Analysis of fluoxetine-induced plasticity mechanisms as a strategy for understanding plasticity related neural disorders
PERSPECTIVE
Analysis of fluoxetine-induced plasticity mechanisms as a strategy for understanding plasticity related neural disorders
Fluoxetine hydrochloride, better known for its commercial name Prozac, is one of the most widely prescribed antidepressant drugs all over the world. This drug was considered a “breakthrough drug” for the treatment of depression because of its very high selectivity as a serotonin reuptake inhibitor and because it presented a lower side-effect profile than previous drugs (Wong et al., 2005). However, the precise mechanisms of fluoxetine action and how it alleviates mood disorders is still largely unknown.
Similarly to its predecessors, the core mechanism of fluoxetine action was considered to be the promotion of monoamine neurotransmitter (serotonin and noradrenalin) function in the brain. This observation constituted the initial basis for the “monoamine hypothesis” for depression, later extended to the “chemical hypothesis”, which proposes that mood disorders are caused by functional and structural changes in specific molecules, and that antidepressant drugs act on counteracting these molecular changes. As information in the nervous system is processed and stored through modulation of chemicals in specific neuronal networks, a more recent and alternative hypothesis, complementary to the chemical one, raised: the “network hypothesis”. This proposes that antidepressants, by modulating several signaling molecules, determine changes at functional and structural level in affected neuronal networks, allowing a better adaptation to environmental conditions. In this framework, antidepressants modulate activity-dependent plasticity processes of specific neuronal networks finally inducing improvements in several mood disorders (Berton and Nestler, 2006; Castrén, 2013).
One of the first demonstration that fluoxetine is able to modulate plasticity processes in the central nervous system was obtained by exploiting the paradigmatic experimental model of plasticity in the mammalian visual cortex (Maya Vetencourt et al., 2008). In this brain area, as in other sensory ones, experience-dependent plasticity is particularly elevated during specific developmental time windows, so-called critical periods, but dramatically declines with age. A brief occlusion of visual experience in one eye (by monocular deprivation, MD) induces a plastic reorganization at functional and structural level in the visual cortex only in young animals, while no major changes are observed when performed in adults. Moreover, MD induces loss of visual functions (amblyopia) which cannot be recovered once at an adult stage because of the decline of plasticity. However, long-term pharmacological treatment of adult rats with fluoxetine hydrochloride (administered for one month in the drinking water) is able to reactivate high levels of plasticity (measured as sensitivity to MD). Furthermore, the pharmacological treatment in combination with the appropriate rehabilitation procedure (patching the open eye) leads to full recovery of visual functions in adult animals rendered amblyopic by MD performed during the critical period.
The efficacy of fluoxetine in potentiating plasticity levels also in neuronal networks related to mood disorders was demonstrated in a study using the fear-conditioning and extinction training paradigm (Karpova et al., 2011). Here, pairing a neutral tone with a mild foot shock, conditions mice to be fearful of that tone and to respond with a freezing behavior when the simple tone is played. Repeated exposure to the tone alone gradually extinguishes the freezing response, and, as in the visual system experimental model, the efficacy of extinction is elevated during a developmentally regulated critical period of plasticity, but declines with time. Again, when combined with the extinction training, the fluoxetine-induced reactivation of juvenile-like plasticity in the affected network leads to stronger and long-lasting beneficial effects at behavioral level.
In conclusion, both studies clearly indicate that the antidepressant fluoxetine can restore in adult animals the plastic potential characteristic of early stages of postnatal life. In turn, this increased plasticity facilitates the recovery at functional and structural level of the specific neuronal networks which were eventually miswired during development, and thus their ability to process and store information in response to environmental stimulation in an appropriate way.
Besides mood disorders, fluoxetine and other antidepressants have been used in restoring, at least partially, functions in humans and/or animal models of stroke, Alzheimer’s disease, traumatic brain injury, Down syndrome, and cervical spinal cord lesion (Castrén and Hen, 2013). To summarize, it is evident that all pathological conditions where reactivation of plasticity may be desired to recover functions at adult stages, may potentially benefit from the application of strategies based on fluoxetine or other antidepressant treatments (LeBlanc and Fagiolini, 2011) (Figure 1).
The cellular and molecular mechanisms underlying the potentiation of plasticity induced by fluoxetine are still poorly understood. Experimental evidence obtained mainly in the plasticity model of the mammalian visual cortex contributed to dissect the primary basic processes of fluoxetine action (Bavelier et al., 2010). The actual view is that visual cortex plasticity is regulated by both functional and structural mechanisms responsible for the opening, duration and closure of the critical period, and also for limiting plasticity levels at later stages of postnatal life. The better characterized functional process involved in the control of plasticity is the maturation of the cortical inhibitory tone, and hence the balance within local circuits between this and the excitatory one (excitatory/inhibitory, E/I balance). The E/I balance is a key player in defining specific developmental thresholds for the opening and closure of the critical period, and for restricting the plastic potential in adulthood. At structural level, the amounts of peri-neuronal nets in the extracellular matrix and the degree of myelination process play prominent roles in the regulation of plasticity by controlling neurite outgrowth and neuronal reorganization. It has been recently suggested that these same factors may act in the adults as functional and structural brakes limiting the expression of plasticity. Thus, an excellent strategy to restore high levels of plasticity in the adults, and to allow recovery of functions, would be to modulate and/or eventually lift these brakes. Indeed, different genetics, pharmacological, and environmental approaches have been successfully used to remove these brakes leading to recovery of juvenile-like levels of plasticity in the cortex, and also enabling improvement of visual functions in adult rodents.
