广东省镇海水库拟柱孢藻(Cylindrospermopsis raciborskii)的季节动态及驱动因子分析
赵 莉,雷腊梅,2,彭 亮,2,韩博平,2
(1:暨南大学水生生物研究中心,广州 510632)(2:广东省水库蓝藻水华防治中心,广州 510632)
广东省镇海水库拟柱孢藻(Cylindrospermopsis raciborskii)的季节动态及驱动因子分析
赵 莉1,雷腊梅1,2,彭 亮1,2,韩博平1,2
(1:暨南大学水生生物研究中心,广州 510632)(2:广东省水库蓝藻水华防治中心,广州 510632)
拟柱孢藻(Cylindrospermopsisraciborskii)是热带地区普遍存在的蓝藻种类,已在广东省很多水库成为优势种类甚至形成水华,作为一种新的有害水华类型,目前对其成因研究甚少. 以广东省江门市镇海水库为研究对象,于2014年11月-2015年10月期间对其进行逐月采样,观测理化因子和浮游植物组成,测定拟柱孢藻的丝体长度,初步探讨该水库拟柱孢藻优势形成的原因. 数据表明,拟柱孢藻是镇海水库的绝对优势种,常年生物量较高,介于5.9~15.5 mg/L之间,平均生物量为11.3 mg/L,占浮游植物总生物量的93.5%. 从季节上看,拟柱孢藻生物量在2-6月相对较高,最高生物量出现在6月,10月和11月生物量最低. 拟柱孢藻的丝体长度具有显著的季节变化,与水温呈极显著负相关. 相关性分析表明拟柱孢藻生物量与总氮、总磷浓度呈显著正相关,与氮磷比呈显著负相关,而逐步回归分析表明拟柱孢藻生物量的变化主要由总磷浓度决定,推测该藻对磷的超强吸收和储存能力在其生物量季节变动中起重要作用.
拟柱孢藻;藻丝体长度;生物量;环境因子;镇海水库

由于我国经济的快速发展,淡水水体富营养化日益严重,导致蓝藻水华频发,其中以微囊藻水华最为常见. 但近年来,鱼腥藻(Anabaena)、束丝藻(Aphanizomenon)、拟柱孢藻等蓝藻水华也屡有报道[13-15]. 拟柱孢藻在我国的广东、湖北、云南、台湾、福建等地区的水体中均有发现[15-18]. 作为热带特征性种类,拟柱孢藻在温带地区的湖北和云南等水体中并不是优势种,在相对温暖的广东、台湾和福建等地区,拟柱孢藻可占据优势,并在广东与台湾地区形成水华. 在福建江东水库,拟柱孢藻在水体稳定的夏、秋季为蓝藻优势种之一[18]. 在台湾,拟柱孢藻从夏季到冬季均占优势,尤其在夏季高温时,该藻占浮游植物的比例可达90%,分析发现低透明度、高温、弱碱性、低DIN的环境条件有利于拟柱孢藻形成优势[17]. 广东省属于亚热带海洋性季风气候,常年温度较高,调查显示该省多个水库均发现拟柱孢藻的分布,其生物量在丰水期高于枯水期[15],在三坑、百花林、显岗等多个水库中发生了拟柱孢藻水华,其相对丰度可达93%以上[19],而目前我国对南亚热带水库拟柱孢藻频繁出现乃至形成水华的原因完全缺乏了解. 为深入分析广东省拟柱孢藻种群优势形成的影响因素,本文以镇海水库为研究对象,对2014年11月-2015年10月间的浮游植物群落结构和理化环境因子进行观测和分析,探讨拟柱孢藻优势形成的生态条件.

图1 镇海水库在广东省的位置与水库采样点设置Fig.1 Location of the sampling site in Zhenhai Reservoir in Guangdong Province
1 材料与方法
1.1 水库概况、采样点与采样时间
镇海水库(22°34′N, 112°33′E)位于广东省江门的开平市北部,水库集雨面积为128 km2,总库容为1.09×108m3,正常库容为7670×104m3,平均水深9.6 m,属于亚热带海洋性季风气候,夏季高温多雨,冬季温和少雨,为广东省重要的大型供水水库. 本次调查的采样点设置在镇海水库的库中区(图1),于2014年11月-2015年10月间每月进行一次采样.
