Dissociative Photoionization of 1,2-Epoxyoctane Studied with Synchrotron Radiation
Yang-yang Wang,Ya-wei Liu,Xu Kang,Xiao-li Zhao,Lin Wang,Chuo-zhao Hu, Fu-yi Liu,Liu-si Sheng,Lin-fan Zhu∗a.Hefei National Laboratory for Physial Sienes at the Mirosale and Department of Modern Physis, University of Siene and Tehnology of China,Hefei 230026,Chinab.Synergeti Innovation Center of Quantum Information and Quantum Physis,University of Siene and Tehnology of China,Hefei 230026,China.National Synhrotron Radiation Laboratory,University of Siene and Tehnology of China,Hefei 230029,China
Dissociative Photoionization of 1,2-Epoxyoctane Studied with Synchrotron Radiation
Yang-yang Wanga,b,Ya-wei Liua,b,Xu Kanga,b,Xiao-li Zhaoa,b,Lin Wanga,b,Chuo-zhao Hua,b, Fu-yi Liuc,Liu-si Shengc,Lin-fan Zhua,b∗
a.Hefei National Laboratory for Physical Sciences at the Microscale and Department of Modern Physics, University of Science and Technology of China,Hefei 230026,China
b.Synergetic Innovation Center of Quantum Information and Quantum Physics,University of Science and Technology of China,Hefei 230026,China
c.National Synchrotron Radiation Laboratory,University of Science and Technology of China,Hefei 230029,China
Dissociative photoionization of 1,2-epoxyoctane was investigated by synchrotron radiation vacuum ultraviolet photons in the energy region of 9.8−16.6 eV under ultrasonic molecular beam.Dissociative fragment ions were measured with reflection time-of-flight mass spectrometer at different photon energies.Appearance potentials of the dominative ion fragments were determined through photoionization efficiency curves.The structures and energies of the parent,ionized and neutral radicals were obtained with G3 calculations. Through comparing the experimental results with the theoretical calculations,we proposed the dissociative channels for the photoionization of 1,2-epoxyoctane.
1,2-Epoxyoctane,Dissociative photoionization,Appearance potential,Dissociation channel
I.INTRODUCTION
Epoxides are widely distributed in nature.With two interesting structural features which are the strong ring strain and the polar covalent bonds with an oxygen atom in the ring,the 1,2-epoxy functional group,in a large number of natural epoxy products,has been found playing a key role in biological growth,heredity,and atrophy[1−3].As a member with long-straight chain,1,2-epoxyoctane(c-(CHCH2O)(CH2)5CH3,C8H16O)has been used to test the effect of the catalysts for rearrangement or used as organic solvents[4−7].Furthermore,1,2-epoxyoctane is also a kind of important industrial chemicals for pesticides,surfactants,etc.
Compared with the electron impact ionization and laser photoionization,the vacuum ultraviolet(VUV) photoionization has obvious advantages in the research of dissociative ionization of atoms and molecules[8,9]. With the features of high brightness,high collimation and continuously adjustable wavelength,synchrotron radiation matches the requirements of molecular photoionization and photodissociation investigations where the wide photon energy region is often needed,and the monoenergetic photons can be easily obtained by using grating monochromator.Under the photon energy higher than the molecular ionization threshold, molecules can be ionized directly or be excited to the superexcited states.The directly ionized or autoionized(from the superexcited state)parent ions may be unstable,and they can dissociate into daughter fragments corresponding to the dissociative photoionization.Photoionization mass spectroscopy is often used to study the molecular photoionization process with time-of-flight(TOF)mass spectrometer to identify the fragmented ions.Then the important photochemical properties such as the photoabsorption cross section, ionization efficiency,molecular ionization energy,appearance potentials(APs)of the fragment ions,dissociation energy,as well as the reaction channels,can be determined.In addition,technique of the ultrasonic jet in photoionization mass spectroscopy can improve the accuracy of the measured ionization energy and appearance potentials,as it can remove the hot-band effect effectively and avoid ion-molecule or ion-ion reactions which will induce the dissociation[10−12].
