Effect of polycystic ovaries on in vitro fertilisation and intra-cytoplasmic sperm injection treatment outcome
Michael Francis Costello, Chiao Yi Michelle Chew, Kristen Lindsay, Alex Wang, Glen McNally
1Obstetrics and Gynaecology, University of New South Wales, Sydney, Australia
2Royal Hospital for Women, Sydney, Australia
3IVF Australia, Sydney, Australia
Effect of polycystic ovaries on in vitro fertilisation and intra-cytoplasmic sperm injection treatment outcome
Michael Francis Costello1,2,3*, Chiao Yi Michelle Chew1, Kristen Lindsay1, Alex Wang1, Glen McNally2
1Obstetrics and Gynaecology, University of New South Wales, Sydney, Australia
2Royal Hospital for Women, Sydney, Australia
3IVF Australia, Sydney, Australia
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Polycystic ovaries
In vitro fertilisation
Intra-cytoplasmic sperm injection Infertility
Ultrasound
Objective: The reproductive performance of women with polycystic ovaries (PCO) with regular ovulatory menstrual cycles undergoing in vitro fertilisation and intra-cytoplasmic sperm injection (IVF/ ICSI) treatment has not been well described. This study aimed to investigate the outcome of IVF/ICSI in ovulatory women with PCO. Methods: A retrospective cohort study of women aged ≤42 years with infertility and regular ovulatory menstrual cycles who underwent their fi rst IVF/ICSI cycle using the long down regulation protocol at IVF Australia-EAST in Sydney between 2000 and 2011. A pre-treatment baseline transvaginal pelvic ultrasound (TVS) had been performed by a single tertiary level diagnostic ultrasound centre. Patients were divided into either group NO (normal ovaries) or group PCO according to the pre-treatment TVS. The primary outcome measure was live birth rate per patient. Results: A total of 200 patients (135 in group NO and 65 in group PCO) were included in the data analysis. There was no diff erence in live birth rate per patient between the two groups (25.2% vs 26.2%) with both raw (OR [95% CI] = 1.05 [0.54–2.07]) and logistic regression adjusted (for maternal age) (Adjusted OR [95% CI] = 0.99 [0.50–1.98]) data. Conclusions: The presence of PCO in ovulatory women did not adversely aff ect IVF/ICSI outcome at our unit. However, the results are not conclusive and further large, well-designed prospective cohort studies are required in order to conf irm our fi ndings.
1. Introduction
Polycystic ovary syndrome (PCOS), which is characterized by features of ovulatory dysfunction, hyperandrogenism and polycystic ovaries (PCO) has been shown to affect a striking 12%–21% of Australian reproductive-age women[1-3]. The reproductive performance of women with PCOS undergoing in vitro fertilisation and intra-cytoplasmic sperm injection (IVF/ICSI) treatment has been well described in a large systematic review and meta-analysis of nine observational studies comparing 458 women with PCOS (793 cycles) with 694 matched controls (1 116 cycles)[4].
In contrast, there are very few studies analysing IVF/ICSI outcome in women with regular ovulatory menstrual cycles with PCO. Ultrasound evidence of PCO affects approximately 20%–30% of the female population[5-7] and up to 34% of women attending fertility clinics[8]. Although the presence of PCO may be considered a normal variant, data suggest that subtle endocrine disturbances, similar to those that are found in PCOS, may be uncovered in up to 1/3 women with ovulatory PCO[9]. The aim of this study was to assess the success rate of women with regular ovulatory menstrual cycles who have ultrasound evidence of PCO undergoing IVF/ICSI treatment.
2. Materials and methods
2.1. Patients
This retrospective cohort study included all patients with various causes of infertility undergoing their first cycle of IVF/ICSI treatment at IVF Australia-East between January 2000 and 2011and who had a transvaginal pelvic ultrasound (TVS) performed by a single tertiary level diagnostic ultrasound centre (Warren and McNally Diagnostic Ultrasound Group, Royal Hospital for Women, Sydney, Australia) prior to embarking on their treatment cycle.
