不同种植年限条件下黄花蒿根际土壤微生物生物量、酶活性及真菌群落组成
李倩,杨水平,崔广林,黄建国*,李隆云*,程玉渊
(1.西南大学资源环境学院,重庆400716;2.重庆市中药研究院,重庆 400065;3.河南省烟草公司南阳市公司, 河南 南阳473000)
不同种植年限条件下黄花蒿根际土壤微生物生物量、酶活性及真菌群落组成
李倩1,3,杨水平1,崔广林2,黄建国1*,李隆云2*,程玉渊3
(1.西南大学资源环境学院,重庆400716;2.重庆市中药研究院,重庆 400065;3.河南省烟草公司南阳市公司, 河南 南阳473000)
试验采集未种植、种植1年、3年和5年的黄花蒿根际土壤,采用常规分析和Illumina MiSeq高通量测序技术,研究了土壤微生物生物量、酶活性及真菌群落组成。结果表明,在人工种植黄花蒿的土壤中,微生物生物量碳氮减少,碳氮比例改变;脱氢酶、脲酶和蔗糖酶活性降低,酸性磷酸酶活性增强;说明黄花蒿释放的化感物质选择性抑制了土壤微生物生长、繁殖和代谢。在不同种植年限的土壤中,主成分分析显示代表不同种植年限土壤真菌群落的点在坐标图中分布距离较远,表明它们的群落组成发生了显著变化(P<0.05)。此外,子囊菌门占土壤真菌的66.10%~95.28%,黄花蒿种植时间影响真菌门类和优势真菌的丰富度。在前20种优势真菌中,有14种共存于不同种植年限的土壤中,每种土壤中存在1~3种独有真菌,说明土壤是决定真菌种群组成的主导因素,又因种植黄花蒿而改变。在栽培1~5年的黄花蒿土壤中,优势菌株中出现蒿属的常见病菌——蒿白粉菌和艾菊柄锈菌,提高相应病害的发生风险。
黄花蒿;土壤酶活性;真菌;高通量测序
重庆市是我国黄花蒿(Artemisiaannua)的主产区,种植面积和青蒿素产量分别占全国的70%和80%[1]。由于土地资源缺乏,黄花蒿连作现象十分普遍。但长期持续连作,黄花蒿生长不佳,青蒿素含量和产量下降;叶白粉病、茎腐病和根腐病等真菌性病害的发生率超过10%,造成巨大的经济损失,严重制约黄花蒿种植业的发展[2-4]。
黄花蒿在生长过程中,主要通过植株残体腐解、根系分泌和雨水淋溶向土壤生态系统释放倍半萜青蒿素类、多甲氧基黄酮类、酚酸类和生物碱类等物质[5-6]。据报道,其主要化感物质——青蒿素在土壤中的半衰期长,对土壤微生物产生持续作用[7],选择性地杀抑枯草芽孢杆菌(Bacillussubtilis)、镶刀菌(Fusariumsp.)、点枝顶孢菌(Elcremoniumstrictum)和黄曲霉(Aspergillasflavus)等土壤微生物[8-9],但对大肠杆菌(Escherichiacoli)、小麦全蚀病菌(Gaeumannomycesgraminis)、禾谷丝核菌(Rhizoctoniazeae)和棉花黄萎病菌(Verticilliumdahliae)无显著影响[10-11]。在人工种植黄花蒿的根际土壤中,细菌、真菌和放线菌的动态变化与青蒿素含量密切相关[12]。在野生黄花蒿生长的土壤中,去氧青蒿素含量与放线菌数量呈极显著负相关(r=-0.528**,n=24);青蒿素含量与细菌和放线菌数量呈显著负相关(r=-0.508*和r=-0.478*,n=24)[13]。Herrmann等[5]发现,在种植黄花蒿的土壤中,可培养细菌数量显著低于未种植土壤。因此,种植黄花蒿可能影响土壤微生物的生长、繁殖、代谢和种群结构。
土壤微生物生物量碳氮和土壤酶活性可定量指示微生物数量、活性及代谢状况[14]。真菌是土壤微生物的重要组分,具有多种多样的生理、生化和生态功能,且多数真菌群落组成与重要病害发生密切相关。长期连续种植人参(Panaxginseng)、三七(Panaxnotoginseng)、地黄(Rehmanniaglutinosa)和丹参(Salviamiltiorrhiza)等药用植物之后,病原真菌增加,真菌病害大面积发生,造成大幅减产降质[15]。在自然栖息环境中,150万种真菌仅有5%~10%被人类认识。目前采用的常规培养法仅能获得土壤微生物总量的1%~3%[16];真菌细胞膜的磷脂脂肪酸高度相似,通常只能检测出18:1ω9c和18:3ω6c[17];聚合酶链式反应-变性梯度凝胶电泳法(polymerase chain reaction-denaturing gradient gel electrophoresis, PCR-DGGE)难于甄别单个真菌的基因序列[18]。迄今为止,对土壤真菌的认识还远远不够。高通量测序技术根据真菌18S rDNA的保守性,经提取、扩增、纯化、定量和均一化真菌DNA序列,再经测序、过滤、优化、聚类,对比基因库中的已知序列,从而鉴别真菌种(属)类,是传统培养方法所获得微生物数量的十倍甚至数百倍,能准确灵敏地检测土壤真菌[19]。为此,本试验利用常规分析和Illumina MiSeq高通量测序技术,研究了重庆市黄花蒿主产区土壤中的微生物生物量、酶活性及真菌群落组成,旨在为克服黄花蒿连作障碍和保持高产优质提供有益信息。
1 材料与方法
1.1 样地概况