Previous studies about photoionization of epoxides mainly focus on ethylene oxide,propylene oxide and their derivatives[1−3].As for 1,2-epoxyoctane,only the electron-impact ionization[13]was reported.In the present work,the appearance potentials of ion fragments of 1,2-epoxyoctane are determined,and the dissociative photoionization of 1,2-epoxyoctane in the photon energy region of 9.8−16.6 eV is investigated.
∗Author to whom correspondence should be addressed.E-mail: lfzhu@ustc.edu.cn
II.EXPERIMENTAL METHOD
Details of the experimental setup and computational methods employed in this work were described in Refs.[10,14−16],here we only give a brief description. The experiment was performed at the beamline U14 of atomic and molecular physics at National Synchrotron Radiation Laboratory in Hefei. The photon energy range of 9.8−16.6 eV was employed with an energy resolution of 10 meV at 12.4 eV.1,2-Epoxyoctane was purchased from Shanghai Aladdin Reagent Co.Ltd.with a claimed purity of 97%without further purification. The sample of 1,2-epoxyoctane was introduced by supersonic expansion through a 1 mm skimmer from the molecular beam chamber into the ionization chamber. We used filtering gases before the interaction chamber to filter out the high-order harmonic component.Argon was used as both filtering gases and carrier gases in the photon energy range of 9.8−11.6 eV because it is untransparent for the corresponding high order harmonic photons with energies higher than 19.6 eV considering the ionization energy 15.76 eV of argon.Similarly neon was used in the photon energy range of 11.6−16.6 eV [14].Although reduced greatly by the filtering gases, the high-order harmonic photons could not be filtered out completely since the filtering gases also attenuate the incident light,and it will be discussed below.The typical pressure of the ionization chamber was 10−4Pa during the experiment.A homemade reflection TOF mass spectrometer was used to collect the fragmented ions produced in the photoionization,and the ion signals amplified by a pre-amplifier were collected by ultrafast data acquisition card and the counts of fragmented ions under different scanning photon energies were finally obtained.The mass resolution of the present work is about 1000.
III.RESULTS AND DISCUSSION
Photoionization mass spectrum of 1,2-epoxyoctane at the photon energy of 15.5 eV is shown in Fig.1(a),along with the one(Fig.1(b))on NIST Chemistry Book measured by the electron impact method[13].It can be seen from Fig.1(a)that the peak of the parent ion C8H16O+at m/z=128 is hardly observed,which implies that the parent ion is instable and it can dissociate quickly.Figure 1(a)also shows several clusters formed by the fragment ions with different carbon atoms,which is similar to the electron impact result[13],although the relative intensities of the mass peaks measured by these two methods are different.It is easily understood since the energy transfer and momentum transfer in the electron impact are variable,while for this work the mass spectrum shown in Fig.1(a)was measured at 15.5 eV, i.e.,the accepted energy and momentum by the 1,2-epoxyoctane are definite.Among the natural isotopes of carbon and hydrogen,the abundance of12C is 98.9% while1H is 99.985%.So in the measured spectra the contributions of the isotopes of13C,14C,2H(D)and3H(T)can be neglected.
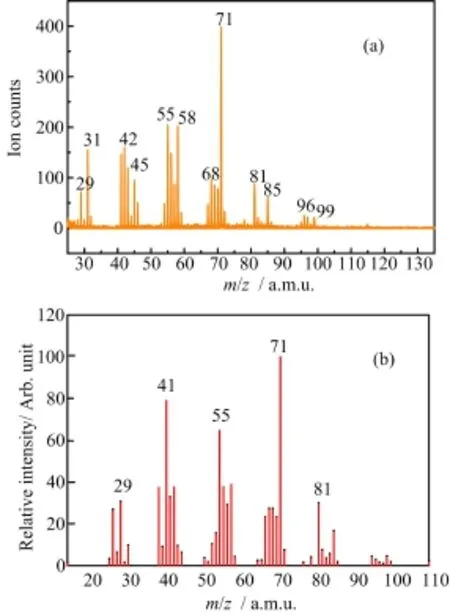
FIG.1 (a)Photoionization massspectrum of1,2-epoxyoctane at the photon energy of 15.5 eV,(b)mass spectrum of 1,2-epoxyoctane on NIST chemistry book[13].
