酰胺型配体铜、锌、银配合物的合成、结构及荧光性质
林龙 李先宏 张波 张战营 吴伟娜 王元
(1河南理工大学材料科学与工程学院,焦作454000)
(2河南理工大学化学化工学院,焦作454000)
酰胺型配体铜、锌、银配合物的合成、结构及荧光性质
林龙*,1李先宏1张波1张战营1吴伟娜*,2王元2
(1河南理工大学材料科学与工程学院,焦作454000)
(2河南理工大学化学化工学院,焦作454000)
合成并通过单晶衍射表征了5个配合物[CuLCl2]·CH3COCH3(1)、[ZnLCl2]·CH3COCH3(2)、[ZnL(NO3)2]·0.5CH3COCH3(3)、[AgL2]ClO4(4)和[AgL2]BF4(5)(L=2-(5-氯-8-喹啉氧基)-1-(吡咯烷-1-基)乙酮)。配合物1和2同构,五配位的中心金属离子采取扭曲的四方锥配位构型,与来自配体L的2个氧原子和1个氮原子及2个氯离子配位。而在配合物3中,锌离子与1个三齿配位的配体L,1个单齿配位的硝酸根和1个双齿配位的硝酸根配位,配位构型为扭曲的八面体。配合物4和5中,中心金属与配体的比例为1∶2。银离子与2个三齿配位的配体L配位,采取扭曲的八面体配位构型。乙腈溶液中,配合物1、2、4和5在410 nm处的最大荧光发射峰与配体L相似。而配合物3由于配体到锌离子之间的能量转移,最大荧光发射峰红移至430 nm。
铜配合物;锌配合物;银配合物;酰胺型配体;荧光
As one of the promising systems of amide open chain ligands,quinolinyloxy acetamides have been proved to be a well suited type of antenna for lanthanide(Ⅱ)ions.In particular,their Sm(Ⅱ)and Eu(Ⅲ)complexes could exhibit the characteristic emission of the Eu(Ⅲ)and Sm(Ⅱ)ions,respectively[1].On the other hand,such ligands have been used for the recognition ofimportant transition metalions,such as Zn(Ⅱ),Cd(Ⅱ)or Hg(Ⅱ),due to the metal-induced fluorescence emission enhancement[2-3].Our previous work also shows that this kind of ligands could coordinate to Cu(Ⅱ)/ Zn(Ⅱ)ions to form stable complexes[4-5].However,their Ag(Ⅰ)complexes have been paid much less attention[4-5]. Thus,in this paper,five Cu(Ⅱ)/Zn(Ⅱ)/Ag(Ⅰ)complexes containing an amide type ligand L,namely,2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethanone,were synthesized and characterized by X-ray diffraction.In addition,the fluorescence properties of all complexes are investigated in detail.
1 Experimental
1.1 Materials and measurements
Solvents and starting materials for synthesis were purchased commercially and used as received.The ligand L was prepared by the reported method[5]. Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.The UV spectra were recorded on a Purkinje General TU-1800 spectrophotometer.Fluorescence spectra were determined on a Varian CARY Eclipse spectrophotometer,in the measurements of emission and excitation spectra the pass width is 5 nm.
1.2 Preparations of the complexes
The ligand L(0.1 mmol)and CuCl2(0.1 mmol) were dissolved in an acetone solution(5 mL).After stirring for about 1 h,the mixture was filtered and set aside to crystallize at room temperature.The crystals suitable for single crystal X-ray analyses are obtained after few days.The syntheses of 2~5 are similar to that of 1,while using ZnCl2,Zn(NO3)2,AgClO4and AgBF4instead of CuCl2,respectively.
1:yellow blocks.Yield:59%(based on L).Anal. Calcd.for C18H21N2O3Cl3Cu(%):C,44.72;H,4.38;N, 5.80.Found(%):C,44.62;H,4.51;N,5.78.IR(KBr, cm-1):ν(C=O)acetone1 710,ν(C=O)1 623,ν(C=N)1585, ν(Ar-O-C)1 250.