1.2 理化因子的测定

1.3 浮游植物的处理与鉴定
用于浮游植物定量镜检的样品当场加10 ml鲁哥试剂固定,带回实验室沉淀浓缩后计数,在进行浮游植物计数过程中,随机测量180根拟柱孢藻丝体长度,同时对藻丝体上异形胞和厚壁孢子进行观察和计数[21]. 浮游植物的定性样品用25#网孔直径为64 μm的浮游生物网,在水体的水平和垂直方向上多次拖网,所得样品当场加福尔马林至终浓度的3%~5%保存. 浮游植物的定性与定量样品均在光学显微镜(OLYMPUS-BX51)10×40倍下进行鉴定和计数.
1.4 数据处理
采用相关性分析和多因素逐步回归对拟柱孢藻的生物量和长度与环境因子之间的关系进行分析,在方差分析(ANOVA)中,取P<0.05为差异显著,P<0.01为差异极显著. 本文所涉及的所有数据的处理和分析均在SPSS 16.0软件中完成,作图均在Origin 8.0和Photoshop 6.0软件中进行.
2 结果与分析
2.1 镇海水库理化因素的动态特征
调查期间,TP浓度介于0.019~0.086 mg/L之间,平均浓度为0.040 mg/L,其浓度在3-6月间维持较高水平;SRP浓度介于0.001~0.018 mg/L之间,平均值为0.006 mg/L,其季节性变化趋势与TP浓度基本一致(图2a). TN浓度介于0.83~1.80 mg/L之间,平均浓度为1.39 mg/L,在2014年10月-2015年6月比较平稳,基本维持在1.58 mg/L,7-10月逐渐降低至0.83 mg/L;DIN浓度介于0.12~0.62 mg/L之间,平均浓度为0.41 mg/L(图2b). 镇海水库TN/TP的范围为19.2~75.8,根据水体中TN/TP高于Redfield比(TN/TP=16),则水体是磷限制的标准,该水库全年均处于磷限制状态.

图2 镇海水库TP和SRP浓度(a), TN和DIN浓度(b), Zeu/Zm和水温(c)的季节动态Fig.2 Seasonal dynamics of TP and SRP concentrations(a), TN and DIN concentrations (b), Zeu/Zm and surface water temperature (c) in Zhenhai Reservoir
镇海水库的Zeu/Zm在0.12~0.34间波动,平均值为0.2,水库在3-4月Zeu/Zm较高,在3月达到最大值,也仅为0.34(图2c),因此该水库浮游植物的生长常年受到光限制. 表层水温在16.7~32.8℃间波动,平均表层水温为25℃,6-9月水温均在30℃以上,8月达到最高值32.8℃,在12月至次年2月,表层水温一直低于20℃,2月为最低水温16.7℃(图2c).
2.2 镇海水库浮游植物群落动态特征
调查期间,镇海水库共检测到浮游植物6门43种(属),其中蓝藻门11种(属),绿藻门23种(属),硅藻门4种(属),甲藻门3种(属),裸藻门1种(属),隐藻门1种(属). 镇海水库的浮游植物群落以蓝藻为主,其平均生物量为11.6 mg/L,占浮游植物总生物量的95.6%,其他各门浮游植物仅占浮游植物总生物量的4.4%(图3).