All patients met the following inclusion criteria: (i) aged 42 years or less at the time of commencement of IVF/ICSI treatment, (ii) infertility, (iii) regular ovulatory menstrual cycles (iv) normal uterine cavity assessed by ultrasound, hysterosalpingogram or hysteroscopy, (v) fi rst stimulated cycle of IVF/ICSI, and (vi) long down regulation protocol. Exclusion criteria consisted of: (i) oocyte donor treatment cycle, (ii) presence of hydrosalpinges, (iii) presence of stage four (severe) endometriosis, and (iv) past history of myomectomy. This study was approved by the IVF Australia Ethics Committee.
2.2. IVF/ICSI treatment
All patients had IVF/ICSI treatment using the long down regulation protocol with gonadotrophin releasing hormone (GnRH) agonist commenced in the mid-luteal phase (or 15 d after starting the combined oral contraceptive pill [OCP] for OCP pre-treated cycles) either as a nasal spray (nafarelin acetate; Pharmacia Australia), 200 μg twice daily, or as a subcutaneous injection (leuprorelin acetate; Abbott Australasia, Cronulla, NSW, Australia), 1 mg daily for at least 10 d, until pituitary down-regulation was confi rmed by a serum estradiol (E2) level of < 120 pmol/L. Follicle-stimulating hormone (FSH) injections (Gonal F; Merck Serono Laboratories, Frenchs Forest, NSW, Australia; or Puregon; MSD Laboratories, Lane Cove, NSW, Australia) were then commenced for ovarian stimulation, with the starting dose being determined by the individual clinician according to the patient’s age, BMI and the presence or absence of PCO on ultrasound. Daily FSH injections and the GnRH agonist were continued until the day of human chorionic gonadotropin (hCG) trigger injection (Profasi or Ovidrel; Merck Serono Laboratories, Sydney) when 2 or more follicles at least 17 mm diameter were seen on ultrasound. Transvaginal egg collection was timed 36 h following the hCG trigger injection.
Two to four hours following egg collection, the oocytes were either inseminated (IVF) or injected (ICSI) with prepared sperm and fertilisation was confirmed 16–18 h later. Depending on whether the patient was having an embryo cleavage or blastocyst stage transfer, the embryos were transferred transcervically on day 2/3 or day 5 after egg collection, respectively. Patients were given vaginal progesterone (Progesterone pessaries 100 mg, Maquarie Street Pharmacy, Sydney, NSW, Australia or Crinone gel, Serono Laboratories, Frenchs Forest, NSW, Australia) beginning on the day after egg collection and continuing daily until the pregnancy test with serum βhCG 16 d following egg collection.
Biochemical pregnancy was defi ned as a positive βhCG at the time of the pregnancy test. Clinical pregnancy was defi ned by the presence of an intrauterine gestational sac and live fetus on TVS at 7 weeks gestation. Ongoing clinical pregnancy was defined as a clinical pregnancy continuing past 12 weeks gestation. Live birth was defi ned as the delivery of a live baby beyond 20 weeks gestation. Severe ovarian hysperstimulation syndrome (OHSS) was defi ned as OHSS requiring hospital admission.
2.3. Pelvic ultrasound assessment
All patients had a TVS in the follicular phase of the menstrual cycle by a single ultrasound practice specialized in gynaecological imaging (Warren and McNally Diagnostic Ultrasound Group) prior to commencing their treatment cycle. The ultrasound machine used was an Ultrasound Systems, GE Logiq 9 Systems (General Electric Medical Systems, Milwaukee, Wisconsin, USA). In addition, the ultrasound images of all patients had been digitally stored (ALI Ultrapacs) and were re-reviewed by a sole reviewer from the single ultrasound practice (Author GM) who was sub-specialised in gynaecological sonography.
All patients’ ultrasounds were assessed for the presence of PCO according to the Rotterdam criteria: presence of 12 or more follicles in either ovary measuring 2–9 mm in diameter, and/or increased ovarian volume (≥ 10 mL) (2).