2:colorless blocks.Yield:68%(based on L). Anal.Calcd.for C18H21N2O3Cl3Zn(%):C,44.56;H, 4.36;N,5.77.Found(%):C,44.67;H,4.49;N,5.62. IR(KBr,cm-1):ν(C=O)acetone1 713,ν(C=O)1 626,ν(C= N)1586,ν(Ar-O-C)1 241.
3:colorless rods.Yield 72%(based on L).Anal. Calcd.for C16.5H18N4O8.5ClZn(%):C,38.92;H,3.56;N, 11.00.Found(%):C,39.11;H,3.80;N,10.76.IR (KBr,cm-1):ν(C=O)acetone1 711,ν(C=O)1 633,ν(C=N) 1 588,ν(Ar-O-C)1 245,ν1(NO3)1 484,ν(NO3)1 384 and 1 291.
4:colorless blocks.Yield 79%(based on L). Anal.Calcd.for C30H30N4O8Cl3Ag(%):C,45.68;H, 3.83;N,7.10.Found(%):C,45.78;H,3.92;N,7.02. IR(KBr,cm-1):ν(C=O)1 642,ν(C=N)1 586,ν(Ar-OC)1 239.
5:colorless plates.Yield 76%(based on L). Anal.Calcd.for C30H30N4O4Cl2BF4Ag(%):C,46.42;H, 3.90;N,7.22.Found(%):C,46.57;H,3.79;N,7.42. IR(KBr,cm-1):ν(C=O)1 636,ν(C=N)1 586,ν(Ar-OC)1 238.
1.3 X-ray crystallography
The X-ray diffraction measurements for complexes 1~5 were performed on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kαradiation(λ=0.071 073 nm) by usingφ-ωscan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[6].The structures were solved by direct methods and refined by fullmatrixleast-square on F2using the SHELXTL-97 program[7].All nonhydrogen atoms were refined anisotropically.All the H atoms were positioned geometrically and refined using a riding model.SQUEEZE procedure was applied to deal with the lattice acetone molecules of complexes 2 and 3.Details of the crystal parameters, data collection and refinements for complexes 1~5 are summarized in Table 1.
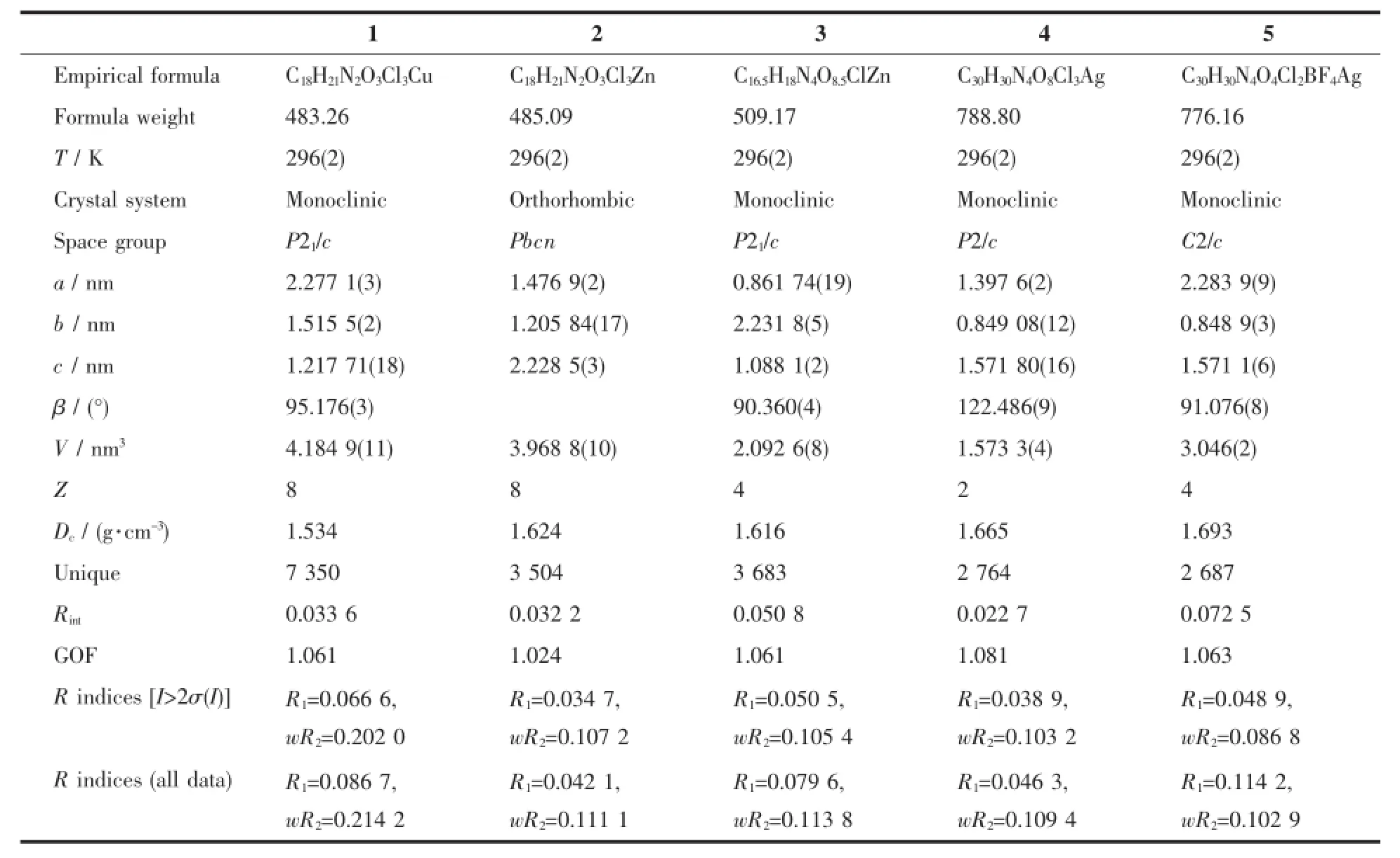
Table 1 Selected crystallographic data for complexes 1~5
CCDC:1484068,1;1484069,2;1484070,3; 1484071,4;1484072,5.
2 Results and discussion
2.1 Crystalstructures of the complexes