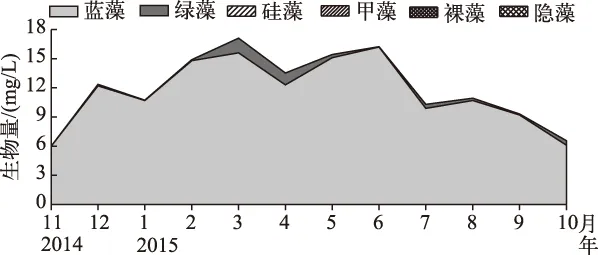
图3 镇海水库浮游植物生物量的季节动态Fig.3 Seasonal dynamic of phytoplankton biomass in Zhenhai Reservoir
拟柱孢藻为蓝藻门的绝对优势种,生物量为5.9~15.5 mg/L,平均值为11.3 mg/L,在调查期间其优势度一直维持较高水平,平均占浮游植物总生物量的93.5%. 从季节上看,拟柱孢藻生物量在2-6月相对较高,最高生物量出现在6月,10月和11月生物量最低(图4a). 拟柱孢藻生物量与TN和TP浓度呈显著正相关(r=0.596,P=0.041;r=0.671,P=0.017),与TN/TP呈显著负相关(r=-0.583,P=0.046)(表1). 将拟柱孢藻生物量与相关的环境因子进一步进行多因素逐步回归分析表明,拟柱孢藻生物量只与TP浓度呈显著正相关(r2=0.4,P=0.017).
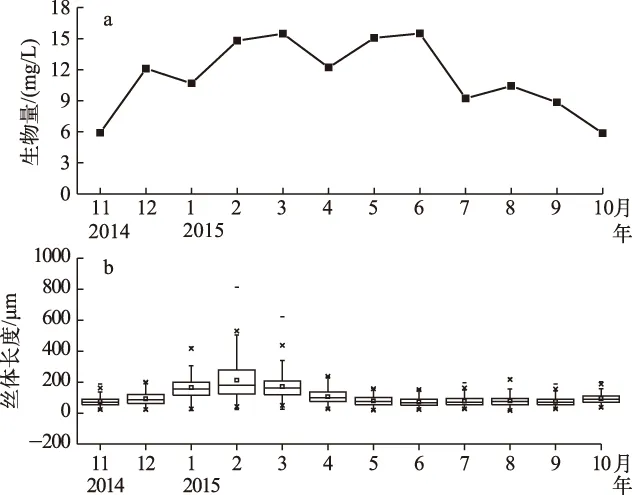
图4 镇海水库拟柱孢藻生物量(a)和丝体长度(b)的季节动态Fig.4 Seasonal dynamic of the C. raciborskii biomass(a) and filamental length(b) in Zhenhai Reservoir
在本次调查期间,基本没有见到含异形胞或厚壁孢子的拟柱孢藻,但其丝体形态呈现明显的季节变化,这主要体现在丝体长度,丝体宽度则无显著差异. 镇海水库拟柱孢藻丝体长度在72.1~212.8 μm内波动,平均长度为108.1 μm. 在表层水温较低的1-3月,藻丝体长度均在160 μm以上,在表层水温最低的2月,藻丝体长度达到最大值212 μm;反之,藻丝体在水温较高的季节较短,长度集中在70~90 μm之间,最小值出现在2015年6月(图4b). 相关性分析表明,拟柱孢藻的丝体长度与TN浓度呈显著正相关(r=0.596,P=0.041),与水温呈极显著负相关(r=-0.734,P=0.007)(表1). 进一步的多因素逐步回归分析表明,拟柱孢藻丝体长度只与水温存在极显著负相关(r2=0.554,P=0.007).
表1 拟柱孢藻生物量或丝体长度与环境因子之间的相关性
Tab.1 Correlation analysis between the environmental factors andC.raciborskiibiomass or filamental length

TempZeu/ZmTNTPTN/TPSRPDINDIN/SRP拟柱孢藻丰度拟柱孢藻生物量nsns0.596∗0.671∗-0.583∗nsnsnsns拟柱孢藻丝体长度-0.734∗∗ns0.596∗nsnsnsnsnsns
*表示显著相关,P<0.05;**表示极显著相关,P<0.01;ns表示无显著相关性,P>0.05.
3 讨论
本次调查中,广东省镇海水库的浮游植物生物量几乎都由拟柱孢藻贡献,其他藻类的生物量所占比例极少. 这种拟柱孢藻常年占据绝对优势的现象极为少见,在巴西Ingazeira水库和Lagoa santa湖有类似的现象发生,Ingazeira水库拟柱孢藻的相对生物量在4-11月可占到96%~100%,生物量最高可达70 mg/L[22];Lagoa santa湖的拟柱孢藻在整个研究期间都为绝对优势种,平均占总浮游植物的70%[7]. 但更多的研究发现拟柱孢藻的优势度往往呈季节性变化,如希腊的Kastoria湖和非洲塞内加尔的Guiers湖的研究发现,拟柱孢藻常年为两个湖泊的优势种,在夏季温度较高时生物量较高,约占浮游植物总生物量的60%[23-24];在法国的Francs-Pêcheurs池塘中,虽然拟柱孢藻最高可达99%,但仅在7-9月水温较高的季节发生[5].