重庆市巴南区东泉镇黄花蒿栽培区位于北纬29°26′,东经106°50′,海拔329 m。年均温度18.7 ℃,气温-1~40.8 ℃;平均年降雨量1100 mm,主要集中在5-7月;雾期60~90 d,日照1100~1300 h;无霜期在300 d以上。样地地貌平坦,土壤均匀一致,土壤类型为三叠纪嘉陵江组石灰岩发育的黄壤。土壤pH 5.91,有机质13.14 g/kg,有效氮72.02 mg/kg,有效磷 20.13 mg/kg,有效钾70.53 mg/kg。
1.2 试验设计
试验开始于2011年,在土壤条件均匀一致的黄花蒿种植区,选取12个小区,第一年随机选取3块种植黄花蒿,其余小区按照当地习惯夏种玉米(Zeamays),冬种油菜(Brassicacampestris)或小麦(Triticumaestivum)。在连作黄花蒿的第3年,随机另选3个小区种植黄花蒿,以此类推,在第5年时分别形成黄花蒿连作5年和3年,种植1年和未种黄花蒿(对照,种植玉米)的土壤,依次用Y5、Y3、Y1和Y0表示。黄花蒿品种为“渝青1号”,每年12月中旬播种育苗,次年4月上旬移栽,按照当地习惯基施N-P2O5-K2O=25-10-5复合肥900 kg/hm2,常规管理。
1.3 样品采集与测定
在黄花蒿现蕾期,每小区按“S”形随机选取10株长势一致的植株,拔出后轻轻抖落多余的土壤至每株剩50 g左右,然后用力抖动取样,合并土壤,拣去杂物,放入冰盒,迅速带回实验室。
将部分土壤晾干,分别用3,5-二硝基水杨酸比色法、次氯酸钠—苯酚钠比色法、磷酸苯二钠比色法和TTC分光光度法测定土壤蔗糖酶、脲酶、酸性磷酸酶和脱氢酶活性[20]。
另将部分新鲜土壤,分别测定微生物生物量碳氮和真菌ITS基因序列。土壤微生物生物量碳(microbial biomass carbon, MBC)和微生物生物量氮(microbial biomass nitrogen, MBN)采用氯仿熏蒸法:0.5 mol/L K2SO4提取,用K2Cr2O7氧化法测定提取液中的微生物生物量碳,靛酚蓝比色法测定微生物生物量氮[16]。用OMEGA公司E.Z.N.A Soil DNA试剂盒抽提土壤基因组,1%琼脂糖凝胶电泳检测DNA的大小及片段完整性,NanoDrop2000检测DNA纯度,TBS-380检测DNA浓度。用ABIGeneAmp®9700PCR仪对真菌ITS区进行PCR扩增,引物为817F: 5′-TTAGCATGGAATAATRRAATAGGA-3′和1196R: 5′-TCTGGACCTGGTGAGTTTCC-3′。PCR扩增程序如下:95 ℃预变性3 min;95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸45 s,共35个循环,72 ℃终止延伸10 min结束。每个样品3次重复,混合同一样品的PCR产物,2%琼脂糖凝胶电泳,AxyPrepDNA凝胶回收试剂盒(AXYGEN 公司)切胶回收PCR产物,QuantiFluorTM-ST蓝色荧光定量系统进行定量。Miseq文库构建和测序均在上海美吉生物科技有限公司进行,测序平台为Illumina MiSeqPE300/PE250。测序结束后,对有效序列进行去杂、修剪、去除嵌合体等过滤处理,得到优化序列,根据序列97%的相似性形成操作分类单元(operational taxonomic units,OUTs),对比Unite库,采用RDP classifier贝叶斯算法,得到OTUs的分类学信息。
1.4 数据处理
用Excel进行试验数据基本计算,SPSS 19.0统计分析,R语言工具和Origin 8.5作图,单因素方差分析(one way-ANOVA)检验不同种植年限处理间差异显著性,CANOCO 4.5主成分分析(principal component analysis,PCA)不同处理间土壤微生物群落组成差异,显著水平设置为P<0.05。真菌门的相对丰度为某门真菌18 S rDNA 读数占真菌18 S rDNA 总读数的百分数。
2 结果与分析
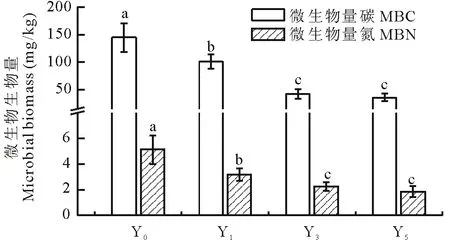
图1 不同种植年限黄花蒿根际土壤微生物生物量碳氮 Fig.1 Changes of microbial biomass carbon and nitrogen in rhizospheric soils for A. annua cultivation Y0:未种植黄花蒿 Uncultivated A. annua;Y1:种植黄花蒿1年 Cultivated A. annua for 1 year;Y3:黄花蒿连作3年 Continuous cropping A. annua for 3 years;Y5:黄花蒿连作5年 Continuous cropping A. annua for 5 years.柱上方同一测定指标有不同小写字母者表示差异显著(P<0.05),下同。Different small letters above the bars within same measurement item indicate a significant difference at P<0.05, the same below.