2.5. Primary outcome
The primary outcome measure was live birth rate per patient.
2.6. Secondary outcome
The secondary outcome measures were (i) ongoing clinical pregnancy rate per patient, (ii) clinical pregnancy rate per patient, (iii) biochemical pregnancy rate per patient, (iv) miscarriage rate per biochemical pregnancy and patient, (v) multiple pregnancy rate per clinical pregnancy and patient, (vi) ovarian stimulation response, and (v) severe OHSS rate per patient.
2.7. Statistical analysis
Continuous variables were tested for normal distribution using Kolmogorov-Smirnov test and subsequently analysed using either the independent samples t-test (normally distributed data) or Mann-Whitney U test (skewed data) to compare two means (normally distributed data) or medians (skewed data), where appropriate. Categorical variables were analysed using the Chi-square test or Fisher’s Exact Test where appropriate. Statistical signifi cance was assumed when P < 0.05. Statistical analysis was performed using SPSS version 21 (SPSS Inc., Chicago, IL).
3. Results
A total of 200 patients undergoing their fi rst stimulated cycle of IVF/ICSI were included in the data analysis. One hundred and thirty fi ve patients had normal ovaries (group NO) and 65 patients had PCO (group PCO) on baseline pelvic ultrasound.
Table 1 compares the baseline demographic and clinical variables between the patients with normal or PCO on ultrasound and demonstrates no diff erences between the two groups in terms of treatment type (IVF or ICSI), treatment year, the time between TVS and IVF/ICSI treatment cycle, the duration of infertility, body mass index, cause of infertility, gravidity, and the number of patients who smoke. However, the PCO group was younger compared to the NO group.
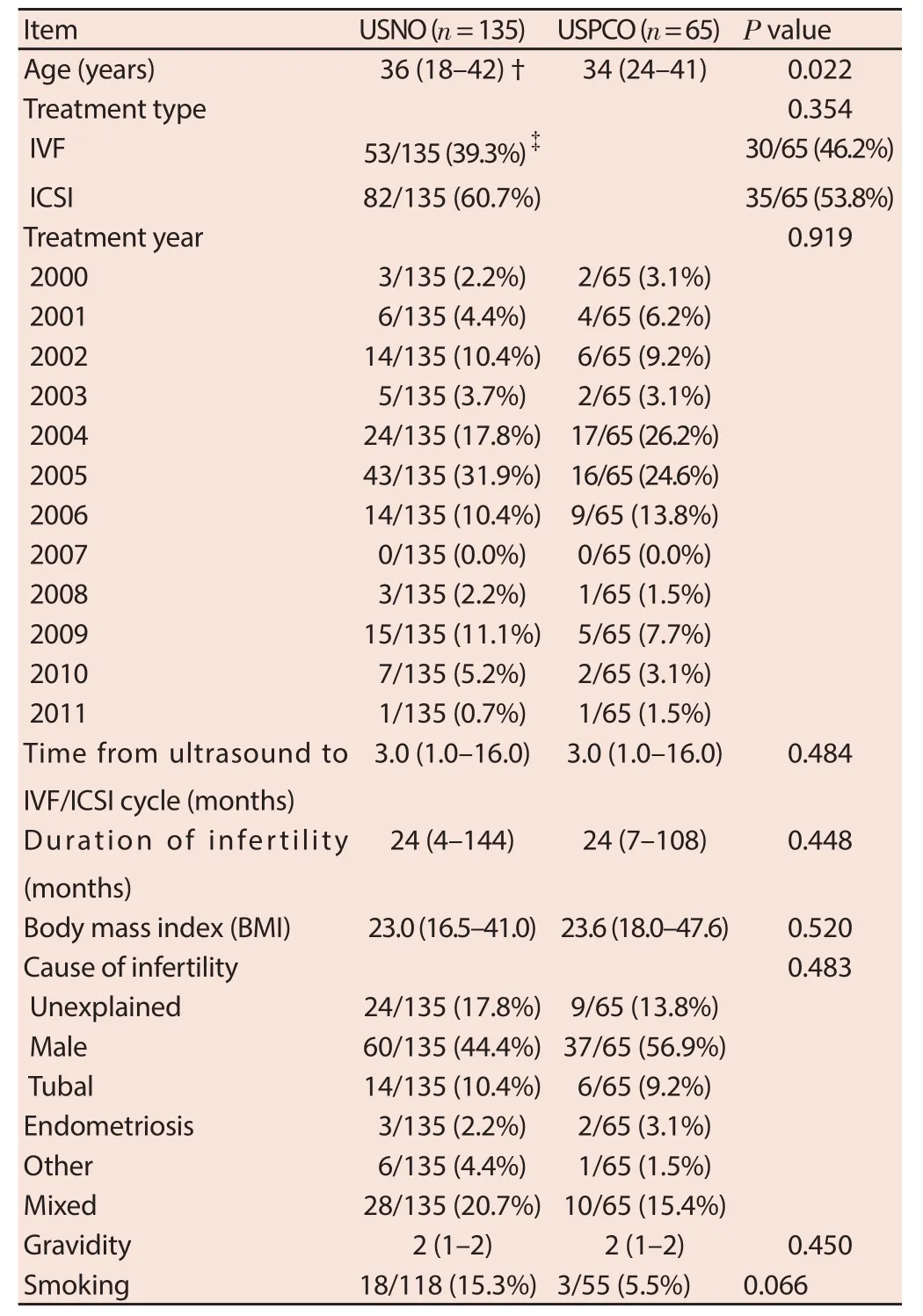
Table 1 Demographic data of patients with ultrasound normal ovaries (USNO) and polycystic ovaries (USPCO).
The IVF/ICSI treatment cycle outcomes between the patients with normal and PCO are compared in Table 2. There was no difference seen in any of the ovarian response parameters, embryological parameters or clinical outcomes between the two groups. Specifi cally, there was no diff erence observed in the primary outcome measure of live birth rate per patient between the two groups (25.2% vs 26.2%) with both raw (OR [95% CI] = 1.05 [0.54– 2.07]) and logistic regression adjusted (for maternal age) (Adjusted OR [95% CI] = 0.99 [0.50–1.98]) data (Table 3).
