Salvianolic acid B protects the myelin sheath around injured spinal cord axons
Zhe Zhu, Lu Ding, Wen-feng Qiu, Hong-fu Wu,, Rui Li,
1 Hand & Foot Surgery and Reparative & Reconstruction Surgery Center, the Second Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Physiology, Guangdong Medical University, Dongguan, Guangdong Province, China
RESEARCH ARTICLE
Salvianolic acid B protects the myelin sheath around injured spinal cord axons
Zhe Zhu1, Lu Ding2, Wen-feng Qiu2, Hong-fu Wu2,*, Rui Li1,*
1 Hand & Foot Surgery and Reparative & Reconstruction Surgery Center, the Second Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Physiology, Guangdong Medical University, Dongguan, Guangdong Province, China
Graphical Abstract
orcid: 0000-0002-1115-3681 (Hong-fu Wu)
Salvianolic acid B, an active pharmaceutical compound present in Salvia miltiorrhiza, exerts a neuroprotective effect in animal models of brain and spinal cord injury. Salvianolic acid B can promote recovery of neurological function; however, its protective effect on the myelin sheath after spinal cord injury remains poorly understood. Thus, in this study, in vitro tests showed that salvianolic acid B contributed to oligodendrocyte precursor cell differentiation, and the most effective dose was 20 μg/mL. For in vivo investigation, rats with spinal cord injury were intraperitoneally injected with 20 mg/kg salvianolic acid B for 8 weeks. The amount of myelin sheath and the number of regenerating axons increased, neurological function recovered, and caspase-3 expression was decreased in the spinal cord of salvianolic acid B-treated animals compared with untreated control rats. These results indicate that salvianolic acid B can protect axons and the myelin sheath, and can promote the recovery of neurological function. Its mechanism of action is likely to be associated with inhibiting apoptosis and promoting the differentiation and maturation of oligodendrocyte precursor cells.
nerve regeneration; spinal cord injury; salvianolic acid B; oligodendrocytes; myelin sheath; neural regeneration
Introduction
The primary cause of spinal cord injury (SCI) is an initial mechanical impact, causing compression and contusion, with damage to nerve cells, myelin, blood vessels, and supporting bone structures. Secondary inflammation and ischemia impact residual neural tissue, further aggravate neuronal death, induce demyelination, and worsen SCI (Papastefanaki and Matsas, 2015). Neuronal and axon regeneration is important after SCI. Myelin sheath and oligodendrocytes protect axons and support axon regeneration (Duncan et al., 2009; Funfschilling et al., 2012; Papastefanaki and Matsas, 2015). Necrosis and apoptosis of the myelin sheath and oligodendrocytes occur in toxic environments due to high metabolic rates, the abundance of iron-containing enzymes, and a reduced amount of reduced glutathione (Oyinbo, 2011). Saving a myelin sheath soon after injury promotes remyelination (Crowe et al., 1997; Grossman et al., 2001; Lytle and Wrathall, 2007). In contrast, slow regeneration ofthe myelin sheath and oligodendrocytes is not sufficient to support axon regeneration (Franklin and Ffrench-Constant, 2008; Franklin and Gallo, 2014; Papastefanaki and Matsas, 2015), which impacts the recovery of neurological function. An extract of Salvia miltiorrhiza has an effect on myocardial ischemia and reperfusion (Wang et al., 2013). Animal experiments have demonstrated that salvianolic acid B (Sal B), an active ingredient of S. miltiorrhiza, has neuroprotective effects against cerebral ischemia and reperfusion (Tian et al., 2009) and SCI (Fu et al., 2014), and can promote the recovery of neurological function in rats. The protective effect of Sal B on injured spinal cord was associated with a reduced inflammatory reaction and an increase in blood supply to the damaged tissue (Fu et al., 2014). Nevertheless, the effects of Sal B on the myelin sheath and oligodendrocytes after SCI remain unclear.
On the basis of the above-mentioned studies, we presumed that Sal B exerted a neuroprotective effect by mitigating damage to the myelin sheath and oligodendrocytes following SCI. Thus, we sought to verify the effects of Sal B on the maturation and differentiation of oligodendrocyte precursor cells using an in vitro approach.