2.1 土壤微生物生物量碳氮
由图1可见,随黄花蒿种植年限增加,土壤微生物生物量碳氮持续降低。其中,微生物生物量碳降幅为30.04%~74.98%;微生物生物量氮降幅为38.01%~63.94%,微生物生物量碳氮比为18.88~31.80。
2.2 土壤酶活性
由表1可见,栽培黄花蒿不同程度地抑制土壤脲酶、脱氢酶和蔗糖酶活性。至栽培第5年,降幅依次为51.82%, 96.15%和53.76%。在种植黄花蒿前后,土壤酸性磷酸酶活性无显著差异,但种植1年的土壤显著低于种植3和5年的土壤。
2.3 土壤真菌
2.3.1 真菌门类 高通量测序从Y0、Y1、Y3和Y5土壤中分别获得了19358,23826,17385和12133个真菌ITS序列数(Reads),依次代表96,69,98和86种真菌(OTUs),归属于子囊菌门、壶菌门、担子菌门、球囊菌门、接合菌门和未知类型,且丰富度因真菌门类和种植黄花蒿而变化(图2)。其中,子囊菌门占绝大多数,超过66.10%,在Y1土壤中的相对丰富度高达95.28%。
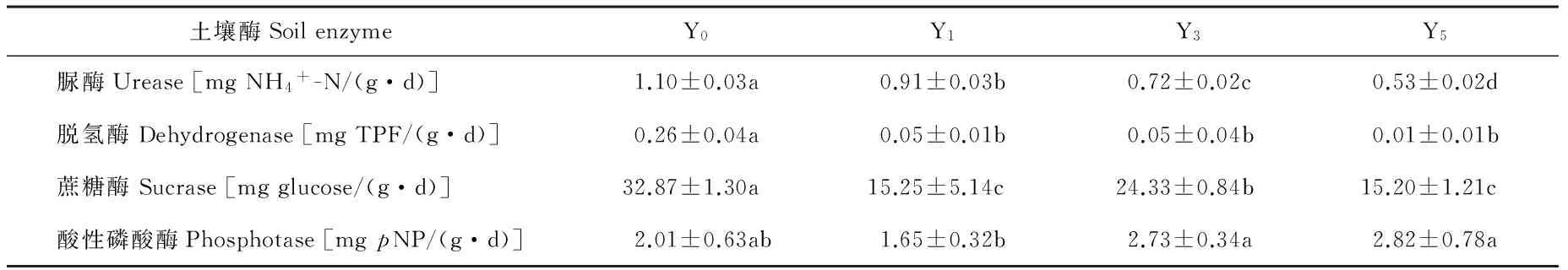
表1 不同种植年限黄花蒿根际土壤酶活性Table 1 Enzyme activities in rhizospheric soils with A. annua cultivated
注:在同行中,不同小写字母表示差异显著(P<0.05)。
Note:In each row, different small letters indicate significant differences among systems atP<0.05.

图2 在门水平上,不同种植年限黄花蒿根际土壤中真菌群落组成Fig.2 Phyla in fungal communities in rhizospheric soils with A. annua grown for different yearsA:子囊菌门Ascomycota;B:壶菌门Chytridiomycota;C:担子菌门Basidiomycota;D:球囊菌门Glomeromycota;E:纤毛亚门Ciliophora;F:后生动物Metazoa;G:接合菌门Zygomycota;H:未分类的真菌Fungi (unclassified).