Intra-observer variability was measured using a test-retest measure. Ten randomly selected patient’s ultrasound images were re-presented to the assessor who was blinded to the original assessment and these judgments were correlated with the initial judgments. Test-retest performance was found to be quite high (r = 0.80) with 9 agreements out of 10. In addition, there was no inter-observer variability in this study as the same assessor performed all of the ultrasound image examinations and measurements.
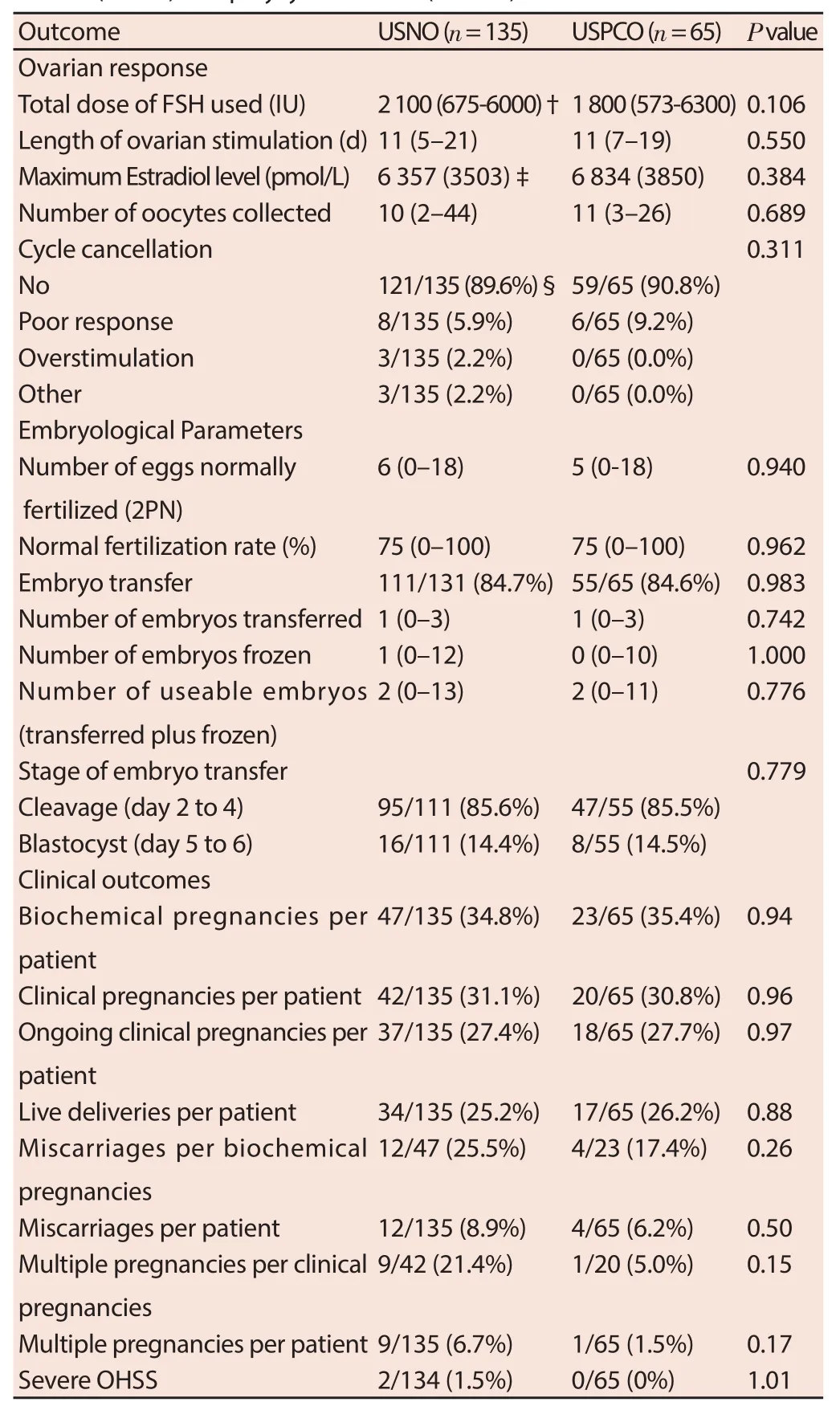
Table 2 IVF/ICSI treatment cycle outcomes of patients with ultrasound normal ovaries (USNO) and polycystic ovaries (USPCO).
4. Discussion
The results of our study demonstrate that there was no diff erence observed in live birth rate per IVF/ICSI treatment cycle in women with regular ovulatory menstrual cycles with ultrasound evidence of PCO compared to normal ovaries (NO). However, the study fi ndingsare not conclusive as one cannot exclude an important eff ect of PCO on IVF/ICSI live birth rate given the wide 95% confidence limits observed.
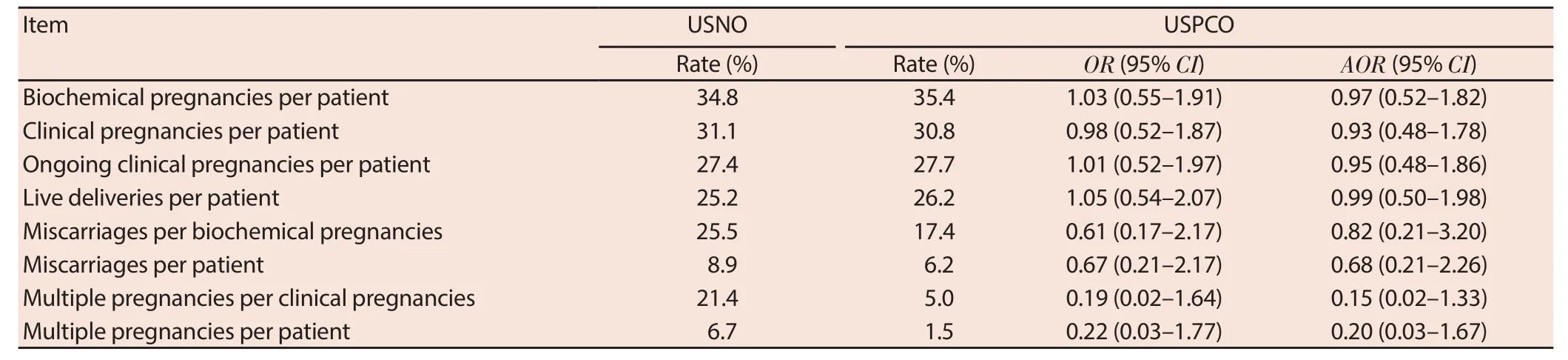
Table 3 Raw and adjusted data for IVF/ICSI treatment cycle clinical outcomes of patients with ultrasound normal ovaries (USNO) and polycystic ovaries (USPCO).
