Sub-nanometer-thick Al2O3Overcoat Remarkably Enhancing Thermal Stability of Supported Gold Catalysts
Chunlei Wang,Junling LuDepartment of Chemical Physics,Hefei National Laboratory for Physical Sciences at the Microscale, iChEM(Collaborative Innovation Center of Chemistry for Energy Materials),CAS Key Laboratory of Materials for Energy Conversion,University of Science and Technology of China,Hefei 230026,China
Sub-nanometer-thick Al2O3Overcoat Remarkably Enhancing Thermal Stability of Supported Gold Catalysts
Chunlei Wang,Junling Lu∗
Department of Chemical Physics,Hefei National Laboratory for Physical Sciences at the Microscale, iChEM(Collaborative Innovation Center of Chemistry for Energy Materials),CAS Key Laboratory of Materials for Energy Conversion,University of Science and Technology of China,Hefei 230026,China
Supported gold nanoparticle catalysts show extraordinarily high activity in many reactions. While the relative poor thermal stability of Au nanoparticles against sintering at elevated temperatures severely limits their practical applications.Here atomic layer deposition(ALD) of TiO2and Al2O3was performed to deposit an Au/TiO2catalyst with precise thickness control,and the thermal stability was investigated.We surprisingly found that sub-nanometerthick Al2O3overcoat can sufficiently inhibit the aggregation of Au particles up to 600°C in oxygen.On the other hand,the enhancement of Au nanoparticle stability by TiO2overcoat is very limited.Diffuse reflectance infrared Fourier transform spectroscopy(DRIFTS)of CO chemisorption and X-ray photoelectron spectroscopy measurements both confirmed the ALD overcoat on Au particles surface and suggested that the presence of TiO2and Al2O3ALD overcoat on Au nanoparticles does not considerably change the electronic properties of Au nanoparticles.The catalytic activities of the Al2O3overcoated Au/TiO2catalysts in CO oxidation increased as increasing calcination temperature,which suggests that the embedded Au nanoparticles become more accessible for catalytic function after high temperature treatment,consistent with our DRIFTS CO chemisorption results.
Au/TiO2,Sintering,Atomic layer deposition,Al2O3overcoat,Nanoparticle stability
I.INTRODUCTION
Since the first discovery that oxide supported gold nanoparticles could be catalytically active in CO oxidation,gold catalysts have drawn extensive attention in many reactions,including CO oxidation[1,2],hydrocarbons oxidations[3],water-gas-shift(WGS)reaction [4],H2O2synthesis[5],preferential CO oxidation in rich hydrogen[6]and selective epoxidation[7].In these reactions,supported Au nanoparticle catalysts show remarkably high activity and selectivity[8,9].
Nonetheless,the applications of Au catalysts in industrial processes are still very limited due to several issues[10,11].One of the most important limitations is sintering of gold nanoparticles under reaction conditions,which causes severe catalyst deactivation due to the decreased number of exposed Au atoms[12] and the much lower activity of Au nanoparticles larger than~5 nm due to the well-known strong size dependence of Au catalysts[3,13].For instance,Valden et al.observed that sintering of the Au nanoparticles occurs rapidly,once the Au/TiO2(110)catalyst was exposed to a mixture of CO and O2at room temperature [14].Lu et al.reported that Ostwald riping of the Au nanoparticles on CeO2(111)mainly proceeds along the step edges of the CeO2(111)support in a mixture of CO and O2at much lower pressure(~1µbar)at room temperature according to the scanning tunneling microscopy(STM)[15].Luengnaruemitchai et al.observed a dramatic decrease of CO conversion from 60% to about 10%within 48 h over CeO2supported Au catalyst in the WGS reaction.In the spent catalyst,an increase of average size of Au nanoparticles from 4 nm to 5.5 nm observed by X-ray powder diffraction(XRD) and transmission electron microscopy(TEM)was suggested to be the main reason for the deactivation[16].
