Key Considerations for the Introduction of a Healthcare Technology Management Program in Remote Locations
J. B. Robson
1.Foundation Director, South Australian Biomedical Engineering (retired), Adelaide, South Australia; 2.Flinders Biomedical Enterprises, Adelaide, South Australia
Key Considerations for the Introduction of a Healthcare Technology Management Program in Remote Locations
J. B. Robson1,2
1.Foundation Director, South Australian Biomedical Engineering (retired), Adelaide, South Australia; 2.Flinders Biomedical Enterprises, Adelaide, South Australia
Establishment of a healthcare technology management program in remote locations requires the same consideration as programs in mainstream healthcare facilities but with an added emphasis on clarity of process, ownership of responsibility, management of resources and communication of roles. The author shares the experiences of clinical engineering personnel from the South Australian Biomedical Engineering Service (SA BME) based in Adelaide who are often required to travel thousands of kilometers by road to provide onsite assistance at over 100 rural and city sites. Effective remote service delivery requires a reliance on site based healthcare workers and local contractors to help manage the clinical engineering issues and risks associated with owning or using healthcare technology with an added role for SA BME staff to manage, foster and regulate this assistance. This paper outlines the policy document published throughout South Australia by the state government’s health department, directing all stakeholders involved in the use and ownership of healthcare technology on their role in the Management of Healthcare Technology. With an emphasis on assuring this technology is safe, effective, available and compliant this policy directive is an example of an essential foundation document that should exist wherever clinical engineering services are applied including sites where no clinical engineering personnel are based. This paper outlines the key elements of this directive, how it was developed and how it is used to achieve clarity of process and ownership of roles. Of essential importance when establishing a healthcare technology management program is its nexus with relevant industry, country and global philosophies. In Australia fundamental technology management principles are collated through Standards Australia the nation's peak non-government Standards organization charged by the Commonwealth Government to meet Australia's need for contemporary, internationally aligned Standards and related services. The particularly relevant Standard is the document titled AS/NZS 3551:2012 “Management programs for medical equipment”. The essential principle of risk management outlined in AS/NZS3551 is described in this paper. It is also important when implementing a healthcare technology management program that an independent audit and/or accreditation program is incorporated and that this program is an integral part of the overall quality program of the healthcare service. This will ensure that the relative importance of a healthcare technology program is considered in competition with the many other priorities of delivering healthcare. This paper outlines a two-step process for quality assurance: a state wide reporting regime and a national accreditation program. Essential to the quality assurance program is the collection and maintenance of relevant information that reports the demographics of the technology fleet and a history of the engineering interventions and quality assurance processes carried out on that fleet. This paper introduces the SA BME approach to record keeping and evidence based decision making. This paper also summarises key process, resource and communication strategies useful when introducing a healthcare technology management program that involves remote locations.
management; safety; standards; accreditation; remote
0 INTRODUCTION
When starting a healthcare technology management program for remote locations, it is valuable to take a moment to consider how the program will fit in with national and international programs and to understand the peculiar forces that isolated sites impose. A program that is built from this prior knowledge is more likely to realize the advantages of a full technology management program over an opportunistic technology repair service and is more likely to gain acceptance from all stakeholders.
It is a key that technology management program is designed in collaboration with other healthcare stakeholders including nursing, clinical, administration and finance and is seen by all as an opportunity rather than a chore or a threat. An opportunity to assure the safety of patients, staff and facilities; an opportunity to assure that the most relevant and effective technology is purchased, is performing to manufacturer specifications and is used effectively; an opportunity to minimize delays in acquiring, installing, using and maintaining the technology; and, an opportunity to show that the technology and its support is compliant with relevant laws and best practices. That is, an opportunity to minimize the risks inherent in the ownership and use of healthcare technology.
The author of this paper has over 40 year experience in clinical engineering. This includes 20 years as a clinical engineering manager at the respected Flinders Medical Centre and Flinders University campus in South Australia and the last 15 years of his career undertaking engagements around Asia and South Australia to establish healthcare technology programs in city, regional and rural settings. The most recent engagement of the author has been with the state government of South Australia where he led the establishment of SA BME as a statewide service developed from the previous 11 separate clinical engineering services including three groups that serviced the clinical, dental and pathology technology across remote rural areas of South Australia as a travelling service. SA BME now supports over 95,000 items of healthcare technology purchased at a value of over $600 million (AUD). The new Royal Adelaide Hospital is due to open in mid-2016 and SA BME is putting the healthcare technology program in place for what is intended to be one of the largest and most technologically advanced hospitals in Australia.