镇海水库中拟柱孢藻丝体长度与温度呈显著负相关,这与以色列和中国台湾水体的研究结果一致[21,17],推测拟柱孢藻可以通过调整藻丝体的长短以适应外界温度的变化. 高温是拟柱孢藻增殖的必要条件[5],因此在低温条件下,拟柱孢藻为积蓄能量不进行分裂繁殖而形成长藻丝体,当温度升高至适宜范围,拟柱孢藻进入快速生长阶段,通过频繁分裂增殖形成短的藻丝体,因此拟柱孢藻丝体长度的季节变化可以反映其生长速率[17]. 在进行拟柱孢藻长度测量的过程中,几乎没有发现厚壁孢子和异形胞,可能是因为镇海水库的水温高于厚壁孢子萌发的最适温度5~10℃[12],氮营养盐浓度较高,不需要通过异形胞固氮来获取生长所需氮源.
高温可能也是导致拟柱孢藻在镇海水库常年发生水华的关键因素. 调查期间,镇海水库水温高且波动小,这样的温度条件有助于增强浮游植物群落的稳定性,并促进拟柱孢藻获得生长优势. 已有研究表明,拟柱孢藻喜高温环境[25],从镇海水库分离出的N8藻株的比生长速率随温度的升高而升高,并在高温下具最适生长速率[26]. 本研究中拟柱孢藻生物量与水温之间并不具显著相关性,这可能是因为南亚热带地区表层水温变化范围小,温度始终处于拟柱孢藻生长所需的适宜范围内. 调查期间,除TP和SRP浓度稍有变化外,镇海水库的表层水温、Zeu/Zm及TN、DIN浓度均无显著的季节变化,多因素逐步回归分析表明除TP浓度外,其他因素与拟柱孢藻生物量无显著相关性,而蓝藻的存在和优势地位通常与水体的稳定性有较高的相关性[27],有人认为拟柱孢藻不仅能够很好地适应稳定的水体条件,而且可以在稳定的水体中一直保持优势地位并形成水华[7,22].
镇海水库浮游植物的生长常年受到光限制,Zeu/Zm代表了光可获得性的大小,当该比值>1时光可获得性较好,湖上层的浮游植物极少受到光限制;当该比值<1时光可获得性较差[28]. 而拟柱孢藻被认为能耐受低光,比其他蓝藻对光照的需求更低[4],由此低的光照给高耐阴性的拟柱孢藻提供竞争优势使得镇海水库易发生拟柱孢藻水华[29],这与Briand、Figueredo、Bouvy等的研究结果基本一致[5,7,22]. 镇海水库属于磷限制性水体,SRP浓度在3-5月较高时也仅达到18 μg/L,其他月份一直低于4 μg/L,但拟柱孢藻却常年都是浮游植物的绝对优势种群,这可能是因为拟柱孢藻具有超强的吸收和储存磷的能力[6],比其他蓝藻(如微囊藻)有更高的吸收和转换效率[4]. 我们的研究也发现,在以磷为限制性底物时,镇海水库分离出的拟柱孢藻N8藻株的半饱和常数比微囊藻低,即该藻在低磷环境中更具生长优势[30]. 此外,当外界环境中的无机磷处于限制条件时,拟柱孢藻有可能利用不同的有机磷源支持其生长[31]. 镇海水库具有常年高温且波动小、低的光可获得性及磷限制性水体的特性,有利于拟柱孢藻在这一环境条件下维持竞争优势与高生物量.
[1] Antunes JT, Leão PN, Vasconcelos VMetal.Cylindrospermopsisraciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species.FrontiersinMicrobiology, 2015, 6: 473. DOI: 10.3389/fmicb.2015.00473.