To the best of our knowledge, there are four other observational studies that have evaluated the impact of PCO, compared to NO on ultrasound, on IVF/ICSI outcome in women with regular ovulatory menstrual cycles[10-13] demonstrating either no effect[11-13] or improved effect[10] in women with PCO on IVF/ICSI outcome in terms of pregnancy or live birth rate.
Engmann and colleagues performed a prospective cohort study in 191 ovulatory women (46 PCO, 145 NO) undergoing IVF or ICSI in the United Kingdom using the long down regulation protocol over 1 to 3 treatment cycles and adjusted the starting FSH dose according to ovarian morphology on ultrasound (NO or PCO)[10]. This study observed that women with PCO had a signifi cant reduction in the total FSH dose used and days of controlled ovarian stimulation (COH) whilst having a signifi cant increase in the maximum E2 level and number of eggs collected averaged over three treatment cycles. There was no diff erence in the fertilization rate averaged over three treatment cycles. The authors did not report the clinical pregnancy or live birth rate per cycle but found a trend towards a higher cumulative clinical pregnancy rate over three cycles (OR = 1.69; 95% CI 0.99–2.90; P = 0.05) and a higher cumulative live birth rate over three cycles (OR = 1.82; 95% CI 1.05–3.16; P = 0.03). There was no diff erence between the two groups in the rate of miscarriage or moderate to severe OHSS.
A retrospective cohort study of 141 ovulatory women (39 PCO, 102 NO) undergoing their first cycle of IVF or ICSI in Iran using the long down regulation protocol, where there was no diff erence in the starting dose of FSH according to ovarian morphology on ultrasound, performed by Esmailzadeh et al., found no diff erence in the total FSH dose used or days of COH between the two groups but there was a signifi cantly higher number of eggs collected in the PCO group[11]. There was no diff erence in the fertilization rate, clinical pregnancy rate or OHSS rate between the two groups.
Sahu et al. conducted a retrospective cohort study of ovulatory women undergoing 154 cycles (51 cycles for women with PCO, 104 cycles for women with NO and the number of women not stated) of ICSI treatment in the United Kingdom using the long down regulation protocol where the starting FSH dose was adjusted according to ovarian morphology on ultrasound[12]. This study showed no diff erence in the days of COH between the two groups, a signifi cant reduction in the total dose of FSH used and a signifi cant increase in the maximum E2 level and number of eggs collected in the PCO group. There was no diff erence observed in the rate of fertilization, clinical pregnancy, miscarriage or moderate to severe OHSS between the PCO and NO’ groups.
Lastly, Swanton and colleagues also performed a retrospective cohort study of 212 ovulatory women (101 PCO, 111 NO) undergoing their fi rst cycle of IVF or ICSI in the United Kingdom using the long down regulation protocol where the starting dose of FSH was not adjusted according to ovarian morphology on ultrasound and the primary outcome measure was the rate of severe OHSS requiring hospitalization[13]. Findings were that of a signifi cant reduction in the total FSH dose used and a signifi cant increase in the maximum E2 level and number of eggs collected in the PCO group. The fertilization rate was signifi cantly reduced in the PCO group. However, there no diff erence seen in the clinical pregnancy, miscarriage or live birth rate per cycle between the two groups but the PCO group had a signifi cantly higher rate of severe OHSS requiring hospitalization.