Materials and Methods
Ethics statement
All animal procedures were conducted in accordance with guidelines reviewed and approved by the Institutional Animal Care and Use Committee of Guangdong Medical University, China, and in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. Precautions were taken to minimize suffering (see anesthesia procedures below) and the number of animals used in each experiment.
Primary culture of oligodendrocyte precursor cells and pharmacological intervention
In accordance with Armstrong’s method (1998), cerebral cortex was taken from 48-hour-old Sprague-Dawley rats [provided by the Experimental Animal Center of Southern Medical University of China; license No. SCXK (Yue) 2011-0015] using an operating microscope (M525F40; Leica, Wetzlar, Germany) under sterile conditions. The tissue was washed with D-Hank’s solution, cut into pieces and digested with 0.25% trypsin at 37°C for 15 minutes. Cells were incubated in oligodendrocyte precursor cell medium, containing Dulbecco’s modified Eagle’s medium/Ham’s F12 and 15% fetal bovine serum, and then placed in a polylysine-coated culture flask for 10 days. The culture flask was centrifuged at 37°C in a swing arm rotor at 200 r/min for 1.5 hours. After removal of supernatant, oligodendrocyte precursor cell medium was added and the cells incubated for 24 hours with shaking at 37°C in a swing arm rotor at 200 r/min for 1.5 hours. The cells were purified using a 74-μm sieve. Purified cells were further cultured for 24 hours. Sal B powder (Xi’an Hongsheng, Xi’an, Shaanxi Province, China) plus physiological saline were prepared into a 5 mg/mL stock solution. Oligodendrocyte precursor cells were incubated with the medium containing 5, 10 or 20 mg/L Sal B for 3 days.
Determination of purification and differentiation of oligodendrocyte precursor cells
Oligodendrocyte precursor cells at 8 hours after purification and before drug intervention were observed with an Axioplan 2 imaging E microscope (Carl Zeiss, Oberkochen, Germany). Immunohistochemistry for A2B5 was conducted to assess the purification of oligodendrocyte precursor cells. Immunohistochemistry for myelin basic protein (MBP) and immunohistochemistry for 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) was performed for Sal B and control (without drug treatment) groups at 3 days after drug administration. The effects of drugs on oligodendrocyte precursor cell differentiation were observed with the Axioplan 2 imaging E microscope (Carl Zeiss). Staining for oligodendrocyte markers, MBP and A2B5: cells were incubated on 7 mm × 22 mm coverslips, fixed with 2% paraformaldehyde at room temperature for 15 minutes, washed three times with phosphate buffered saline (PBS) for 5 minutes each, treated with 2% H2O2at room temperature for 30 minutes, washed three times with PBS for 5 minutes each, and blocked with 5% goat serum at 4°C for 2 hours. The samples were incubated with 5% goat serum-diluted rabbit anti-rat A2B5 antibody (1:100; Millipore Corp, Billerica, MA, USA) and rabbit anti-rat MBP antibody (1:100; Epitomics, Burlingame, CA, USA) at 4°C overnight, washed three times with PBS for 5 minutes each at room temperature, incubated with biotinylated goat anti-rabbit IgG for 30 minutes, then with avidin-biotin complex reagent (Neobioscience, Shenzhen, China) at 37°C for 30 minutes, washed twice in PBS, and washed once in Tris buffer. All samples were immersed in substrate solution, placed in the dark for 20 minutes, stained with hematoxylin, dehydrated through a graded alcohol series, permeabilized with xylene, and mounted with neutral resin. Immunohistochemistry for CNPase was carried out as follows: cells were incubated on 7 mm × 22 mm coverslips, washed with PBS, blocked and permeabilized with PBS containing 0.3% Triton X-100 and 10% natural goat serum for 30 minutes, treated with rabbit anti-rat CNPase antibody (1:100; Sigma, Carlsbad, CA, USA) at 4°C overnight, washed three times with PBS, treated with rhodamine (goat anti-rabbit, 1:200; Sigma) at room temperature for 1 hour, washed twice with PBS, treated with 4′,6-diamidino-2-phenylindole (DAPI) (1:10,000; Sigma) for 10 minutes, washed with PBS, dried in the air, and mounted.