In each case where the author has led the introduction of a health technology management service the emphasis has been on establishing a policy foundation and processes to assure the safety, effectiveness, availability and compliance of the technology on an on-going basis.
This paper is intended to share the author’s experience and knowledge on strategies that have worked well and can be adapted to both mainstream and remote environments.
1 ESTABLISH A SOLID POLICY FOUNDATION
1.1 Stakeholder engagement and policy development
Local engagement: Healthcare technology management programs cannot be established in isolation from key site based stakeholders or imposed on the basis of work undertaken elsewhere. If nothing else, the program needs to be understood and willingly adopted by the local nursing and clinical staff and needs to have the legal, financial and strategic support of at least the on-site hospital administrator and desirably the regional, state or national authorities as well. Of course, it is also beneficial that the program is locally tailored to be considerate of on-site demographics including the model of care, the patient case mix, the nature of the technology fleet, the availability of expertise and the level of resources that can realistically be made available.
From experience, a good way to initiate a healthcare technology management program is to develop a list of key stakeholder groups and to identify an appropriate method of their engagement or representation. It will serve no one well to have large committees of disparate views at the start of the process and for the first round of engagement it would be practical to limit the involvement to those with influential opinions and those who will be most likely to refer to the plan on a regular basis after it has been adopted viz. Nursing Staff, Clinical Staff, Allied Health staff, and Clinical Engineering staff. Even among these groups a practical core group will be easily identified to develop an initial draft policy document. The author proposes that the clinical engineering staff be the leaders of the policy document development and be the eventual authors/custodians of the policy documentation into the future.
It would be beneficial and expedient if clinical engineering staff prepared a proposed table of contents for a written policyprior to meeting with the core stakeholders by interacting with peer groups and viewing what clinical engineers and relevant regulatory bodies elsewhere have produced. The author offers a suggested policy content below (Figures 1&2) as a starting point. It is proposed that this skeleton document be shared with the core stakeholder group for consideration/editing on an iterative face to face basis. It is important to have frequent periodic and personal interaction with the core group to build the necessary relationships as an investment for the time implementation needs to occur. It is desirable to sort out any strong differences in opinion during this first stage because it is probable that the next draft would be suitable for wider circulation with a broader group of stakeholder employees. Subsequent rounds of circulation are likely to attract a more detailed level of scrutiny and it is important that the bigger picture issues have already been determined, agreed and incorporated in the early draft.
Once a practical, realistic and achievable draft policy is established (in the experience of the author this will take a regular effort over a period of approximately 6 months), it is time to engage the highest level of administrative authority relevant to the policy’s existence. In the authors experience, adoption by such an authority has generally proven surprisingly easy to achieve since there is now stakeholder momentum and that a good well written policy will reasonably be seen as a prudent risk management strategy protecting both the authority and the organization during times of litigation. Through this authority it is then recommended that the draft be circulated more widely with groups such as Human Resources, Financial, Legal, Risk Management, Industrial Relations, Health and Safety, and Procurement.
The pen-ultimate draft resulting from the above process should then be circulated from the office of the highest authority to all employees and associates affected by the policy with the manager of the clinical engineering service being the point of contact during a consultation period of at least 3 months. A final draft would then be put forward for formal adoption and circulation.
National and international alignment: During development of the initial draft policy the clinical engineering service should seek to include and align the wording with national or international guidelines or legislation if it exists. Whereas these guidelines vary country by country the Australian model can serve as a good example. Like most countries, Australia promotes a generic framework dependent on expert interpretation by trained personnel using an evidence based risk management approach that can be adapted depending on the local environment.
AS/NZS 3551: “Management Programs for medical equipment” is the relevant national Standard in Australia and is characterized by the following:
(1) It is a guideline only and would need to be referenced in contracts or legislation for it to become a legal requirement.
(2) Provides a definition for medical technology.
(3) Puts an onus on the organization to ensure that the safety and performance of the medical technology is maintained by an effective management program.
(4) Proposes the nomination of a staff member to be designated with authority for the technology management program. Requires this delegate to determine technology warranty, training and maintenance regimes.
(5) Requires the users of medical technology to know how to safely and effectively use the technology and to assure the device is only used for the purpose intended by the manufacturer.
(6) Gives examples of protocols, procedures and strategies for managing medical technology from procurement to disposal including rigorous acceptance testing programs prior to first use.
(7) Outlines a risk management approach that can be used to guide the medical equipment management program.