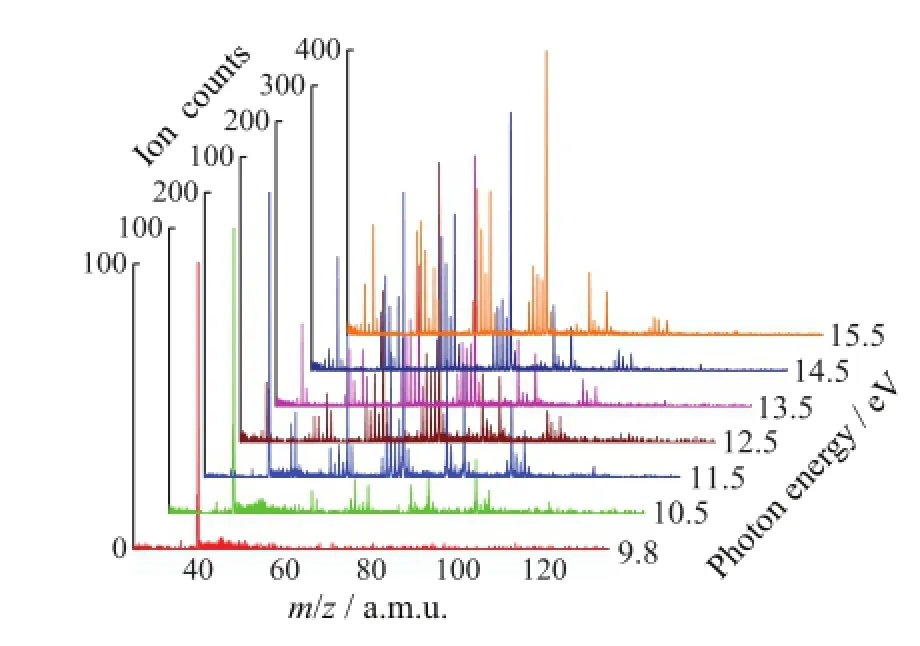
FIG.2 Photoionization mass spectra of 1,2-epoxyoctane at different photon energies.
Figure 2 shows the photoionization mass spectra of 1,2-epoxyoctane at different photon energies from 9.8 eV to 15.5 eV.It can be seen that with higher energy of the VUV photons,more clusters of fragments were detected.Due to the high-order harmonic component,the Ar+(m/z=40)was observed due to the ionization from the carrier gas when the photon energy is lower than 11.5 eV.The saturated vapor pressure of 1,2-epoxyoctane was 0.2 kPa at 25°C[17],and the pressure of the carrier gas argon were 0.18 MPa.From Fig.2 the counts of Ar+at low energies showed that theeffect of the high-order harmonic photons was negligible.From Fig.1 and Fig.2,we can see that the intensity of the mass peak m/z=71 is the highest,which is in agreement with the spectrum in NIST database carried out by electron impact[13].
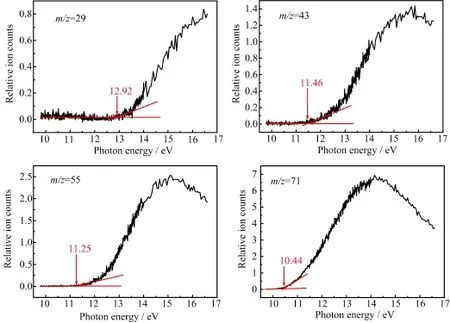
FIG.3 PIE curves of mass peaks at m/z=29,43,55,and 71.
The intensity curve of a specific ion along with the excitation photon energy is called the photoionization efficiency(PIE)curve.PIE curves of some main mass peaks are shown in Fig.3.the appearance potentials are determined by the linear extrapolation method[3,18, 19]relating to the rising section of the PIE curve and are shown in Table I.Choosing different rising ranges may lead to different values of APs.The experimental errors of the APs are 0.05 eV considering the different choices of the rising regions.From the obtained experimental APs of the fragmented ions,possible dissociation channels of the parent can be determined with the aids of the calculated energies of the parent and its daughter fragments.Then we will discuss the dissociative photoionization channels of 1,2-epoxyoctane.