[2] Briand JF, Leboulanger C, Humbert JFetal.Cylindrospermopsisraciborskii(cyanobacteria) invasion at mid-latitudes: Selection, wide physiological tolerance, or global warming?JournalofPhycology, 2004, 40(2): 231-238. DOI: 10.1111/j.1529-8817.2004.03118.x.
[3] Bonilla S, Aubriot L, Soares MCSetal. What drives the distribution of the bloom-forming cyanobacteriaPlanktothrixagardhiiandCylindrospermopsisraciborskii?FEMSMicrobiologyEcology, 2012, 79(3): 594-607. DOI: http://dx.doi.org/10.1111/j.1574-6941.2011.01242.x.
[4] Wu ZX, Shi JQ, Li RH. Comparative studies on photosynthesis and phosphate metabolism ofCylindrospermopsisraciborskiiwithMicrocystisaeruginosaandAphanizomenonflos-aquae.HarmfulAlgae, 2009, 8(6): 910-915. DOI: 10.1016/j.hal.2009.05.002.
[5] Briand JF, Robillot C, Quiblier-Llobéras Cetal. Environmental context ofCylindrospermopsisraciborskii(Cyanobacteria) blooms in a shallow pond in France.WaterResearch, 2002, 36(13): 3183-3192. DOI: 10.1016/S0043-1354(02)00016-7.
[6] Isvánovics V, Shafik HM, Présing Metal. Growth and phosphate uptake kinetics of the cyanobacterium,Cylindrospermopsisraciborskii(Cyanophyceae) in throughflow cultures.FreshwaterBiology, 2000, 43: 257-275. DOI: 10.1046/j.1365-2427.2000.00549.x.
[7] Figueredo CC, Giani A. Phytoplankton community in the tropical lake of Lagoa Santa (Brazil): Conditions favoring a persistent bloom ofCylindrospermopsisraciborskii.Limnologica-EcologyandManagementofInlandWaters, 2009, 39(4): 264-272. DOI: 10.1016/j.limno.2009.06.009.
[8] Saker ML, Eaglesham GK. The accumulation of cylindrospermopsin from the cyanobacteriumCylindrospermopsisraciborskiiin tissues of the Redclaw crayfish Cherax quadricarinatus.Toxicon, 1999, 37(7): 1065-1077. DOI: 10.1016/S0041-0101(98)00240-2.
[9] Herrero A, Muro-Pastor AM, Valladares Aetal. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria.FEMSMicrobiologyReviews, 2004, 28(4): 469-487. DOI: http://dx.doi.org/10.1016/j.femsre.2004.04.003.
[10] Padisák J, Reynolds CS. Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to cyanoprokaryotes.Hydrobiologia, 1998, 384: 41-53. DOI: 10.1023/A:1003255529403.
[11] Figueredo CC, Rückert GV, Cupertino Aetal. Lack of nitrogen as a causing agent ofCylindrospermopsisraciborskii, intermittent blooms in a small tropical reservoir.FEMSMicrobiologyEcology, 2014, 87(3): 557-567. DOI:10.1111/1574-6941.12243.
[12] Moore D, O’Donohue M, Garnett Cetal. Factors affecting akinete differentiation inCylindrospermopsisraciborskii(Nostocales, Cyanobacteria).FreshwaterBiology, 2005, 50(2): 345-352. DOI: 10.1111/j.1365-2427.2004.01324.x.
[13] Li ZL, Yu JW, Yang Metal. Cyanobacterial population and harmful metabolites dynamics during a bloom in Yanghe Reservoir, North China.HarmfulAlgae, 2010, 9(5): 481-488. DOI: 10.1016/j.hal.2010.03.003.
[14] Liu Y, Chen W, Li Detal. First report of aphantoxins in China—waterblooms of toxigenicAphanizomenonflos-aquaein Lake Dianchi.EcotoxicologyandEnvironmentalSafety, 2006, 65(1): 84-92. DOI: 10.1016/j.ecoenv.2005.06.012.