The embryological (fertilization rate) and clinical (pregnancy and/ or live birth rate) outcome results of our study were similar to the results of the previously published four studies comparing ovulatory women with PCO versus NO on ultrasound undergoing IVF with ICSI treatment[10-13]. Our study demonstrated no difference in clinical pregnancy rate per cycle between the two groups which is consistent with the three studies that assessed this outcome[11-13] and also the fourth study that examined the cumulative clinical pregnancy rate over three treatment cycles[10]. Our study also observed no diff erence in live birth rate per cycle between the two groups and this fi nding was also reported in the only other study examining this outcome measure[13]. However, the cumulative live birth rate over three treatment cycles was higher in the PCO group in the study by Engmann et al[10].
Interestingly, only one of the previous studies comparing PCO with NO and reporting on clinical pregnancy or live birth rate per cycle presented the results with 95% confi dence intervals[11]. This study by Esmaizadeh et al., showing no diff erence in clinical pregnancy rate between the two groups, also reported wide 95% confi dence intervals and thus also was unable to exclude an important diff erence between PCO and NO on IVF/ICSI outcome.
However, the results of our ovarian response outcomes diff ered to those of the two other studies which, similar to our study, adjustedthe start dose of FSH according to ovarian morphology on ultrasound (PCO or NO)[10,12]. The study by Swanton and colleagues did not adjust the starting dose of FSH according to the presence of PCO as this study specifi cally measured the rate of severe ovarian OHSS as the primary outcome[13].
Our study demonstrated no diff erence in the length of ovarian stimulation between the PCO and NO groups, which agreed with the fi ndings of Sahu et al. but not Engmann et al. whose study showed a signifi cant reduction by 0.8 d in the PCO group[10,12]. Both the other studies found a signifi cantly higher maximum E2 level and number of eggs collected (+ 3 eggs) in the PCO group whereas our study showed a non-signifi cant increase in both these outcomes (+ 1 egg) in the PCO group. The reason for this diff erence in fi ndings in the ovarian response outcomes for our study cannot be easily explained but may be related to diff erences in patient demographic characteristics including definition of PCO, start dose of FSH determination and ovarian stimulation between the studies.
There are a number of strengths to this study including large sample size; strict inclusion and exclusion criteria to limiting potential confounding bias; analysis of data from the fi rst treatment cycle only to overcome problems of lack of independence, biased assessment of outcomes, and prognostic heterogeneity; and reporting on the clinically important outcome measure of live birth. In addition, the diagnosis of patients with or without PCO had low intra-observer variability and no inter-observer variability as there was a single experienced assessor examining the ultrasound images.
However, there are also a number of limitations to this study. The observational nature of this study is disadvantaged methodologically by an inability to control completely for selection and confounding biases[14]. The PCO group in our study was younger compared to the NO group and this fi nding is consistent with the fact that the number of ovarian follicles decreases with age[15]. However, the lack of diff erence in treatment outcome that remained after adjusted analysis makes confounding bias unlikely[16].
In conclusion, the present study demonstrates that the presence of PCO in ovulatory women undergoing IVF/ICSI did not adversely affect live birth rate which is consistent with the findings in the literature to date. However, the results of our study are not conclusive and further large, well-designed prospective cohort studies are required in order to confi rm these fi ndings.
Declare of interest statement
We declare that we have no confl ict of interest.
Acknowledgments
The authors wish to thank the staff from IVFA-East, Ultrasound Department and Department of Reproductive Medicine at the Royal Hospital for Women, Sydney for their assistance in this study. This research required no fi nancial support and the authors declare that they have no confl icts of interest for this study and article.