Since one peak may contain different kinds of fragments with the same molecular weight,we need to compare the experimental APs with the theoretical calculations to find the specific dissociation channel. The theoretical APs were calculated by the Gaussian-3(G3)method[20]in Gsussian-09 program[21].The molecular orbitals were calculated by ab initio method as follows: firstly the structure of 1,2-epoxyoctane and the daughter fragments were optimized at the MP2(Full)/6-31G(d)level,then the energies of the parent,neutral and ionized fragments were calculated at the MP4/6-311D(d,p),MP4/6-311+G(d,p),MP4/6-311G(2df,p)and MP2/6-311+G(3df,2p)levels,finally the zero-point vibrational energies were corrected by the calculations at the HF/6-31G(d)level.Table II shows the calculated results of the major radicals.Then the theoretical dissociation energies,APs and other information of the fragmented ions can be determined by these G3 calculations.
It is well known that the dissociation channels of polyatomic molecules are complicated.For perspicuousness, we simply classify the dissociative ionization channels of 1,2-epoxyoctane into three types:bond cleavage reaction,reaction involving transition structure and reaction producing multiple radicals according to the fragmented species.If the reaction produces one ion accompanying with more than one radical,it is called reaction producing multiple radicals.If the reaction produces one ion and one radical with these fragments retaining their skeleton as in 1,2-epoxyoctane,we call it bond cleavage reaction.If the structure of the ion and radical is different from that in 1,2-epoxyoctane,it is called reaction involving transition structure.
Firstly,we will discuss dissociative ionization channels of bond cleavage reaction.Herein we take the mass peak at m/z=57 as an example.The peak m/z=57 may contain two types of ions as C4H9+and C3H5O+,which can be attributed to the simple bond cleavage reactions of the parent ion,and the corresponding neutral radicals are C4H7O and C5H11respectively.Possible dissociation channels are:
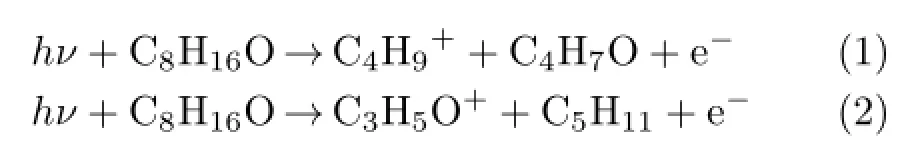
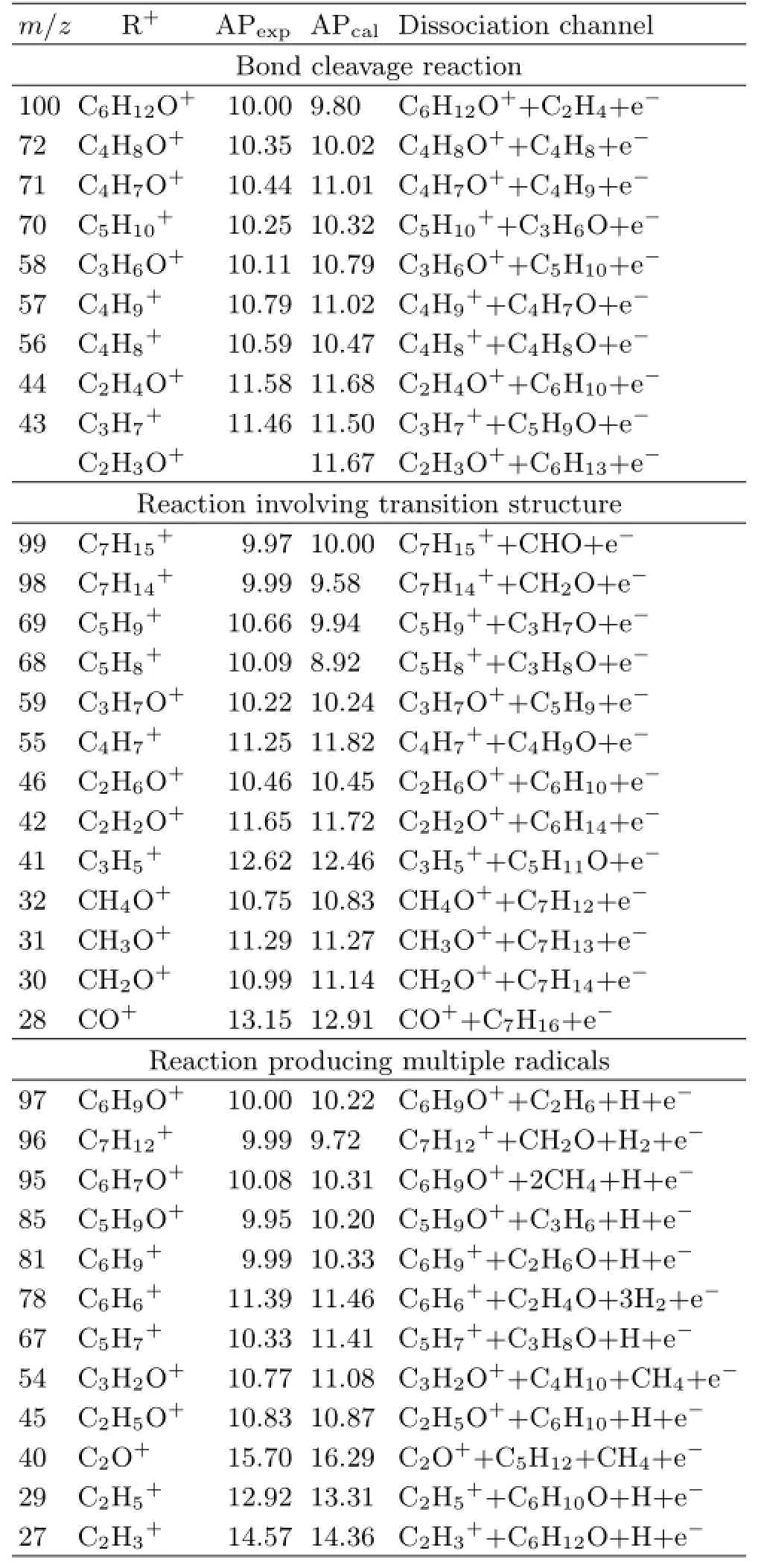
TABLE I Experimental and calculated APs(in eV)of main fragmented ions and possible dissociative ionization channels of hν+C8H16O.The experimental errors of the APs are 0.05 eV.
For channels(1)and(2),according to the calculated energies of the fragment ions and radicals in Table II, the APs of C4H9+and C3H5O+are as follows:
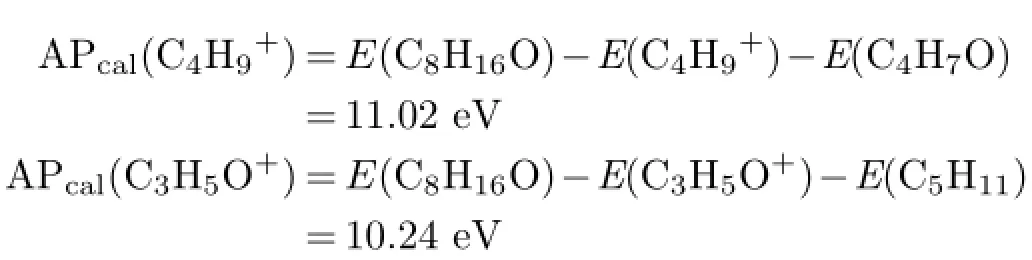
As the experimental AP shown in Fig.3 is 10.79 eV, the discrepancies of APcal(C4H9+)is smaller.Therefore the dissociation channel shown by formula(1)is speculated to be more reasonable.
It should be pointed out that in the discussion above we ignored the influences of kinetic shift,internal energy and reverse activation barriers,and the experimental APs corresponds to the vertical APs which are the uplimit values.