Establishing a rat model of SCI and pharmacological intervention
Twenty female Sprague-Dawley rats weighing 230-250 g were provided by the Experimental Animal Center of Southern Medical University of China [license No. SCXK (Yue) 2011-0015]. The rats were randomly assigned to treatment and control groups. Rats were intraperitoneally injected with 10% chloral hydrate 400 mg/kg (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). A rat model of contusion was established by heavy impact (Metz et al., 2000). Aftershaving, the rats were fixed in the prone position. After sterilization, T8-11vertebral segments were removed, and the spinal cord was exposed. The spinal dura mater was left intact. A 10 g 2 cm-diameter metal rod was vertically dropped from a 25 mm height to impact the exposed spinal cord and to cause SCI (Ohta et al., 2004). Within the first week after SCI, the rats were assisted to urinate, and were intramuscularly injected with 50 mg/kg cefazolin, twice a day.
According to the animal experiment (Ye et al., 2011), 20 mg/L Sal B was optimal for postoperative functional recovery. The rats in the treatment group were intraperitoneally administered 20 mg/kg Sal B dissolved in 1 mL of physiological saline, once a day, for 8 consecutive weeks. The rats in the control group were given an equal volume of physiological saline.
Motor function assessment
Locomotor activity was evaluated using the Basso, Beattie, and Bresnahan locomotor rating scale (Basso et al., 1995), once a week. Paralysis is 0 points and normal locomotion is 21 points. The trunk, lower limb and tail activities were observed at 2 days after surgery. The rats were placed in a large enough space to allow free movement for 5 minutes, and their activities were video recorded. Two independent examiners, blind to the experiment, observed hindlimb movements and assessed the animals’ locomotor function.
Spinal cord slice preparation
Eight weeks after surgery, three rats from each group were intraperitoneally injected with 600 mg/kg 10% chloral hydrate, and perfused with physiological saline and 4% paraformaldehyde through the heart. T7-12segments were placed in 4% paraformaldehyde for 6 hours, and immersed in 30% sucrose. Frozen sections were embedded with embedding medium. The samples were sliced into 25 μm-thick longitudinal sections.
Immunohistochemistry for MBP
Twenty-five-micron-thick sections were antigen retrieved with 10 μg/mL proteinase K in a 37°C water bath for 10 minutes. One section from every four was incubated with rabbit anti-rat MBP antibody (1:400; Millipore) at 4°C overnight, washed in PBS, incubated with mouse anti-rabbit conjugated to AlexaFluor 568 secondary antibody (1:400; Invitrogen) at room temperature for 2 hours, washed with PBS, air-dried and mounted. Staining was observed under a fluorescence microscope (Carl-Zeiss Axioplan 2 imaging E).
Toluidine blue staining and electron microscopy
Four rats from each group were perfused with 0.9% physiological saline or 0.25% glutaral + 4% paraformaldehyde and the spinal cords dissected. Three animals were processed for toluidine blue staining and one was prepared for electron microscopy. The spinal cord was cut into 10 mm-long longitudinal blocks taking the injury site as the center. The samples were fixed with glutaraldehyde and osmic acid, embedded with epoxy resin, and then sliced into 1 μm-thick semithin sections. These sections were stained with toluidine blue (Sigma), mounted and observed with a microscope. At least three sections of each rat were observed. Myelinated nerve fibers were quantified in ten fields of each section. The percentage of myelinated nerve fibers relative to all fibers was calculated. Fifty-nanometer-thick ultrathin sections were stained with 2% uranyl acetate (Merck Drugs & Biotechnology, New Jersey, USA) and lead citrate (SPI-Chem, West Chester, USA), and observed with a transmission electron microscope (Philips CM120, Philips, The Netherlands).