Regarding fluoxetine, it has been shown that this pharmacological treatment restores plasticity in the adults by reducing intra-cortical inhibition (extracellular basal levels of GABA), thus modulating the E/I balance, and increasing the expression of brain-derived neurotrophic factor, a neurotrophin participating in several neuronal processes as synaptic development, maturation, and plasticity. These effects are specifically dependent on the 5-HT1A serotonin receptor, the BDNF receptor TrkB, and the MAPK-Erk1/2 signaling pathway. Moreover, a modulation at epigenetic level occurs, as an increase of the acetylation status of the H3 histone at BDNF promoter regions, and a decrease in the expression of the Hdac5 deacetylase gene. More recently, evidence for the activity-dependent transcription factor NPAS4 (Neuronal PAS Domain Protein 4)as a key mediator of visual system plasticity in the adult rat has been reported. Fluoxetine treatment increases NPAS4 expression at both mRNA and protein level with a parallel reduction of the H3 histone methylation status at the NPAS4 promoter. Moreover, the experimental down-regulation of NPAS4 at the cortical level prevents the fluoxetine-induced plastic outcome. As for the known effects at the structural level, it has been shown that fluoxetine treatment can also induce morphological remodeling in the adult mouse visual cortex by increasing the dynamics of interneuron branch tips (Maya-Vetencourt and Pizzorusso, 2013).
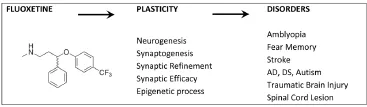
Figure 1 Mechanism of action of fluoxetine on plasticity and experimental models of pathologies.
Many advances in our understanding of the fundamental mechanisms controlling plasticity levels have been initially obtained by means of small-scale experimental approaches. In the last two decades, largescale approaches comparing mRNA expression levels between distinct experimental conditions by using innovative genomic approaches or subtractive hybridization have been carried out. Also proteomic approaches, which allow identification of the ultimate effector molecules in the biology of cells, have been applied to complement and add new information to previous data. However, despite the recent straightforward advances in the technologies applied to functional proteomics, these methods are still underexploited in the field of experience-dependent plasticity in the visual cortex. Notable contributions have been made by L. Arckens’ group in the developing cat visual cortex and in the mouse following monocular enucleation (by fluorescent two-dimensional difference gel electrophoresis, DIGE, also following reversed phase chromatography, RP-HPLC), and by C.N. Levelt’s group on mouse visual cortex synaptosomes during development and following manipulation of the visual input (by isobaric tag for relative and absolute quantitation, iTRAQ). These groups have contributed to the identification of candidate genes in the control of cortical plasticity, as for instance the collapsin response mediator (CRMPs) family of proteins which, modulating neurite growth and guidance, may instruct neuronal cells to form meaningful networks (Van den Bergh et al., 2006); and also to better define general considerations on the role of experience on cortical development, as that dark rearing does not simply delay development but activates specific signaling cascades related to the control of plasticity (Dahlhaus et al., 2011).
Nevertheless, only a few works, mainly in the hippocampal area, have used proteomic approaches to study in vivo the effects of fluoxetine on protein expression. This motivated us to develop in our laboratory a study using two-dimensional 2D gel differential analysis followed by mass spectrometry with the aim of identifying possible protein candidates mediating the outcome of fluoxetine treatment on adult plasticity (Ruiz-Perera et al., 2015). The study was performed in the mouse visual cortex which has recently become a predominant model for investigating the principles that underlie experience-dependent plasticity, as the structural and functional organization of visual networks is generally conserved and very similar across mammals, and in this species powerful tools and recently developed techniques, as transgenesis or optogenetics, can be applied.