Secondly,reaction involving transition structure may follow similar analysis. Possible fragment ions of m/z=99 are C7H15+and C6H11O+. If the ion is C6H11O+,the generated neutral radical through bond cleavage is C2H5,and the dissociation channel is:

With the G3 energies from Table II,the calculated AP can be obtained through
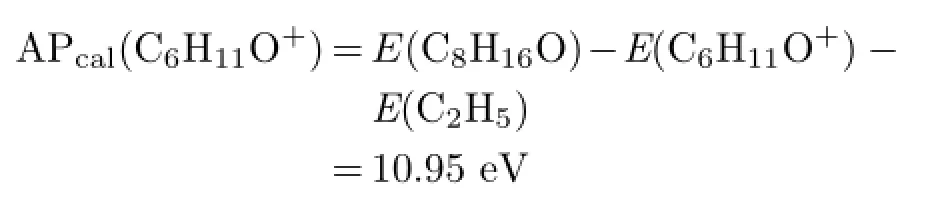
Fragment ion of C7H15+and the corresponding radical CHO may come from hydrogen transfer as:
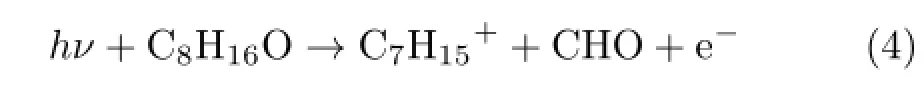
And the calculated AP is
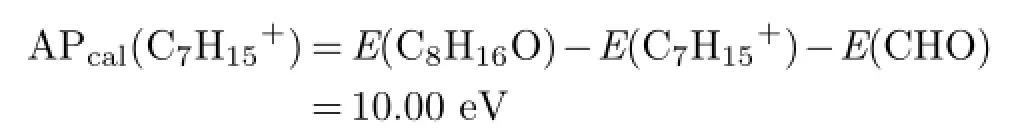
We find a smaller difference between APcal(C7H15+) and the experimental value of 9.97 eV,and the dissociation channel may be concluded as channel(4).
Thirdly,reaction producing multiple radicals will be analyzed.Herein we take m/z=45 as an example and the corresponding reactions are more complex.The fragmented ion can only be C2H5O+,and the reaction may be
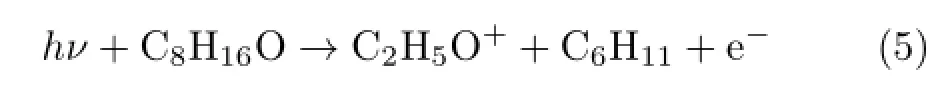
The energy computed on the basis of Table II is

APexp(C2H5O+)from the experiment is 10.83 eV and is much higher than the value of AP1cal(C2H5O+).Possible explanation is that more than one neutral radicals are generated.The relevant reaction and the theoretical calculations are like
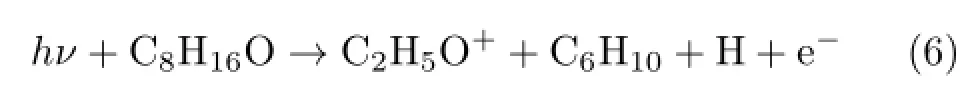

TABLE II Calculated energies of major fragmented ions and radicals by G3 method.
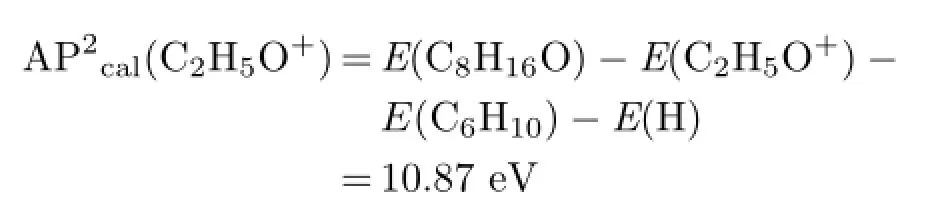
AP2cal(C2H5O+)is much close to APexp(C2H5O+),and we can treat channel(6)as the proper reaction.