(8) Defines periodic testing protocols and appropriate test intervals and test limits.
(9) Defaults to the manufacturers’ instructions and specifications in the absence of substantial contradictory evidence.
(10) Identifies the importance of record keeping as a basis for evidence based decision making.
(11) Stresses the importance of regular communication between all stakeholders.
(12) Outlines the alignment with international Standards.
Policy content: The written policy formally adopted by the healthcare organization aims to provide stakeholders with both a broad understanding of the policy intent and with detail that can be used to resolve issues of uncertainty.
To achieve the first objective, it is recommended that an easily read policy statement be the first and most prominent component of the document.
As an example, the policy statement and content adopted by the South Australian government is shown in Figures 1&2.

Figure 1 Example of a policy statement.
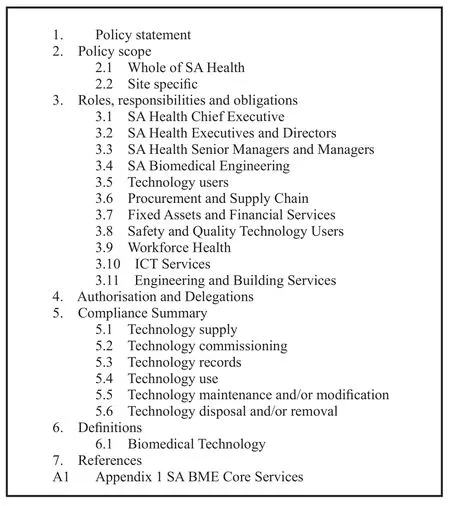
Figure 2 Example of policy content.
1.2 On-going policy relevance and practical adherence
Establish professional relationships: During the development and adoption of a healthcare technology management program the clinical engineering provider will have established or renewed professional and personal contact with the major stakeholders of the policy. The author recommends that this contact be actively reinforced and further formalized. One example successfully deployed by the author is to keep a register of device managers and associated clinical engineering staff members responsible for ongoing clinical engineering issues for each geographical location or client group in the health service. The device managers contribute local clinical and operational knowledge essential in the development of a risk based approach to technology management, especially when resources are limited[1]. Device managers are provided with a regular report on the healthcare technology in their area and with the related procedures and protocols put in place to successfully deliver a technology management program. This has the benefit of establishing a single point of contact between the client group and the clinical engineering provider and thereby lessens the chance of human error due to mixed communications. Additionally, periodic reports on adherence to the policy should be made available as required to the device managers and to the relevant managers within the organizational and statewide hierarchy.
Collect and maintain a database of evidence: An essential element of any technology management program is the creation and maintenance of a database of information on the technology supported. Such a record is useful for both planning and scheduling the clinical engineering interventions and for providing evidence on compliance with the healthcare technology policy of the organization. The author recommends that the establishment of a database is given careful consideration and is not undertaken without peer group collaboration. A database structure that is consistent across sites and uses industry wide definitions for the data collected is significantly more use for benchmarking activities and compliance verification than a system developed and deployed in isolation. SA BME benefits enormously from a single database of information across more than one hundred sites that records a South Australia health system wide risk rating score against each of over 95,000 devices. The author strongly recommends a similar singular record approach as part of any healthcare technology program.
Audit and accreditation: A technology management system that relies on information recorded in a database for the purposes of regulatory compliance will only be defendable if there is a process of independent audit which is preferably part of a health system wide quality process. In Australia, the Commission on Safety and Quality in Health Care is a government agency that publishes a set of National Safety and Quality Healthcare Standards (NSQHS) as a nationally consistent statement about the level of care consumers can expect from health service organizations. All Australian hospitals are required to be accredited against NSQHS Standards on a periodic basis. It is therefore, important thatany healthcare technology management program in Australia is designed to be consistent with these Standards and, in particular, mandates the collection of evidence that is useful in obtaining accreditation during the periodic audit inspections.
Evidence based decision making: It is common within the clinical engineering discipline that there will be insufficient resources to allow all desired engineering demands to be fulfilled in a timely fashion. Therefore, clinical engineering resources need to be expended on the most important or highest risk requests at any point in time. Generally, the decision making process for prioritizing engineering interventions is made by experienced senior personnel in consultation with operational/clinical experts taking into account many parameters that may vary in relative importance each time there is a new request. In the author’s opinion, the priority of unplanned interventions will always need to be made by clinical engineers, but the scheduling of routine planned maintenance can be automated by recording a risk rating against each asset in the database and using this information as a basis for deciding which interventions are given priority within the resources limits. The latest risk ranking deployed by the author is provided in Table 1 as an example:

Table 1 Risk rating recorded in each device record
2 CONSIDERATIONS FOR REMOTE LOCATIONS
2.1 Variations to policy
Part of the skill of writing an appropriately worded policy is that there should be no variation to the overall intent of the healthcare technology management policy in remote locations when compared with mainstream sites.