Here, we first reported that the antidepressant fluoxetine is able to restore high levels of functional plasticity in the adult visual cortex also of mice, similarly to what previously observed in rats. The proteomic analysis allowed the identification of 24 differentially expressed proteins in fluoxetine-treated animals vs. controls. Mass spectrometry identification followed by bioinformatics analysis revealed that the identified proteins are involved in the control of cytoskeleton organization, endocytosis, molecular transport, intracellular signaling, redox cellular state, metabolism, and protein degradation. Altogether, our results indicate a complex effect of fluoxetine on neuronal signaling mechanisms potentially involved in restoring plasticity in the adult brain. Part of the proteins and signaling molecules identified in our study were previously characterized as potential key players in the control of neuronal plasticity processes in other areas, indicating that our data are in line with previous works. While it is fundamental to perform further studies to confirm the relevance of each of the identified proteins as candidates in the regulation of plasticity in the visual cortex, at least one group of proteins emerges as particularly relevant, the group associated to the control of cytoskeleton organization. In this study, we found that fluoxetine modulates the level of three of these proteins, the Actin-related protein 2, the Profilin-2 and the small Rho-GTPase CDC42 (Cell division control protein 42). Recently, with a similar proteomic approach we identified another protein modulated by fluoxetine in the mouse visual cortex and associated to the control of the cytoskeleton, Cofilin-1 (Bornia et al., 2015, unpublished result). It is interesting to note that these four proteins interact with the Arp2/3 complex which plays a predominant role controlling morphological plasticity of dendritic spines. As these same proteins have been associated with the regulation of plastic processes in other areas, as the hippocampus and the forebrain, it is likely that they are partners of the molecular machinery mediating the fluoxetine-induced plastic structural modifications.
In conclusion, we believe that the visual cortex experimental paradigm is particularly useful to identify specific genes or signaling pathways involved in the response of neuronal networks to the environment, i.e., the mechanisms controlling experience-dependent plasticity process, not only in normal, but also in pathological conditions. A wider application of large-scale approaches for identifying the molecular mechanisms through which fluoxetine reactivates plasticity in the adult and corrects visual dysfunctions, may eventually lead to the identification of innovative therapeutic approaches favoring the functional recovery in several neuronal disorders.
Agencia Nacional de Investigación e Innovación (ANII, Fondo Clemente Estable, FCE_6834 to FMR) and Programa de Desarollo de las Ciencias Basicas, Pedeciba, Uruguay.
Francesco Mattia Rossi*
Laboratorio de Neurociencias “Neuroplasticity Unit”, Facultad de Ciencias, UdelaR, Iguá 4225, C.P. 11400, Montevideo, Uruguay
*Correspondence to: Francesco Mattia Rossi, Ph.D., fmrossi@fcien.edu.uy.
Accepted: 2016-01-12
orcid: 0000-0001-9855-5925 (Francesco Mattia Rossi)
Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK (2010) Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 30:14964-14971.
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137-151.
Castrén E (2013) Neuronal network plasticity and recovery from depression. JAMA Psychiatry 70:983-989.
Castrén E, Hen R (2013) Neuronal plasticity and antidepressant actions. Trends Neurosci 36:259-267.
Dahlhaus M, Wan Li K, van der Schors RC, Saiepour MH, van Nierop P, Heimel JA, Hermans JM, Loos M, Smit AB, Levelt CN (2011) The synaptic proteome during development and plasticity of the mouse visual cortex. Mol Cell Proteomics 10:M110.005413. Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, Antila H, Popova D, Akamine Y, Bahi A, Sullivan R, Hen R, Drew LJ, Castren E (2011) Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 334:1731-1734.
LeBlanc JJ, Fagiolini M (2011) Autism: a “critical period” disorder? Neural Plast 2011:921680.
Maya-Vetencourt JF, Pizzorusso T (2013) Molecular mechanisms at the basis of plasticity in the developing visual cortex: epigenetic processes and gene programs. J Exp Neurosci 7:75-83.
Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E, Maffei L (2008) The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320:385-388.
Ruiz-Perera L, Muniz M, Vierci G, Bornia N, Baroncelli L, Sale A, Rossi FM (2015) Fluoxetine increases plasticity and modulates the proteomic profile in the adult mouse visual cortex. Sci Rep 5:12517.
Van den Bergh G, Clerens S, Firestein BL, Burnat K, Arckens L (2006) Development and plasticity-related changes in protein expression patterns in cat visual cortex: a fluorescent two-dimensional difference gel electrophoresis approach. Proteomics 6:3821-3832.
Wong DT, Perry KW, Bymaster FP (2005) Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov 4:764-774.
10.4103/1673-5374.180731 http://www.nrronline.org/
How to cite this article: Rossi FM (2016) Analysis of fluoxetine-induced plasticity mechanisms as a strategy for understanding plasticity related neural disorders. Neural Regen Res 11(4):547-548.
- 中国神经再生研究(英文版)的其它文章
- Gait deterioration due to neural degeneration of the corticoreticular pathway: a case report
- Complement components of nerve regeneration conditioned fluid influence the microenvironment of nerve regeneration
- Electrical stimulation of dog pudendal nerve regulates the excitatory pudendal-to-bladder reflex
- Supplementary motor area deactivation impacts the recovery of hand function from severe peripheral nerve injury
- Combined use of Y-tube conduits with human umbilical cord stem cells for repairing nerve bifurcation defects
- Senegenin inhibits neuronal apoptosis after spinal cord contusion injury