2.3.2 优势真菌 在黄花蒿栽培前后的土壤中,前20种优势真菌的丰富度合计占真菌总量的93.37%以上。黄花蒿种植年限不同,其优势真菌种(属)类和丰富度也不一样(表2)。值得注意的是,与未种植黄花蒿的土壤相比,栽培土壤中仍有14种共有真菌,它们是不可培养的粪壳菌、待定真菌-1、不可培养的皱褶球壳菌、待定粪壳菌、肉座菌目、刺盾炱目、待定子囊菌-1、格孢菌目 IRB20-2、不可培养球囊菌、踝节菌属、伞菌目、待定伞菌、长喙壳目和柱隔孢属。
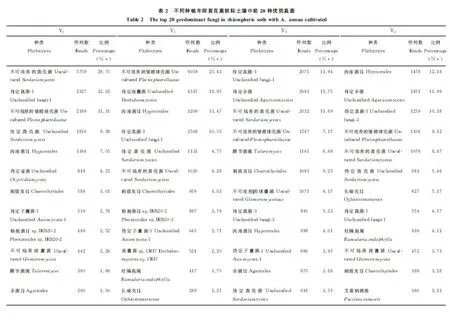
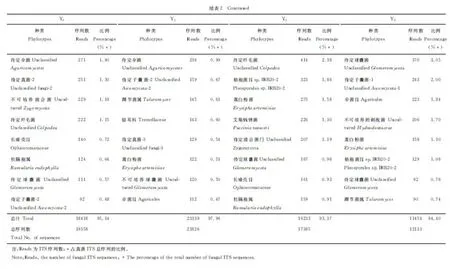
除上述共同存在的真菌之外,在Y0和Y1土壤中,均检测到待定子囊菌-2;在Y0和Y3土壤中,待定纤毛菌和待定球囊菌相同;在Y0和Y5土壤中,均发现了待定球囊菌。在Y1、Y3和Y5土壤中,均存在待定真菌-3和蒿白粉菌;在Y3和Y5土壤中,均出现了待定真菌-3、蒿白粉菌、艾菊柄锈菌和待定球囊菌。
在黄花蒿栽培前后土壤中,检测到各自1~3种独有真菌,它们是Y0土壤中的待定壶菌、待定真菌-2和不可培养接合菌;Y1土壤中的待定座囊菌、座囊菌CRI7和银耳科;Y3土壤中的待定接合菌门;Y5土壤中的不可培养的刺孢菌和待定球囊菌。
2.3.3 真菌群落组成主成分分析 图3是黄花蒿种植前后,土壤真菌群落的主成分变异情况。Principal component 1和Principal component 2分别表示不同群落间65.83%和29.12%的变异度。代表Y0、Y1与Y3和Y5土壤真菌群落组成的点在坐标图中分布距离较远。其中,Y0位于象限III的左下方,Y1位于象限IV和Y轴右侧的X轴上,Y3和Y5分别散落于Y轴左侧的X轴上和左上方(象限Ⅱ)。
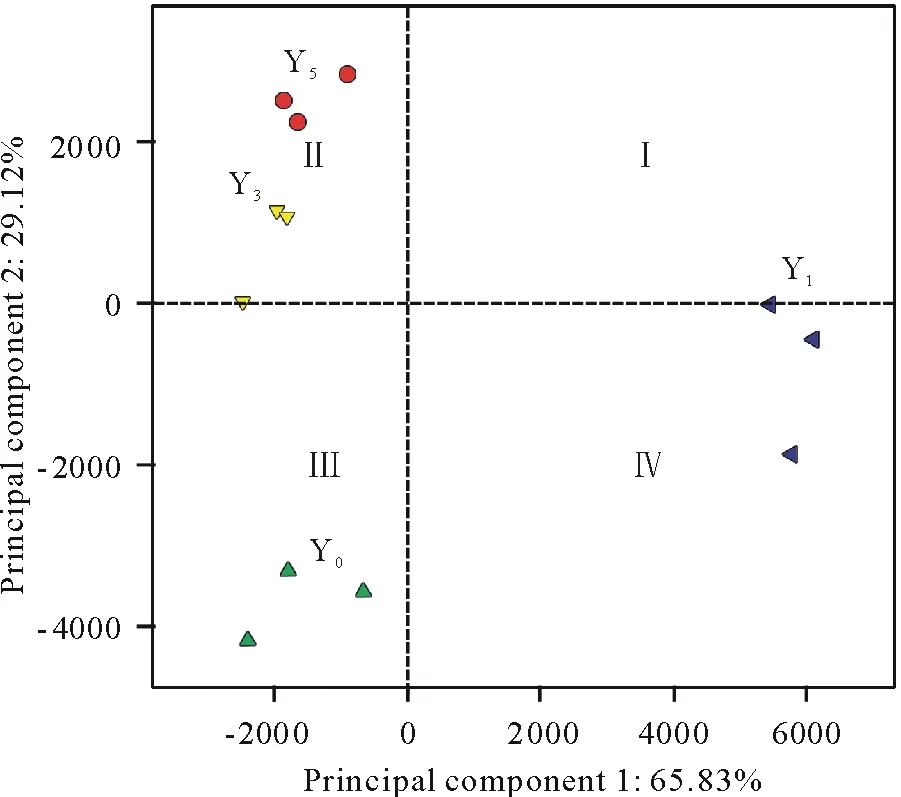
图3 不同种植年限黄花蒿根际土壤真菌群落组成主成分分析Fig.3 Principal component analysis of fungal communities in rhizospheric soils with A. annua grown
3 讨论
在人工种植黄花蒿的土壤中,微生物生物量碳氮显著降低,种植时间愈长,降幅越大,说明长期持续种植黄花蒿降低了土壤微生物数量,类似Herrmann等[5]和刘飞等[21]的研究结果。在不同种植年限的黄花蒿根际土壤中,微生物生物量碳氮比相差1.68倍,意味着土壤微生物组成改变,其原因可能是黄花蒿释放的青蒿素类化合物选择性地抑制了微生物生长繁殖。据报道,土壤微生物是土壤酶的主要来源,它们的数量和种群结构影响土壤酶活性[22-23]。傅慧兰等[24]发现,随种植年限增加,豆科作物根系分泌物在土壤中持续积累,作用于微生物和酶分子空间结构,使脲酶、过氧化氢酶和转化酶活性降低,酸性磷酸酶活性增强。与之类似,在种植黄花蒿的土壤中,脲酶、脱氢酶和蔗糖酶活性降低;但连续种植3年以上则使酸性磷酸酶活性增强。在持续种植棉花(Gossypiumspp.)、番茄(Lycopersiconesculentum)、辣椒(Capsicumannuum)等的土壤中,土壤微生物和酶活性改变是发生连作障碍的重要原因之一[25-27]。因此,黄花蒿释放的化感物质影响土壤微生物生物量碳氮和酶活性,可能导致土壤结构形成、养分转化供应、毒物降解等生物化学过程紊乱,不利于黄花蒿生长发育,使青蒿素含量和产量降低。