Complexes 1 and 2 are isostructural,while crystallize in monoclinic and orthorhombic,space group P21/c and Pbcn,respectively.As shown in Fig. 1a and 1b,in each complex,the metal ion is fivecoordinated by one amide ligand with NO2donor set and two chloride anions.It is noted that there are two independent complex molecules in the asymmetric unitof1.According to the Addison rule[8],the geometric indexτis 0.178 or 0.235 and 0.303 in complexes 1 and 2,respectively,indicating that the coordination geometry ofmetalion is a distorted tetragonalpyramid. By contrast,the Zn(Ⅱ)ion in complex 3(Fig.1c)is surrounded by one tridentate ligand L,one monodentate and one bidentate nitrate anions,giving a distorted octahedral coordination geometry.In addition,the second O atom,O7,of the monodentate NO3-group, forms a weak bond with the Zn(Ⅱ)ion(Zn1-O7 0.286 5 nm),thus the coordination octahedral is largely deviated from the ideal one.Except with different lattice solvent molecules,the structures of complexes 1~3 are similar as those derived from the same ligand L and metal salts in acetonitrile solution[5].
Complexes 4 and 5 are isostructuraland the ratio of metal ion and ligand is 1∶2,while with perchlorate and tetrafluoroborate as counter ions,respectively.As shown in Fig.1d and 1e,the asymmetric unit of each complex contains a halfofthe molecule with the Ag(Ⅰ)ion situated on the two-fold rotational axis.The Ag(Ⅰ)ion is coordinated by two amide ligands with NO2donor set,and possesses a distorted octahedral coordination geometry.The Ag-N/O bond lengths in complexes 4 are similar as those found in complex 5, and comparable to the Ag(Ⅰ)complexes with same donor sets[9].As expected,there are no classical hydrogen bonds in the crystals of 1~5.