[15] Lei L, Peng L, Huang Xetal. Occurrence and dominance ofCylindrospermopsisraciborskiiand dissolved cylindrospermopsin in urban reservoirs used for drinking water supply, South China.EnvironmentalMonitoringandAssessment, 2014, 186(5): 3079-3090. DOI: 10.1007/s10661-013-3602-8.
[16] Wu Z, Shi J, Xiao Petal. Phylogenetic analysis of two cyanobacterial generaCylindrospermopsisandRaphidiopsisbased on multi-gene sequences.HarmfulAlgae, 2011, 10(5): 419-425. DOI: 10.1016/j.hal.2010.05.001.
[17] Yamamoto Y,Shiah FK. Factors related to the dominance ofCylindrospermopsisraciborskii(cyanobacteria) in a shallow pond in northern Taiwan.JournalofPhycology, 2012, 48(4): 984-991. DOI: 10.1111/j.1529-8817.2012.01184.x.
[18] Tian YQ, Huang BQ, Yu CCetal. Dynamics of phytoplankton communities in the Jiangdong Reservoir of Jiulong River, Fujian, South China.ChineseJournalofOceanologyandLimnology, 2014, 32: 255-265. DOI: 10.1007/s00343-014-3158-7.
[19] Jiang Qiming, Hou Wei, Gu Jiguangetal. Nutritional status and population characteristics of cyanobacteria in small and medium sized reservoirs in Guangzhou, southern China.EcologyandEnvironmentalSciences, 2010, 19(10): 2461-2467. [江启明, 侯伟, 顾继光等. 广州市典型中小型水库营养状态与蓝藻种群特征. 生态环境学报, 2010, 19(10): 2461-2467.]
[20] Lin Shaojun, He Lijing, Huang Peishengetal. Comparison and improvement on the extraction method for chlorophyll a in phytoplankton.EcologicScience, 2005, 24(1):9-11. DOI: 1008-8873(2005)01-009-03.[林少君, 贺立静, 黄沛生等. 浮游植物中叶绿素a提取方法的比较与改进. 生态科学, 2005, 24(1): 9-11.]
[21] Alster A, Kaplan-Levy RN, Sukenik Aetal. Morphology and phylogeny of a non-toxic invasiveCylindrospermopsisraciborskiifrom a Mediterranean Lake.Hydrobiologia, 2010, 639(1): 115-128. DOI: 10.1007/s10750-009-0044-y.
[22] Bouvy M, Molica RJR, Oliveira SDetal. Dynamics of a toxic cyanobacterial bloom (Cylindrospermopsisraciborskii) in a shallow reservoir in the semi-arid region of northeast Brazil.AquaticMicrobialEcology, 1999, 20(3): 285-297. DOI: 10.3354/ame020285.
[23] Berger C, Ba N,Gugger Metal. Seasonal dynamics and toxicity ofCylindrospermopsisraciborskiiin Lake Guiers (Senegal, West Africa).FEMSMicrobiologyEcology, 2006, 57(3): 355-366. DOI: http://dx.doi.org/10.1111/j.1574-6941.2006.00141.x.
[24] Moustaka-Gouni M, Vardaka E, Tryfon Eetal. Phytoplankton species succession in a shallow Mediterranean lake (L. Kastoria, Greece): Steady-state dominance ofLimnothrixredekei,MicrocystisaeruginosaandCylindrospermopsisraciborskii.Hydrobiologia, 2007, 575(1): 129-140. DOI: 10.1007/s10750-006-0360-4.
[25] Padisák J.Cylindrospermopsisraciborskii(Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: Worldwide distribution and review of its ecology.ArchivfürHydrobiologieSupplementbandMonographischeBeitrage, 1997, 107(4): 563-593.
[26] Yu Ting, Dai Jingjun, Lei Lameietal. Effects of temperature, irradiance and nitrate on the growth ofCylindrospermopsisraciborskiiN8.JLakeSci, 2014, 26(3):441-446.DOI: 10.18307/2014.0315.[于婷, 戴景俊, 雷腊梅等. 温度、光照强度及硝酸盐对拟柱孢藻(CylindrospermopsisraciborskiiN8)生长的影响. 湖泊科学,2014, 26(3):441-446.]