土壤真菌是土壤微生物的重要组分,具有多种多样的生理、生化和生态功能,其内转录间隔区(ITS)序列保守性极高,因此,本试验以此为分子标记,鉴定黄花蒿种植土壤中的真菌种类。结果表明,在不同黄花蒿种植年限的土壤中,真菌门类及优势真菌的丰富度差异显著;真菌群落组成主成分变异表明,种植黄花蒿改变了土壤真菌群落组成。但无论是否种植黄花蒿,在土壤前20种优势真菌中,仍有14种共有真菌,不同种植年限之间仅3~5种真菌不一样,说明土壤是决定真菌群落组成主导因素,但因种植黄花蒿而发生不同程度地变化。
在种植黄花蒿1年的土壤中,子囊菌门比对照显著增加,相对丰富度高达95.28%。研究表明,多数子囊菌为植物病原真菌,可造成白术(Atractylodesmacrocephala)、地黄、三七和桔梗(Platycodongrandiflorus)等多种药用植物发生根腐和茎腐病等[28]。随黄花蒿种植年限增加,土壤中的球囊菌、座囊菌、接合菌和刺孢菌也逐渐成为优势真菌。众所周知,座囊菌易引起香蕉和梨树等果树叶斑病;接合菌导致果蔬、食品等霉变腐烂;刺孢菌侵染兰科作物形成炭疽病[29-30]。所以,连作黄花蒿可能提高某些作物发生真菌病害的几率。田间调查发现,新地种植黄花蒿,一般很少发生病虫害;短期连作黄花蒿,根腐病和茎腐病病害危害率为3%~5%,长期连作病虫害发生率持续上升[31]。在未种植黄花蒿的土壤中,优势菌株中未检测到蒿属的特有病原真菌——蒿白粉菌和艾菊柄锈菌;但种植黄花蒿后,蒿白粉菌和艾菊柄锈菌均成为优势菌株。通常在连作条件下,某些植物会持续向病原微生物提供特有的营养物质,促进其生长繁殖,造成病害大量发生[32]。Jessing等[7]发现,青蒿素类物质能够选择性杀抑土壤微生物,而部分微生物却以它们为碳源和营养物质。因此,连作黄花蒿持续释放青蒿素类物质可能促进蒿白粉菌和艾菊柄锈菌生长繁殖,提高相应病害的发生风险。反之,在黄花蒿-马铃薯轮作体系中,由于中断了青蒿素类物质进入土壤,黄花蒿白粉病和茎腐病发生率则显著下降[33]。
总之,人工栽培黄花蒿显著降低土壤微生物生物量碳氮,改变土壤酶活性和真菌群落组成,选择性增加病原菌数量。
References:
[1] Li L Y, Su S, Wu Y K. The Industrialization Production and Management of High QualityArtemisiaannua[M]. Chongqing: Chongqing Press, 2009. 李隆云, 舒抒, 吴叶宽. 优质青蒿产业化生产与经营[M]. 重庆: 重庆出版社, 2009.