Western blot assay
Fresh spinal cord was obtained from three rats of each group 30 minutes after SCI, including 0.5 cm of tissue from the distal and proximal ends of the injury site. All samples were triturated with a pipette, lysed with radioimmunoprecipitation assay buffer, and centrifuged at 10,000 r/min. The supernatant was collected and total protein concentration was measured by the bicinchoninic acid assay. Samples were stored at -20°C. Thirty micrograms of protein were heated at 100°C for 5 minutes and then electrophoresed on a 10% sodium dodecyl sulphate-polyacrylamide gel. The proteins were then electro-transferred onto polyvinylidene difluoride membranes (Amersham, Piscataway, NJ, USA). The membranes were washed, blocked with 5% skimmed milk in Tris-buffered saline for 1 hour, incubated with rabbit anti-rat caspase-3 primary antibody (1:1,000; Sigma) at 4°C overnight, washed, incubated with horseradish peroxidase-labeled mouse anti-rabbit IgG secondary antibody (1:5,000, Sigma) for 30 minutes, and visualized by enhanced chemiluminescence. β-Actin (1:1,000, rabbit anti-rat; Sigma) served as an internal reference. The absorbance of scanned bands was determined using Image J (National Institutes of Health, Rockville, MD, USA). The results are expressed as the ratio of the target protein band intensity to that for β-actin.
Statistical analysis
The data were analyzed using GraphPad Prism 5 software (GraphPad Software, Inc., CA, USA), and are expressed as the mean ± SD. One-way analysis of variance with Bonferroni post hoc and Student’s t-test were used to compare intergroup differences. A value of P < 0.05 was considered statistically significant.
Results
Sal B promoted the maturation and differentiation of oligodendrocyte precursor cells
Purified oligodendrocyte precursor cells were round, elliptical and uniform, and had bipolar or tripolar cell bodies (Figure 1A). The percentage of A2B5-positive oligodendrocyte precursor cells was more than 95% (Figure 1B), indicating a high purification rate of oligodendrocyte precursor cells.
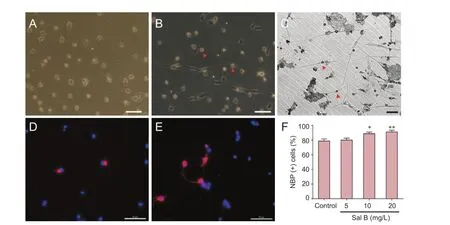
Figure 1 Effects of different concentrations of Sal B on oligodendrocyte precursor cell differentiation.
Three days after intervention with different concentrations of Sal B, oligodendrocyte precursor cells underwent immunohistochemical staining for MBP and CNPase to observe cell differentiation. The percentage of cells differentiating into oligodendrocytes was largest after treatment with 20 mg/L Sal B (Figure 1F). The number and thickness of cell processes were increased (Figure 1C). In the 20 mg/L Sal B group, a large number of oligodendrocyte precursor cells were positive for CNPase (Figure 1E). In the control group, CNPase staining was low (Figure 1D). Thus, 20 mg/kg was used as a therapeutic dose in the following in vivo experiments.
Sal B improved motor function in SCI rats
Basso, Beattie, and Bresnahan scores were significantly higher in the 20 mg/kg Sal B group compared with the control group at 3 weeks after intervention with Sal B (P < 0.01 or P< 0.05; Figure 2A).
Sal B enhanced remyelination in the injured spinal cord
Immunofluorescence staining for MBP demonstrated that at 8 weeks after SCI, in the control group, the quantity of myelin sheath around axons in the injured spinal cord was reduced, annuli were not intact and damaged broken myelin sheath was visible (Figure 2B1). In the Sal B-treated group many annuli were observed, and myelin sheath was intact (Figure 2B2). Also, the number of MBP-immunoreactive cells was more in the 20 mg/kg Sal B group than in the control group.
Toluidine blue staining revealed that the myelin sheath was broken and separated from axons in the control group (Figure 2B3). The number of myelinated nerve fibers was more in the 20 mg/kg Sal B group than in the control group. The myelin sheath was multi-layered, and the structure of the myelin sheath was intact in the 20 mg/kg Sal B group (Figure 2B4, B7).
In the control group, transmission electron microscopy showed axons to be shrunk and a marked gap was observed between axons and the myelin sheath (Figure 2B5). In the 20 mg/kg Sal B group, the morphology of the myelin sheath and axons was noticeably improved. Axonal atrophy was mitigated, and the myelin sheath was multilayered (Figure 2B6).