2.2 IR spectra
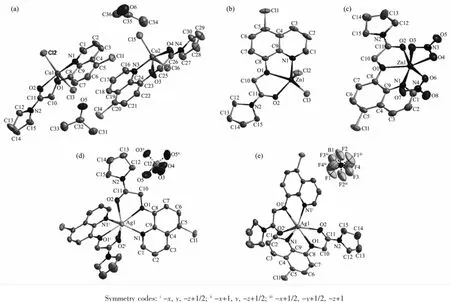
Fig.1 Molecular structures of complexes 1~5(a~e)shown with 30%probability displacement ellipsoids
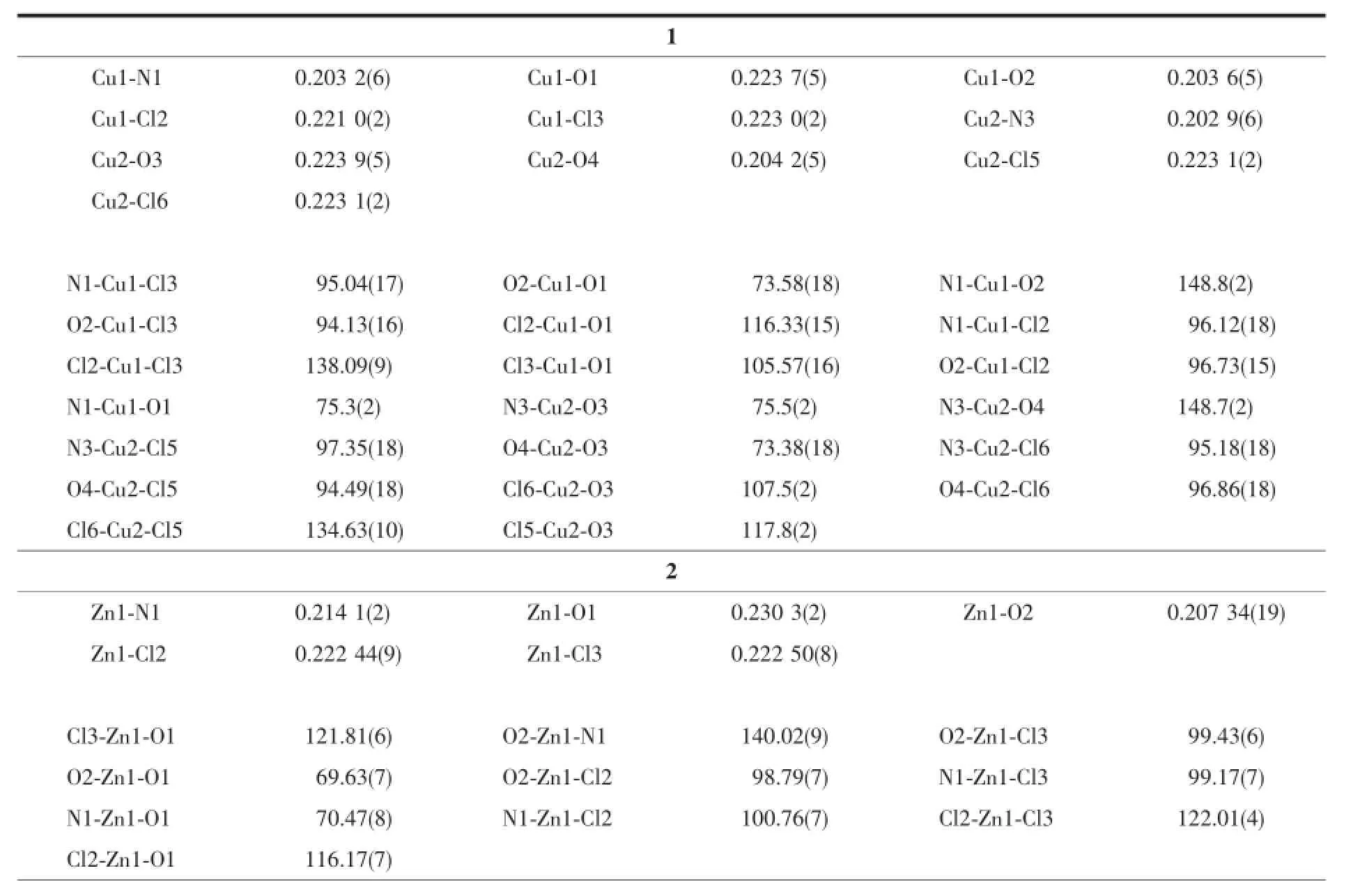
Table 2 Selected bond lengths(nm)and angles(°)in complexes 1~5
The IR spectra of free ligand L show three band at1 682,1 598 and 1 254 cm-1,attributable toν(C=O), ν(C=N)andν(Ar-O-C),respectively[5].They shifts to lower wavenumber in the complexes 1~5,indicating that carbonyl oxygen,ethereal oxygen and quinolinenitrogen atoms take part in coordination[9].In addition, the intense absorption bands in the spectra ofcomplex 3 associated with the asymmetric stretching appear at 1 384 or 1 291 cm-1(ν4)and 1 484 cm-1(ν1),clearly establishing the existence ofmonodentate and bidentate NO3-ligands,respectively[8].Theν(C=O)bands in complexes 1~3 ataround 1 710 cm-1are corresponding to the crystal acetone molecules.It is in accordance with the result of the crystal structure study.

Continued Table 2
2.3 UV spectra
The UV spectra complexes 1~5 in acetonitrile solution(concentration:1×10-5mol·L-1)were measured at room temperature(Fig.2).The UV spectra of complexes 1~5 are quite similar as that of the ligand L with two bands at244 nm(ε=156 000 