[2] Feng S X, Ma X J, Yan Z G,etal. Studies on the rotation model ofArtemisiaannua. China Journal of Chinese Meteria Medica, 2009, 34(4): 488-490. 冯世鑫, 马小军, 闫志刚, 等. 黄花蒿轮作模式的研究. 中国中药杂志, 2009, 34(4): 488-490.
[3] Duke S O, Vaughn K C, Croom E M,etal. Artemisinin, a constituent of annual worm wood (Artemisiaannua), is a selective phytotoxin. Weed Science, 1987, 35(4): 499-505.
[4] Duke S O, Dayan F E, Romagni J G,etal. Natural products as sources of herbicides: current status and future trends. Weed Research, 2000, 40(1): 99-111.
[5] Herrmann S, Jessing K K, Jorgensen N O G,etal. Distribution and ecological impact of artemisinin derived fromArtemisiaannuaL. in an agricultural ecosystem. Soil Biology & Biochemistry, 2013, 57: 164-172.
[6] Jessing K K, Cedergreen N, Mayer P,etal. Loss of artemisinin produced byArtemisiaannuaL. to the soil environment. Industrial Crops and Products, 2013, 43: 132-140.
[7] Jessing K K, Cedergreen N, Jensen J,etal. Degradation and ecotoxicity of the biomedical drug artemisinin in soil. Environmental Toxicology and Chemistry, 2009, 28(4): 701-710.
[8] Lu H, Zou W X, Meng J C,etal. New bioactive metabolites produced byColletotrichumsp., an endophytic fungus inArtemisiaannua. Plant Science, 2000, 151(1): 67-73.
[9] Dhingra V, Rao K V, Narasu M L. Current status of artemisinin and its derivatives as antimalarial drugs. Life Science, 2000, 66(4): 279-300.
[10] Tang H Q, Hu J, Yang L,etal. Terpenoids and flavonoids fromArtemisiaspecies. Planta Medica, 2000, 66(4): 391-393.
[11] Shoeb H A, Tawfik A F, Shibl A M,etal. Antimicrobial activity of artemisinin and its derivatives against anaerobic-bacteria. Journal of Chemotherapy, 1990, 2(6): 362-367.
[12] Luo S Q. Study on Correlation between Soil Microorganism and Anti-malaria-related Compouds ofArtemisiaannuaL.[D].Chongqing: Southwest University, 2013. 罗世琼. 黄花蒿土壤微生物与抗疟相关成分的关联性研究[D]. 重庆: 西南大学, 2013.
[13] Li Q, Yuan L, Luo S Q,etal. Artemisinin and flavonoids in wildArtemisiaannuaand surrounding soil and the influence on soil microbes. Acta Prataculturae Sinica, 2015, 24(11): 29-37. 李倩, 袁玲, 罗世琼, 等. 野生黄花蒿植株和土壤中的青蒿素、黄酮含量变化及其对土壤微生物的影响. 草业学报, 2015, 24(11): 29-37.
[14] Lin X G, Hu J L. Scientific connotation and ecological service function of soil microbial diversity. Acta Pedologica Sinica, 2008, 45(5): 892-900. 林先贵, 胡君利. 土壤微生物多样性的科学内涵及其生态服务功能. 土壤学报, 2008, 45(5): 892-900.
[15] Tan G Y, Yang Z L, Yuan Z L,etal. Research advances in continuous cropping obstacle in medicinal plants and its management. Journal of Northwest A & F University: Natural Science Edition, 2012, 40(4): 197-204. 檀国印, 杨志玲, 袁志林, 等. 药用植物连作障碍及其防治途径研究进展. 西北农林科技大学学报: 自然科学版, 2012, 40(4): 197-204.
[16] Lin X G. Research on the Principle and Method of Soil Microorganisms[M]. Beijing: Higher Education Press, 2010.
[17] Zhang Q F, Liu B, Lin Y Z,etal. The diversity of phospholipid fatty acid (PLFA) biomarker for the microbial community in soil. Acta Ecologica Sinica, 2009, 29(8): 4127-4137. 张秋芳, 刘波, 林营志, 等. 土壤微生物群落磷脂脂肪酸PLFA生物标记多样性. 生态学报, 2009, 29(8): 4127-4137.
[18] Xia W W, Jia Z J. Comparative analysis of soil microbial communities by pyrosequencing and DGGE. Acta Microbiologica Sinica, 2014, 54(12): 1489-1499. 夏围围, 贾仲君. 高通量测序和DGGE分析土壤微生物群落的技术评价. 微生物学报, 2014, 54(12): 1489-1499.