What may vary is the level of individual responsibility of hospital staff charged with assuring compliance with the policy and this is due to the reduced patient load and case mix rather than being due to the remoteness. For example, a single delegate identified in the policy may have multiple levels of authority under the policy and be responsible for a broader range of technologies. 2.2 Variations to technology back-up strategies
At a remote location there is increased risk to the patient caused by the unavailability of an item of healthcare technology. At a mainstream site the patient may be able to be moved to an alternate site, use alternate technology or the clinical engineering provider may be able to provide a fast turn-around time on device repairs – not always possible at a remote site. Any item of technology that is critical to patient safety should have an associated failure plan and where practically possible redundant devices should be purchased and kept on site as a back-up.
2.3 Variations to clinical engineering operations
By agreement the clinical engineering provider at remote locations may also outsource some of their responsibilities under the policy to other healthcare professionals or to local contractors compared to what may typically be undertaken by in-house clinical engineering staff based at mainstream sites (for example, the management of medical gases or the maintenance of hospital beds). However, in the author’s opinion, the accountability has not been outsourced and the clinical engineering provider must retain oversight and put in place a quality management process for any outsourced responsibilities defined in the policy documents.
A good strategy at remote sites is for the clinical engineering provider to put a greater emphasis on preventive maintenance through a schedule of periodic planned visits. A close working relationship with the original equipment manufacturers should be maintained and a knowledge of likely technology failures understood. In South Australia, all remote sites are part of a scheduled roster of maintenance visits at which time engineering parts that are known to have a high failure rate are replaced preemptively. This regime requires a disciplined approach to planning the supply of parts in advance and suitably equipped vehicles that can be used as mobile workshops. During these visits, all healthcare technology at the site is inspected and where appropriate maintained to assure performance and safety.
It is also prudent at each remote site to establish priority courier arrangements that can be deployed without a lengthy or difficult process. Often, in urgent situations, it is not feasible to send a technical resource to a remote location to undertake a fault diagnosis and repair. In these situations, the delivery of the device to a mainstream center is the best alternative and this should be seen as straightforward and easy to arrange by local staff.
2.4 Communication and clarity of process
The relationship between the clinical engineering provider and the remote site hospital staff is even more important than at a mainstream site. A single protocol for raising service requests needs to be understood by all and a web based help desk that can automatically show the status of work in progress and the schedule of upcoming visits is highly recommended.
Two problems that are easily solved through goodcommunication and strong relationships are: the over stating of a breakdown priority by clinical staff, and, simple technology faults that go unreported.
3 CONCLUSION
The establishment of a technology management plan for healthcare technology should follow the same process as that developed for mainstream sites. The plan needs to have a solid policy foundation that formally assigns prescribed levels of responsibility and accountability to healthcare personnel and to the clinical engineering provider. Remote sites benefit from an emphasis on clarity of process, ownership of responsibility, management of resources and communication of roles.
REFERENCE
1 Robson J,Yeo P,Riches M,et al.Risk management and biomedical devices.Conf Proc IEEE Eng Med Biol Soc,2005,1:166-169.
更正声明
本刊2016年1月刊第71-73页《颈动脉超声检查及超敏C反应蛋白检测对2型糖尿病患者急性心梗的诊断价值》一文,通讯作者为李杰,山东大学齐鲁医院超声科主任医师
邮箱:jieli301@163.com
R197.39 [Document code] A
10.3969/j.issn.1674-1633.2016.02.001 [Article ID] 1674-1633(2016)02-0001-05
Dr. John Robson, Flinders Biomedical Enterprises, Room 3E124, Flinders Medical Centre, Flinders Drive, Bedford Park, SA 5042, Australia.
E-mail:johnrobson@fbe.com.au
本刊2016年1月刊第76页,表2的表题应为“多层螺旋CT对主动脉夹层动脉瘤的病情评价结果 n(%)”,特此更正。
本刊2016年1月刊第127页第2大标题“呼吸机的质量检测”下第2行应为“应用美国FLUKE呼吸机检测仪”,特此更正。

