydrazine-induced colon cancer in diabetic and non-diabetic mice: effect on tumor angiogenesis and cell proliferation: PLoS One. 2014; 9: e100562.
33.Rao CV, Janakiram NB, Mohammed A, Madka V, Qian L, Brewer M, et al. Lack of chemopreventive effects of metformin in azoxymethane-induced rat colon carcinogenesis. Cancer Prev Res. 2011; 4: B42.
34.Madka V, Zhang Y, Brewer M, Rao C, Janakiram N, Mohammed A, et al. Chemopreventive efficacy of bisphosphonates, Zometa and Fosamax, alone or in combination with metformin in AOM-induced rat colon cancer model. Cancer Prev Res. 2013; 6: A14.
35.Anisimov VN, Pozharisskiĭ KM, Dil’man VM. Effect of phenformin on the blastomogenic action of 1, 2-dimethylhydrazine in rats. Vopr Onkol. 1980; 26: 54–8.
36.Popovich IG, Zabezhinski MA, Egormin PA, Tyndyk ML, Anikin IV, Spasov AA, et al. Insulin in aging and cancer: antidiabetic drug diabenol as geroprotector and anticarcinogen. Int J Biochem Cell Biol. 2005; 37: 1117–29.
37.Markowitz SD, Bertagnolli MM. Molecular basis for colorectal cancer. N Engl J Med. 2009; 361: 2449–60.
38.Feng YH, Wu CL, Shiau AL, Lee JC, Chang JG, Lu PJ, et al. microRNA-21-mediated regulation of Sprouty2 protein expression enhances the cytotoxic effect of 5-fluorouracil and metformin in colon cancer cells. Int J Mol Med. 2012; 29: 920–6.
39.Zhang PP, Li H, Tan XH, Chen LL, Wang SM. Association of metformin use with cancer incidence and mortality: A metaanalysis. Cancer Epidemiol. 2013; 37: 207–18.
40.Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res. 2010; 3: 1077–83.
41.Higurashi T, Takahashi H, Endo H, Hosono K, Yamada E, Ohkubo H, et al. Metformin efficacy and safety for colorectal polyps: a double-blind randomized controlled trial. BMC Cancer. 2012; 12: 118.
42.Mei ZB, Zhang ZJ, Liu CY, Liu Y, Cui A, Liang ZL, et al. Survival benefits of metformin for colorectal cancer patients with diabetes: a systematic review and meta-analysis. PLoS One. 2014; 9: e91818.
43.Cardel M, Jensen SM, Pottegård A, Jørgensen TL, Hallas J. Longterm use of metformin and colorectal cancer risk in type II diabetics: a population-based case-control study. Cancer Med. 2014; 3: 1458–66.
44.Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol. 2012; 29: 1314–27.
45.Nangia-Makker P, Yu YJ, Vasudevan A, Farhana L, Rajendra SG, Levi E, et al. Metformin: a potential therapeutic agent for recurrent colon cancer. PLoS One. 2014; 9: e84369.
46.Zhang YF, Guan MP, Zheng ZJ, Zhang Q, Gao F, Xue YM. Effects of metformin on CD133+ colorectal cancer cells in diabetic patients. PLoS One. 2013; 8: e81264.
47.Zhang ZJ, Zheng ZJ, Kan HD, Song YQ, Cui W, Zhao GM, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011; 34: 2323–8.
48.Currie CJ, Poole CD, Gale EAM. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009; 52: 1766–77.
49.Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: A systematic review and meta-analysis. Oncologist. 2013; 18: 1248–55.
50.Smiechowski B, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiol Biomarkers Prev. 2013; 22: 1877–83.
51.Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016; 17: 475–83.
52.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang HY, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800, 000 individuals. BMC Cancer. 2011, 11: 20.
Cite this article as:Bekusova VV, Patsanovskii VM, Nozdrachev AD, Trashkov AP, Artemenko MR, Anisimov VN, et al. Metformin prevents hormonal and metabolic disturbances and 1,2-dimethylhydrazine-induced colon carcinogenesis in non-diabetic rats. Cancer Biol Med. 2017; 14: 100-7. doi: 10.20892/j.issn.2095-3941.2016.0088
Viktoria V. Bekusova
E-mail: v.bekusova@spbu.ru
Received October 26, 2016; accepted November 28, 2016. Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
 Cancer Biology & Medicine2017年1期
Cancer Biology & Medicine2017年1期
- Cancer Biology & Medicine的其它文章
- Erratum to Bcl-2 expression is a poor predictor for hepatocellular carcinoma prognosis of andropause-age patients
- Truth telling for patients with esophageal squamous cell carcinoma in Henan, China
- Roles of Rap1 signaling in tumor cell migration and invasion
- Estimation of lung cancer burden in Australia, the Philippines, and Singapore: an evaluation of disability adjusted life years
- A pilot study of radiologic measures of abdominal adiposity: weighty contributors to early pancreatic carcinogenesis worth evaluating?
- Association of genotypes of rs671 within ALDH2 with risk for gastric cardia adenocarcinoma in the Chinese Han population in high- and low-incidence areas