[19] Rhodes J, Beale M A, Fisher M C. Illuminating choices for library prep: a comparison of library preparation methods for whole genome sequencing ofCryptococcusneoformansusing Illumina Hiseq. PLoS One, 2014, 9(11): e113501.
[20] Guan S Y. Soil Enzyme and Its Research Methods[M]. Beijing: Agricultural Press, 1986. 关松荫. 土壤酶及其研究方法[M]. 北京: 农业出版社, 1986.
[21] Liu F, Wu X L, Cui G L,etal. Study on the relationship between the number of rhizosphere microorganisms and artemisinin content ofArtemisiaannua. Lishizhen Medicine and Materia Medica Research, 2010, 21(1): 37-38. 刘飞, 伍晓丽, 崔广林, 等. 青蒿根际微生物数量动态及其与青蒿素含量的关系研究. 时珍国医国药, 2010, 21(1): 37-38.
[22] Yang X J, Wang Y S, Duan L D,etal. Changes of soil microbial biomass and enzymatic activities among restoration stages of Langshan Forest Park, Hunan Province. Acta Prataculturae Sinica, 2014, 23(1): 142-148. 杨贤均, 王业社, 段林东, 等. 湖南崀山森林公园不同植被条件下土壤微生物量及酶活性研究. 草业学报, 2014, 23(1): 142-148.
[23] Sui Y Y, Jiao X G, Gao C S,etal. The relationship among organic matter content and soil microbial biomass and soil enzyme activities. Chinese Journal of Soil Science, 2009, 40(5): 1036-1039. 隋跃宇, 焦晓光, 高崇生, 等. 土壤有机质含量与土壤微生物量及土壤酶活性关系的研究. 土壤通报, 2009, 40(5): 1036-1039.
[24] Fu H L, Yang Z M. Effect of soybean continuous cropping on soil enzyme activity. Journal of Plant Nutrition and Fertilizer, 1996, (4): 374-377. 傅慧兰, 杨振明. 大豆连作对土壤酶活性的影响. 植物营养与肥料学报, 1996, (4): 374-377.
[25] Liu J G, Zhang W, Li Y B,etal. Effects of long-term continuous cropping system of cotton on soil physical-chemical properties and activities of soil enzyme in oasis in Xinjiang. Scientia Agricultura Sinica, 2009, 42(2): 725-733. 刘建国, 张伟, 李彦斌, 等. 新疆绿洲棉花长期连作对土壤理化性状与土壤酶活性的影响. 中国农业科学, 2009, 42(2): 725-733.
[26] Sun Y Y, Jiang G Y, Liu J G,etal. Effects of continuous cropping tomato for processing on soil enzyme activities and microbial flora. Acta Ecologica Sinica, 2010, 30(13): 3599-3607. 孙艳艳, 蒋桂英, 刘建国, 等. 加工番茄连作对农田土壤酶活性及微生物区系的影响. 生态学报, 2010, 30(13): 3599-3607.
[27] Liu L, Huang B J, Sun J,etal. Relationship between soil microbial quantity, enzyme activity and soil fertility in hot pepper greenhouse soils of different continuous cropping years. Soils and Fertilizers Sciences in China, 2013, (2): 5-10. 刘来, 黄保健, 孙锦, 等. 大棚辣椒连作土壤微生物数量、酶活性与土壤肥力的关系. 中国土壤与肥料, 2013, (2): 5-10.
[28] Kang Z S. Current status and development strategy for research on plant fungal diseases in China. Plant Protection, 2010, 36(3): 9-12. 康振生. 我国植物真菌病害的研究现状及发展策略. 植物保护, 2010, 36(3): 9-12.
[29] Wang G F, Huang J S, Xie Y X,etal. Advances in research on sigatoka disease of banana. Journal of Fruit Science, 2006, 23(1): 96-101. 王国芬, 黄俊生, 谢艺贤, 等. 香蕉叶斑病的研究进展. 果树学报, 2006, 23(1): 96-101.
[30] Li J H, Zhang L H. Study on appearance-character and comprehensive prevention ofOrchidanthracnose. Northern Horticulture, 2013, 18(18): 108-110. 李景蕻, 张丽华. 兰花炭疽病的发生特点及综合防治. 北方园艺, 2013, 18(18): 108-110.
[31] Jiang Y S, Qi X X, Chen Z Y,etal. Main problems and strategy of artificial plantingArtemisiaannua. Lishizhen Medicine and Materia Medica Research, 2007, 18(9): 2184-2185. 蒋运生, 漆小雪, 陈宗游, 等. 黄花蒿人工栽培种中存在的主要问题及对策. 时珍国医国药, 2007, 18(9): 2184-2185.