In order to improve the thermal stability of Au/TiO2catalyst,two different strategies have been reported. One is to modify the support to strengthen the metalsupport interaction between the Au particles and the support[17−19].For instance,Yan and his co-workers achieved an ultra-stable Au catalyst up to 500°C by modifying the TiO2surface with aluminum oxide via a surface sol-gel process[13]. Zhang et al. modified a FeOxsupport with hydroxyapatite(HAP, Ca10(PO4)6(OH)2),wherein Au nanoparticles were able to be stabilized by the OH−and PO43−group of HAPfor calcination at as high as 600°C[20].Another common method is to deposit an oxide protective layer onto Au nanoparticles.Encapsulation of metal nanoparticles with an oxide protecting layer using techniques such as chemical vapor deposition and sol-gel chemistry provides an effective way to improve the stability of metal nanoparticles[21−25].However,the thick protective layer(usually tens of nanometers thickness)often results in a large decrease in catalytic activity due to mass transfer resistance.A method of atomically precise control over the thickness and composition of protective layers is highly desirable to preserve catalytic activity to the greatest extent while improving the stability.For instance,Zhu et al.reported an Au/TiO2based catalyst with enhanced thermal stability through post modification of Au by amorphous SiO2layer using sol-gel chemistry[26].In this case,the thickness of oxide films is often lack of precise control which might cause a large decrease in catalytic activity due to mass transfer resistance.As a consequence,it could be very critical to achieve precise control over the oxide overcoat thickness to preserve catalytic activity to the greatest extent while improving the stability.
∗Author to whom correspondence should be addressed.E-mail: junling@ustc.edu.cn
Atomic layer deposition(ALD),a thin film growth technique through self-limiting binary reactions between gaseous precursors and the substrate[27,28], has shown great advantages in applying ultrathin and conformal coatings on supported metal catalysts to improve the thermal stability of metal catalysts[29−31]. Nonetheless,applying ALD oxide overcoat onto supported Au catalysts has been very limited.Ma et al. first explored to apply SiO2overcoat on Au/TiO2using ALD,therein,the stability of Au nanoparticles was remarkably improved by the thin SiO2overcoat,however,sintering of Au nanoparticles still happened once the sample was calcined at above 500°C[32].Therefore,SiO2overcoat is not a proper material to improve the thermal stability of supported Au particles.Biener et al.demonstrated that an Al2O3film grown by ALD with even 1 nm thickness can stabilize the nanoporous bulk gold up to 1000°C,much more sufficient than an 2 nm thick TiO2overcoat grown by ALD which can stabilize the nanoporous bulk gold up to 600°C[33]. We recently demonstrated that TiO2ALD overcoat on Au/Al2O3catalyst significantly improved the CO oxidation activity,where we found that TiO2overcoat was preferentially grown at the low-coordination sites of Au nanoparticles[34].However,applying TiO2and Al2O3ALD overcoat onto supported Au catalysts for improving thermal stability of Au nanoparticles has not been reported.
In the present work,we applied different cycles of TiO2and Al2O3overcoat on an Au/TiO2catalyst using ALD and compared the stability of the resulting TiO2and Al2O3overcoated Au/TiO2catalysts after calcination at 500 and 600°C,respectively.We surprisingly found that sub-nanometer-thick Al2O3overcoat can sufficiently inhibit the aggregation of Au particles during calcination at as high as 600°C.On the other hand,the enhancement of Au nanoparticle stability by TiO2overcoat is not obvious.Al2O3overcoated Au/TiO2catalysts in CO oxidation reaction showed that the catalytic activity was almost completely lost, which suggests that the ultrathin Al2O3ALD overcoat on Au nanoparticles is very dense,making the embedded Au nanoparticles almost inaccessible for catalytic function. Nonetheless,high temperature calcination could make the Al2O3ALD overcoat become porous and activate the catalyst to a large extent.