We find that the mass peak of m/z=78 is special. The matched components may be C6H6+and C5H2O+. As we can not find a stable structure of C5H2O+,we consider C6H6+as the daughter ion.The skeleton of the parent 1,2-epoxyoctane has a long-straight chain, however we can hardly speculate a chain structure for the fragment C6H6+.Guessing the C6H6+as benzene ion which has a cyclic structure with a continuous π bond,we try a dissociation channel like
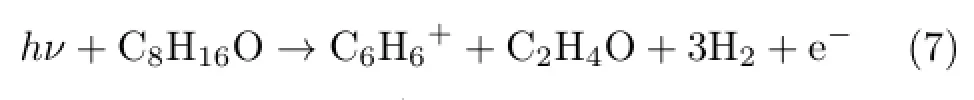
Calculated AP of C6H6+is
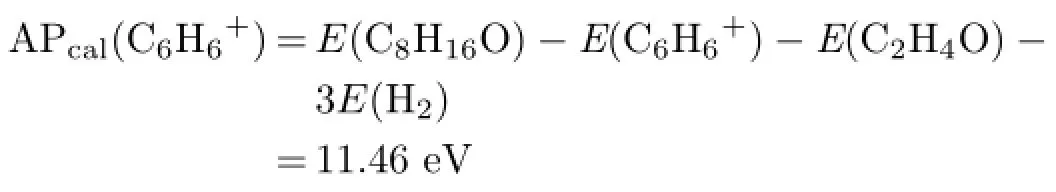
APexp(C6H6+)is 11.39 eV and it is in good agreement with APcal(C6H6+)within experimental error. Therefore channel(7)can be regarded as a reasonable dissociation channel.
Similarly,dissociation channels of other fragmented ions can also be determined by comparing the theoretical calculations with the experimental results as discussed above. Main dissociative ionization channels of 1,2-epoxyoctane have been classified into the abovementioned types and listed in Table I.
IV.CONCLUSION
Dissociative photoionization of 1,2-epoxyoctane was studied with vacuum ultraviolet photoionization and supersonic molecular beam.With the help of Gaussian-09 program,energies of the ionic and neutral fragments generated from the dissociative photoionization processes were calculated.Appearance potentials of the fragmented ions have been determined by the photoionization efficiency curves,most of them were reported for the first time.Dissociative photoionization channels of 1,2-epoxyoctane were elucidated based on the experimental results and theoretical calculations.The presently determined physical quantities provide a supplement to the fundamental thermodynamic parameters.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.U1332204,No.11274291, and No.11320101003)and the USTC-NSRL Association Funding(No.KY2030040002). G3 calculations were carried out at the Supercomputing Center of University of Science and Technology of China.
[1]F.Y.Liu,F.Qi,H.Gao,L.S.Sheng,Y.W.Zhang, S.Q.Yu,K.C.Lau,and W.K.Li,J.Phys.Chem.A 103,4155(1999).
[2]F.Y.Liu,L.S.Sheng,F.Qi,H.Gao,C.X.Li,Y.W. Zhang,S.Q.Yu,K.C.Lau,and W.K.Li,J.Phys. Chem.A 103,8179(1999).
[3]F.Y.Liu,C.X.Li,G.H.Wu,H.Gao,F.Qi,L.S. Sheng,Y.W.Zhang,S.Q.Yu,S.H.Chien,and W.K. Li,J.Phys.Chem.A 105,2973(2001).
[4]H.M.Krieg,J.C.Breytenbach,and K.Keizer,J.Membrane Sci.180,69(2000).
[5]H.M.Krieg,A.L.Botes,M.S.Smith,J.C.Breytenbach,and K.Keizer,J.Mol.Catal.B 13,37(2001).
[6]R.V.Grieken,D.P.Serrano,J.A.Melero,and A. Garcia,J.Mol.Catal.A 222,167(2004).
[7]D.P.Serrano,R.V.Grieken,J.A.Melero,and A. Garcia,Appl.Catal.A 319,171(2007).
[8]C.Y.Ng,Int.J.Mass Spectrom.200,357(2000).
[9]Y.W.Zhang,Physics 24,682(1995).(in Chinese).
[10]Q.X.Li,Q.Ran,C.X.Chen,S.Q.Yu,X.X.Ma, L.S.Sheng,Y.W.Zhang,and W.K.Li,Int.J.Mass Spectrom.Ion Process.153,29(1996).
[11]F.Qi,X.Yang,S.H.Yang,H.Gao,L.S.Sheng,Y.W. Zhang,and S.Q.Yu,J.Chem.Phys.107,4911(1997).
[12]F.Qi,S.H.Yang,L.S.Sheng,H.Gao,Y.W.Zhang, and S.Q.Yu,J.Chem.Phys.107,10391(1997).