[27] Komárková J, Komárek O, Hejzlar J. Evaluation of the long term monitoring of phytoplankton assemblages in a canyon-shape reservoir using multivariate statistical methods.Hydrobiologia, 2003, 504(1/2/3): 143-157. DOI: 10.1023/B:HYDR.0000008514.45771.aa.
[28] Bormans M, Ford PW, Fabbro L. Spatial and temporal variability in cyanobacteria populations controlled by physical processes.PlanktonRes, 2005, 27: 61-70. DOI: 10.1093/plankt/fbh150.
[29] Reynolds CS,Huszar V, Kruk Cetal. Towards a functional classification of the freshwater phytoplankton.JournalofPlanktonResearch, 2002, 24(5): 417-428. DOI: 10.1093/plankt/24.5.417.
[30] Dai Jingjun, Peng Liang, Yu Tingetal. The effects of phosphorus and nitrogen on the growth ofCylindrospermopsisraciborskiiN8 isolated from the Zhenhai Reservoir.ActaHydrobiologicaSinica, 2015, 39(3): 533-539. DOI: 10.7541/2015.70.[戴景俊, 彭亮, 于婷等. 镇海水库拟柱孢藻的分离鉴定和氮磷对其生长的影响. 水生生物学报, 2015, 39(3): 533-539.]
[31] Bai F, Liu R, Yang Yetal. Dissolved organic phosphorus use by the invasive freshwater diazotroph cyanobacterium,Cylindrospermopsisraciborskii.HarmfulAlgae, 2014, 39: 112-120. DOI: 10.1016/j.hal.2014.06.015.
Seasonal dynamic and driving factors of Cylindrospermopsis raciborskii in Zhenhai Reservoir, Guangdong Province
ZHAO Li1, LEI Lamei1,2**, PENG Liang1,2& HAN Boping1,2
(1:InstituteofHydrobiology,JinanUniversity,Guangzhou510632,P.R.China)(2:GuangdongCenterforControlandPreventionofReservoirCyanobacterialBlooms,Guangzhou510632,P.R.China)
Cylindrospermopsisraciborskii, thought to be an originally tropical species, is found to become dominant and even formed blooms in many reservoirs of Guangdong Province. The dominance of this species presents a new threat to the region but little is known about its bloom forming mechanism. With the above reasons, the study aims to determine the factors that may lead to the successful dominance and bloom formation ofC.raciborskii. TheC.raciborskiifilamental length, phytoplankton species composition, and different environmental physico-chemical parameters were examined monthly during the period of November 2014 and October 2015 in Zhenhai Reservoir. Our results showed thatC.raciborskiidominated absolutely in the reservoir during the sampling period. Its biomass ranged from 5.9 to 15.5 mg/L, with a mean value of 11.3 mg/L, accounting for nearly 93.5% of the total phytoplankton biomass.C.raciborskiibiomass was relatively high from February to June in 2015. The highest levels were recorded in June and the lowest in both October and November. Filamental length ofC.raciborskiiexhibited an obvious seasonal regularity. Correlation analysis shows a highly significant negative correlation between filamental length ofC.raciborskiand water temperature. The biomass ofC.raciborskiiwas significant-positively correlated with total nitrogen and total phosphorus, and significant-negatively correlated with the ratio of nitrogen to phosphorus. Further analysis with continuously multiple factors analysis indicated that total phosphorus concentration was the key factor affectingC.raciborskiibiomass. We speculate that the strong absorption to phosphorus and the high P-storage capacity ofC.raciborskiihave played an important role in the seasonal variation of biomass.
Cylindrospermopsisraciborskii; filamental length; biomass; environmental factors; Zhenhai Reservoir
*广东省科技计划项目(2013B091300015)、广东省水利科技创新项目(2016-29)和国家自然科学基金项目(41403061)联合资助. 2016-02-28收稿; 2016-05-04收修改稿. 赵莉(1990~), 女, 硕士研究生; E-mail:1729251839@qq.com.
*通信作者; E-mail: tleilam@jnu.edu.cn.
J.LakeSci.(湖泊科学), 2017, 29(1): 193-199
DOI 10.18307/2017.0121
©2017 byJournalofLakeSciences