[1] Zawadaki JK, Dunaif A. Diagnostic criteria for polycystic ovarian syndrome: towards a rational approach. In: Dunaif A, Given JR, Haseltine F, Merriam GR, editors. Boston: Blackwell Scientifi c; 1992: 377-384.
[2] Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81(1): 19-25.
[3] March WA, Moore VM, Willson KJ, Phillips D, Norman R, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25(2): 544-551.
[4] Heijnen EM, Eijkemans MJ, Hughes EG, Laven JSE, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update 2006; 12(1): 13–21. [5] Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995; 10(8): 2107–2111.
[6] Farquhar CM, Birdsall M, Manning P, Mitchell JM, France JT. The prevalence of polycystic ovaries on ultrasound scanning in a population of randomly selected women. Aust N Z J Obstet Gynaecol 1994; 34(1): 67–72.
[7] Polson DW, Adams J, Wadsworth J, Franks S. Polycystic ovaries–a common fi nding in normal women. Lancet 1988; 1(8590): 870–872.
[8] Balen AH, Tan SL, MacDougall J, Jacobs HS. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum Reprod 1993; 8(6): 959–964.
[9] Carmina E, Wong L, Chang L, Paulson RJ, Sauer MV, Stanczyk FZ, et al. Endocrine abnormalities in ovulatory women with polycystic ovaries on ultrasound. Hum Reprod 1997; 12(5): 905–909.
[10] Engmann L, Maconochie N, Sladkevicius P, Bekir J, Campbell S, Tan SL. The outcome of in-vitro fertilization treatment in women with sonographic evidence of polycystic ovarian morphology. Hum Reprod 1999; 14(1): 167–171.
[11] Esmaizadeh S, Faramarzi M, Jorsarai G. Comparison of in vitro fertilization outcome in women with and without sonographic evidence of polycystic ovarian morphology. Eur J Gynaecol Reprod Med 2005; 121(1): 67-70.
[12] Sahu B, Ozturk O, Ranierri M, Serhal P. Comparison of oocyte quality and intracytoplasmic sperm injection outcome in women with isolated polycystic ovaries or polycystic ovarian syndrome. Arch Gynaecol Obstet 2008; 277(3): 239-244.
[13] Swanton A, Storey L, Mcveigh E, Child T. IVF outcome in women with PCOS, PCO and normal ovarian morphology. Eur J Gynaecol Reprod Med 2010; 149(1): 68-71.
[14] Greenhalgh T. Assessing the methodological quality of published papers. Br Med J 1997; 315(7103): 305-308.
[15] Domingues TS, Rocha AM, Serafini PC. Tests for ovarian reserve: reliability and utility. Curr Opin Obstet Gynecol 2010; 22(4): 271-276.
[16] Normand ST, Sykora K, Li P, Mamdani M, Rochon PA, Anderson GM. Reader’s guide to critical appraisal of cohort studies: 3. Analytical strategies to reduce confounding. Br Med J 2005; 330(7498): 1021-1023.
ent heading
10.1016/j.apjr.2016.04.011
*Corresponding author: Dr Michael Costello, School of Women's and Children's Health, Division of Obstetrics and Gynaecology, Level 1 Women's Health Institute, Royal Hospital for Women, Locked Bag 2000, Randwick, Sydney, NSW, Australia, 2031.
Tel: 61-2-9382 6677
Fax: 61-2-9382 6444
E-mail: mfcostello@unsw.edu.au
 Asian Pacific Journal of Reproduction2016年3期
Asian Pacific Journal of Reproduction2016年3期
- Asian Pacific Journal of Reproduction的其它文章
- A rare cause of infertility: A late complication of female genital mutilation
- Prenatal progesterone exposure of male rats induces morphometric and histological changes in testes
- A new looming of Zika virus
- Alteration in oestus cycle and implantation in Mus musculus administered aqueous wood ash extract of Azadirachta indica (neem)
- Ameliorative potentials of quercetin against cotinine-induced toxic effects on human spermatozoa
- Effects of honey to mobilize endogenous stem cells in efforts intestinal and ovarian tissue regeneration in rats with protein energy malnutrition