Sal B suppressed caspase-3 expression in the injured spinal cord
Caspase-3 expression was significantly lower in the 20 mg/kg Sal B group compared with the control group 30 minutes after SCI (Figure 2C).
Discussion
Early intervention is very important for myelin sheath and oligodendrocyte recovery after injury. Previous studies showed that the number of oligodendrocytes was reduced by half at the injury site 1 day after SCI, so it is very important for nerve cell survival to reduce secondary injury as early as possible, and treatment within 24 hours could obtain good outcomes (Fehlings et al., 2012; Papastefanaki and Matsas, 2015). Thus, in this study, the time of intervention with Sal B was immediately after SCI.
Oligodendrocyte apoptosis can be observed at and surrounding the site of injury in humans and mice several weeks to several years after chronic injury (Kakulas, 1999; Guest et al., 2005; Totoiu and Keirstead, 2005; Lasinene et al., 2008). Demyelination and axonal degeneration impact the recovery of neurological function (Hagg and Oudega, 2006; Jiang et al., 2007; Serarslan et al., 2009). The caspase family plays a key role in apoptosis (Poter and Janicke et al., 1999; Saikumar et al., 1999; Hishikawa et al., 2000; Adjan etal., 2007; Huey et al., 2008). Therefore, caspase-3 was used to measure the effect of Sal B on apoptosis following SCI. This study observed an anti-apoptotic effect of Sal B.
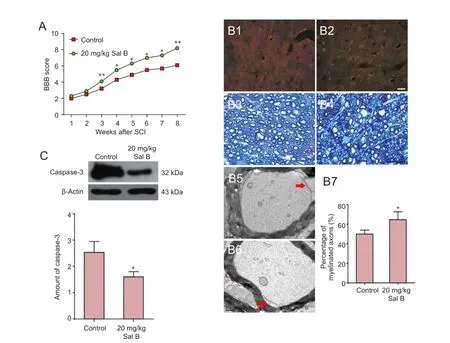
Figure 2 Effects of 20 mg/kg Sal B on remyelination, caspase-3 expression and motor function in rats with spinal cord injury.
Sal B contributed to oligodendrocyte precursor cell differentiation in vitro, indicating that Sal B may promote remyelination after SCI. Oligodendrocyte precursor cells have been shown to produce hypoxia-inducible factor (HIF) under ischemia and hypoxia, and HIF1/2α can inhibit oligodendrocyte precursor cell maturation and myelination, which impact the recovery of neurological function (Yuen et al., 2014). Sal B has been shown to increase the blood supply to nerve tissue during neurological recovery (Fu et al., 2014). Our in vivo results confirmed that Sal B relieved secondary injury to the spinal cord at early stages and improved motor function recovery and promoted remyelination.
In conclusion, Sal B can promote the maturation and differentiation of oligodendrocyte precursor cells, reduce damage to the myelin sheath around axons following SCI, suppress apoptosis, and contribute to the recovery of motor function.
Author contributions: RL conceived and designed the study. ZZ wrote the paper, provided data and ensured the integrity of the data. LD participated in statistical analysis. WFQ provided technical or data support. HFW conceived and designed the study, obtained funding. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Adjan VV, Hauser KF, Bakalkin G, Yakovleva T, Gharibyan A, Scheff SW, Knapp PE (2007) Caspase-3 activity is reduced after spinal cord injury in mice lacking dynorphin: differential effects on glia and neurons. Neuroscience 148:724-736.
Armstrong RC (1998) Isolation and characterization of immature oligodendrocyte lineage cells. Methods 16:282-292.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1-21.
Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS (1997) Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 3:73-76.
Duncan ID, Brower A, Kondo Y, Curlee JF Jr, Schultz RD (2009) Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci U S A 106:6832-6836.
Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A, McKerracher L (2011) A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma 28:787-796.
Franklin RJ, Ffrench-Constant C (2008) Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839-855.
Franklin RJ, Gallo V (2014) The translational biology of remyelination: past, present, and future. Glia 62:1905-1915.
Fu J, Fan HB, Guo Z, Wang Z, Li XD, Li J, Pei GX (2014) Salvianolic acid B attenuates spinal cord ischemia-reperfusion-induced neuronal injury and oxidative stress by activating the extracellular signal-regulated kinase pathway in rats. J Surg Res 188:222-230.
Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517-521.
Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol. 2001; 168:273-282.
Guest JD, Hiester ED, Bunge RP (2005) Demyelination and schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol 192:384-393.
Hagg T, Oudega M (2006) Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma 23:264-280.
Hishikawa K, Nakaki T, Fujii T (2000) Connective tissue growth factor induces apoptosis via caspase-3 in cultured human aortic smooth muscle cells. Eur J Pharmacol 392:19-22.
Huey KA, Roy RR, Zhong H, Lullo C (2008) Time-dependent changes in caspase-3 activity and heat shock protein 25 after spinal cord transection in adult rats. Exp Physiol 93:415-425.
Jiang S, Bendjelloul F, Ballerini P, D’Alimonte I, Nargi E, Jiang C, Huang X, Rathbone MP (2007) Guanosine reduces apoptosis and inflammation associated with restoration of function in rats with acute spinal cord injury. Purinergic Signal 3:411-421.
Kakulas BA (1999) A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med 22:119-124.
Lasiene J, Shupe L, Perlmutter S, Horner P (2008) No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J Neurosci 28:3887-3896.
Lytle JM, Wrathall JR (2007) Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur J Neurosci 25:1711-1724.
Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V (2000) Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma 17:1-17.
Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, Chou H, Ishikawa N, Matsumoto N, Iwashita Y, Mizuta E, Kuno S, Ide C (2004) Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol 187:266-278.
Oyinbo CA (2011) Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 71:281-299.
Papastefanaki F, Matsas R (2015) From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia 63:1101-1125.
Poter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis .Cell Death Differ 6:99-104.
Saikumar P, Dong Z, Mikhailov V, Denton M, Weinberg JM, Venkatachalam MA (1999) Apoptosis: definition, mechanisms, and relevance to disease. Am J Med 107:489-506.
Serarslan Y, Bal R, Altug ME, Kontaş T, Keleş ON, Unal D, Unal B (2009) Effects of trimetazidine on crush injury of the sciatic nerve in rats: a biochemical and stereological study. Brain Res 1247:11-20.
Tian J, Fu F, Li G, Gao Y, Zhang Y, Meng Q, Li C, Liu F (2009) Protections of SMND-309, a novel derivate of salvianolic acid B, on brain mitochondria contribute to injury amelioration in cerebral ischemia rats. Phytomedicine 16:726-733.
Totoiu MO, Keirstead HS (2005) Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 486:373-383.
Wang ZS, Luo P, Dai SH, Liu ZB, Zheng XR, Chen T (2013) Salvianolic acid B induces apoptosis in human glioma U87 cells through p38-mediated ROS generation. Cell Mol Neurobiol 33:921-928.
Ye ZZ, Deng YB, Wu HF, Gan DH (2011) Neuroprotective effects of salvianolic acid B on secondary spinal cord damage. Neural Regen Res 6:188-192.
Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E, Rowitch DH (2014) Oligodendrocyte encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158:383-396.
Copyedited by Allen J, Frenchman B, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.179068 http://www.nrronline.org/
How to cite this article: Zhu Z, Ding L, Qiu WF, Wu HF, Li R (2016) Salvianolic acid B protects the myelin sheath around injured spinal cord axons. Neural Regen Res 11(3):487-492.
Funding: This study was supported by a grant of Guangdong Medical University of China, No. XB1380.
Accepted: 2016-02-20
*Correspondence to: Rui Li, M.D. or Hong-fu Wu, Ph.D., 13304321102@qq.com or hongfuw@126.com.
- 中国神经再生研究(英文版)的其它文章
- NEURAL REGENERATION RESEARCH ABOUT JOURNAL
- Recovery of an injured corticospinal tract during the early stage of rehabilitation following pontine infarction
- Cartilage oligomeric matrix protein enhances the vascularization of acellular nerves
- Altered microRNA expression profiles in a rat model of spina bifida
- Verapamil inhibits scar formation after peripheral nerve repair in vivo
- Substance P combined with epidermal stem cells promotes wound healing and nerve regeneration in diabetes mellitus