L·mol-1·cm-1) and 316 nm(ε=19 500 L·mol-1·cm-1)][5],each ofthem features two main bands located at244 nm(ε=212 886, 149 054,99 927,73 871 and 50 073 L·mol-1·cm-1forcomplexes 1~5,respectively)and 318 nm(ε=40 755, 29 625,11 770,9 390 and 6 450 L·mol-1·cm-1for complexes 1~5,respectively).Such two bands should be assigned to characteristicπ-π*transitions centered on quinoline ring and the acetamide unit of the amide ligand L,respectively[5,8].
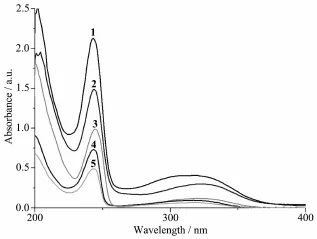
Fig.2 UV spectra of 1~5 in acetonitrile solution at room temperature
2.4 Fluorescence spectra
The fluorescence spectra of complexes 1~5 have also been studied in acetonitrile solution(concentration: 1×10-5mol·L-1)atroom temperature.The results show that the emission spectra of complexes 1,2,4 and 5exhibit only one main peak at 410 nm when excited at 320 nm,which is similar as that of the ligand L[5]. However,the emission band of complex 3 red-shifts to 430 nm under same tested condition,indicating the energy transferring from the ligand L to the Zn(Ⅱ)ion[8]. It should be noted that complexes 2 and 3 exhibit quite different fluorescence emission even they have same centre Zn(Ⅱ)ion,primarily related with the anion effect(chloride for 2,while nitrate for 3)[10].