[13]NIST Mass Spec Data Center,S.E.Stein,director,“Mass Spectra”in NIST Chemistry WebBook,NIST Standard Reference Database Number 69,P.J.Linstrom and W.G.Mallard,Eds.,Gaithersburg MD:National Institute of Standards and Technology,20899, http://webbook.nist.gov,(retrieved May 10,2015).
[14]Y.Y.Wang,X.Kang,Y.W.Liu,W.Q.Xu,L.Wang, C.Z.Hu,J.Chen,F.Y.Liu,L.S.Sheng,X.L.Zhao,P. F.Zhang,and L.F.Zhu,Int.J.Mass Spectrom.359, 1(2014).
[15]Y.W.Zhang,Synchrotron Rad.News 1,12(1988).
[16]F.Qi,L.S.Sheng,Y.W.Zhang,S.Q.Yu,and W.K. Li,Chem.Phys.Lett.234,450(1995).
[17]SciFinder,version 2015.5;Columbus,OH:Chemical Abstracts Service(2015).RN 2984-50-1(accessed May 10,2015),Calculated using Advanced Chemistry Development(ACD/Labs)Software V11.02(c○1994-2015 ACD/Labs).
[18]F.Qi,S.H.Yang,L.S.Sheng,W.Q.Ye,H.Gao,Y. W.Zhang,and S.Q.Yu,J.Phys.Chem.A 101 7194 (1997).
[19]L.S.Sheng,F.Qi,H.Gao,Y.W.Zhang,S.Q.Yu,and W.K.Li,Int.J.Mass Spectrom.Ion Process.161,151 (1997).
[20]L.A.Curtiss,K.Raghavachari,P.C.Redfern,V.Rassolov,and J.A.Pople,J.Chem.Phys.109,7764 (1998).
[21]M.J.Frisch,G.W.Trucks,H.B.Schlegel,G.E. Scuseria,M.A.Robb,J.R.Cheeseman,G.Scalmani, V.Barone,B.Mennucci,G.A.Petersson,H.Nakatsuji,M.Caricato,X.Li,H.P.Hratchian,A.F.Izmaylov,J.Bloino,G.Zheng,J.L.Sonnenberg,M. Hada,M.Ehara,K.Toyota,R.Fukuda,J.Hasegawa, M.Ishida,T.Nakajima,Y.Honda,O.Kitao,H.Nakai, T.Vreven,J.A.Montgomery Jr.,J.E.Peralta,F. Ogliaro,M.Bearpark,J.J.Heyd,E.Brothers,K.N. Kudin,V.N.Staroverov,R.Kobayashi,J.Normand, K.Raghavachari,A.Rendell,J.C.Burant,S.S.Iyengar,J.Tomasi,M.Cossi,N.Rega,J.M.Millam,M. Klene,J.E.Knox,J.B.Cross,V.Bakken,C.Adamo, J.Jaramillo,R.Gomperts,R.E.Stratmann,O.Yazyev, A.J.Austin,R.Cammi,C.Pomelli,J.W.Ochterski, R.L.Martin,K.Morokuma,V.G.Zakrzewski,G.A. Voth,P.Salvador,J.J.Dannenberg,S.Dapprich,A. D.Daniels,O.Farkas,J.B.Foresman,J.V.Ortiz,J. Cioslowski,and D.J.Fox,Gaussian 09 Revision D.01, Wallingford,CT:Gaussian Inc.(2009).
(Dated:Received on March 21,2016;Accepted on May 7,2016)
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Preparation of Bio-hydrogen and Bio-fuels from Lignocellulosic Biomass Pyrolysis-Oil
- Combination Computing of Support Vector Machine,Support Vector Regression and Molecular Docking for Potential Cytochrome P450 1A2 Inhibitors
- Working Condition Real-Time Monitoring Model of Lithium Ion Batteries Based on Distributed Parameter System and Single Particle Model
- Hydrodeoxygenation of Anisole over Ni/α-Al2O3Catalyst
- Highly Efficient and Selective Removal of Pb(II)ions by Sulfur-Containing Calcium Phosphate Nanoparticles
- Efficient Removal Phenol Red over Ternary Heterostructured Ag-Bi2MoO6/BiPO4Composite Photocatalyst