[32] Ehlers B K. Soil microorganisms alleviate the allelochemical effects of a thyme monoterpene on the performance of an associated grass species. PLoS One, 2011, 6(11): 1-5.
[33] Feng S X, Ma X J, Yan Z G,etal. Effects of crop rotation ofArtemisiaannuaand autumn species such as potatoes and other. Southwest China Journal of Agricultural Sciences, 2013, 26(1): 79-83. 冯世鑫, 马小军, 闫志刚, 等. 黄花蒿与马铃薯等秋种作物轮作的效应分析. 西南农业学报, 2013, 26(1): 79-83.
Microbial biomass, enzyme activity and composition of the fungal community in rhizospheric soil cropped with Artemisia annua for several years
LI Qian1,3, YANG Shui-Ping1, CUI Guang-Lin2, HUANG Jian-Guo1*, LI Long-Yun2*, CHENG Yu-Yuan3
1.CollegeofResourceandEnvironment,SouthwestUniversity,Chongqing400716,China; 2.InstituteofChongqingChineseMedicine,Chongqing400065,China; 3.HenanNanyangTobaccoCompany,Nanyang473000,China
Artemisiaannua(Qinghao, Asteraceae) is widely grown in Chongqing, China, for extracting the antimalarial drug, artemisinin. Many research studies focus on the release of allelochemicals into soils via leaching with rainfall percolation, on root exudation, and on decomposition of dead plant residues in the growing process ofA.annuaand on the inhibition of the growth and development of adjacent and subsequent crops by these allelochemicals, particularly artemisinin. Soil microbes play roles in nutrient transformation, organic matter recycling, toxicant decomposition, and hormone efflux, among others. However, little is known about the influence of continuous cultivation of this medicinal plant on soil microorganism populations. Therefore, rhizospheric soils cropped withA.annuafor 1, 3, and 5 years were collected and analyzed by routine methods and Illumina MiSeq pyrosequencing to study microbial biomass, enzyme activity and fungal community components. Microbial biomass carbon (C) and nitrogen (N), and enzyme activities (dehydrogenase, urease and invertase) decreased, while C∶N in microbes varied, and acid phosphatase activity increased in soils with this medicinal plant compared that in the soil without this plant. These results suggest that allelochemicals released fromA.annuainto the rhizosphere inhibited the metabolism, growth and reproduction of microorganisms. Principal component coefficients of fungal communities in soils varied significantly, indicating great changes of fungal community structures. In soil fungal communities, Ascomycota was the largest group, accounting for 66.10%-95.28% of the total taxa detected, and there was a significant change in the abundance of both fungal phyla and the top species duringA.annuacultivation. Among the predominant fungi, 14 species were found in all soils, and only 1-3 unique species existed in each soil, suggesting that the soil was the most important factor governing the composition of the fungal community, but that community structure is also changed byA.annuacultivation.ErysipheartemisiaeandPucciniatanaceti, two pathogenic fungi which only infectA.annua, were found in the soils cropped withA.annua. The presence of these two pathogenic fungi in soils would increase the risk of disease incidence inA.annua. Therefore, rotation is advisable when croppingA.annua. Although our study provided some information about fungal community composition and diversity in the soil cropped withA.annua, a large number of microorganisms detected remain unidentified, and the functions of microbes classified is also not clear. The results confirm that the understanding of soil microbial communities remains very poor. Further study should focus on determining the identity and function of bacterial members of the microbial community, as these could be important in maintaining soil quality and function in cropping systems.Key words:Artemisiaannua; soil enzyme activity; fungi; high throughput sequencing
10.11686/cyxb2016091
http://cyxb.lzu.edu.cn
2016-03-08;改回日期:2016-05-11
国家973计划项目(2013CB127405),国家科技惠民计划项目(2013GS 500102),重庆市科技研发基地项目(cstc 2014ptyjd10001),重庆市自然科学基金(cstc2011jjA 0861)和中央高校基金(SWU113094)资助。
李倩(1987-),女,河南郑州人,博士。E-mail:qianqingzi@qq.com*通信作者Corresponding author. E-mail: huang99@swu.edu.cn, lilongyun8@163.co
李倩, 杨水平, 崔广林, 黄建国, 李隆云, 程玉渊. 不同种植年限条件下黄花蒿根际土壤微生物生物量、酶活性及真菌群落组成. 草业学报, 2017, 26(1): 34-42.
LI Qian, YANG Shui-Ping, CUI Guang-Lin, HUANG Jian-Guo, LI Long-Yun, CHENG Yu-Yuan. Microbial biomass, enzyme activity and composition of the fungal community in rhizospheric soil cropped withArtemisiaannuafor several years. Acta Prataculturae Sinica, 2017, 26(1): 34-42.
——青蒿素