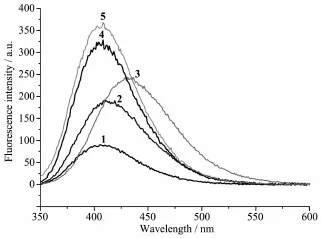
Fig.3 Fluorescence emission spectra of 1~5 in acetonitrile solution at room temperature
[1]MAO Pan-Dong(毛盼东),XU Jun(徐君),WU Wei-Na(吴伟娜),etal.Chinese J.Inorg.Chem.(无机化学学报),2016,32: 677-682
[2]Song K C,Kim J S,Park S M,et al.Org.Lett.,2006,8:3413 -3416
[3]Zhou X,Li P,Shi Z,et al.Inorg.Chem.,2012,51:9226-9231
[4]CAI Hong-Xin(蔡红新),WU Wei-Na(吴伟娜),WANG Yuan (王元).Chinese J.Inorg.Chem.(无机化学学报),2013,29: 845-849
[5]MAO Pan-Dong(毛盼东),YAN Ling-Ling(闫玲玲),WU Wei -Na(吴伟娜),etal.Chinese J.Inorg.Chem.(无机化学学报), 2016,32:1476-1480
[6]Sheldrick G M.SADABS,University of Göttingen,Germany, 1996.
[7]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of Göttingen, Germany,1997.
[8]LI Xiao-Jing(李晓静),WU Wei-Na(吴伟娜),XU Zhou-Qing (徐周庆),et al.Chinese J.Inorg.Chem.(无机化学学报), 2015,31:2256-2271
[9]Wang J,Qi Q,Cheng L,et al.Inorg.Chem.Commun.,2015, 58:5-8
[10]Tavman A,Çinarli A.Inorg.Chim.Acta,2014,421:481-488
Syntheses,Crystal Structures and Fluorescence Properties of Cu(Ⅱ)/Zn(Ⅱ)/Ag(Ⅰ)Complexes with an Amide Type Ligand
LIN Long*,1LI Xian-Hong1ZHANG Bo1ZHANG Zhan-Ying1WU Wei-Na*,2WANG Yuan2
(1School of Materials Science and Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
(2College of Chemistry and Chemical Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Five complexes,[CuLCl2]·CH3COCH3(1),[ZnLCl2]·CH3COCH3(2),[ZnL(NO3)2]·0.5CH3COCH3(3), [AgL2]ClO4(4)and[AgL2]BF4(5)(L=2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethanone),were synthesized and characterized by X-ray diffraction.Complexes 1 and 2 are isostructural,and in each of them the fivecoordinated metal ion is in a distorted tetragonal pyramid with a NO2donor set from one ligand L and two chloride anions.However,the Zn(Ⅱ)ion in complex 3 is coordinated with one tridentate ligand L,one monodentate and one bidentate nitrate anions,giving a distorted octahedral coordination geometry.The structures of 1~3 are quite similar as those of the acetonitrile solvates derived from the same ligand L and metal salts.By contrast,the ratio ofthe metalion and ligand L is 1∶2 in complexes 4 and 5,the central Ag(Ⅰ)ion in each complex is six-coordinated with two independent ligands with N2O donor set,thus possesses a distorted octahedral coordination geometry.In CH3CN solution,the emission spectra ofcomplexes 1,3,4 and 5 exhibit similar peak at 410 nm as the ligand L.However,the emission band ofcomplex 3 red-shifts to 430 nm because ofenergy transferring from the ligand L to the Zn(Ⅱ)ion.CCDC:1484068,1;1484069,2;1484070,3;1484071,4;1484072,5.
Cu(Ⅱ)complex;Zn(Ⅱ)complex;Ag(Ⅰ)complex;amide type ligand;fluorescence
O614.121;O614.24+1;O614.122
A
1001-4861(2016)09-1653-06
10.11862/CJIC.2016.204
2016-06-11。收修改稿日期:2016-08-01。
国家自然科学基金(No.21001040)、河南省科技厅基础与前沿项目(No.162300410011)和河南省教育厅自然科学基金(No.12B150011,14B150029)资助。
*通信联系人。E-mail:linlong@hpu.edu.cn,wuwn08@hpu.edu.cn;会员登记号:S06N6704M1112。