II.EXPERIMENTS
A.Au/TiO2catalyst synthesis
An Au/TiO2catalystwasprepared usingthe deposition-precipitation (DP) method with urea [35,36],Titania Degussa P25(BET surface area SBET=45 m2/g,nonporous,70%anatase and 30% rutile,purity>99.5%)wereused asthesupport. HAuCl4·4H2O(Sinopharm Chemical Reagent Co,Ltd., Au content of 47.8%)was used as the Au precursor. Typically,3 g of TiO2was added to 100 mL aqueous solution,which contained HAuCl4(4.2×10−3mol/L) and urea(0.42 mol/L)at an initial pH of 2. The mixture was vigorously stirred at 75°C for 8 h in the absence of light to avoid the gold precursor decomposition. Next,the suspension was centrifuged and thoroughly washed with deionized water for at least six times to remove chlorine residual.Finally,the obtained material was dried at 80°C in air overnight and further calcined at 250°C in 10%O2in He to obtain the catalyst.The gold contents in Au/TiO2catalyst were 2.5%determined by an inductively coupled plasma atomic emission spectrometer(ICP-AES).
B.TiO2and Al2O3ALD overcoating
TiO2ALD overcoating on Au/TiO2catalysts was performed in a viscous flow reactor(GEMSTAR-6TM Benchtop ALD,Arradiance)at 150°C by alternatively exposing titanium isopropoxide(TTIP,99.7%,Sigma-Aldrich)and Millipore water for different ALD cycles. The TTIP precursor was heated to 80°C to get a reasonable vapor pressure,while the water source was kept at room temperature[34].The timing sequence of TiO2ALD was 10,200,8,and 250 s for TTIP exposure,N2purge,water exposure,and N2purge,respectively.The resulting catalysts are denoted as xcTi-Au/TiO2(the number of ALD cycles,x=20 and 50,and Ti was used to represent the TiO2overcoat).Al2O3ALD coating on the Au/TiO2catalyst was performed by alternative exposures to trimethylaluminum(TMA,Sigma Aldrich, 99%)and Millipore water at 150°C for different cycles [29,30,37],the TMA precursor was kept at room tem-perature.The timing sequence of Al2O3ALD was 8, 200,6,and 250 s for TMA exposure,N2purge,water exposure,and N2purge,respectively.The resulting catalysts are denoted as xcAl-Au/TiO2(the number of ALD cycles,x=2 and 4,and Al was used to represent Al2O3overcoat).All the catalysts with and without overcoats were calcined at 500 and 600°C in 10%O2in Ar for 1 h to examine the thermal stability of Au catalysts.
C.Characterization
TEM measurements were performed on a JEOL-2010 instrument operated at 200 kV.X-ray photoelectron spectroscopy(XPS)measurements were taken on a Thermo-VG Scientific Escalab 250 spectrometer equipped with an aluminum anode(Al Kα=1486.6 eV). The binding energies in XP spectra were referenced to the C 1s binding energy at 284.8 eV.These three facilities,as well as ICP-AES,belong to the Instruments' Center for Physical Science,University of Science& Technology of China.
Diffuse reflectance infrared Fourier transform spectroscopy(DRIFTS)measurements of CO chemisorption were carried out on a Nicolet iS10 spectrometer, equipped with an MCT detector and a low temperature reaction chamber(Praying Mantis Harrick).After loading a sample to the reaction chamber,the sample was first calcined at a specified temperature in 10%O2in He for 30 min and then reduced in 10%H2in He for another 30 min.A background spectrum was collected after cooling the sample to room temperature in He.Subsequently,the sample was exposed to 10%CO in He at a flow rate of 20 mL/min for about 15 min until saturation.In order to avoid any variations caused by the decline of CO chemisorption peak during He purge[34],here we collected the DRIFT spectra of all samples during the flow of 10%CO in He.The CO chemisorption spectra were obtained by subtracting the CO chemisorption DRIFT spectrum of TiO2to remove the gas phase CO.All DRIFT spectra were collected with 256 scans at a resolution of 4 cm−1.
D.Catalytic activity
The activities of Au/TiO2and xcAl-Au/TiO2catalysts in CO oxidation reaction were conducted using a fixed-bed tubular quartz reactor at atmospheric pressure. The inner diameter of the reactor was about 0.8 cm. For the reaction test,50 mg of uncoated Au/TiO2catalyst was diluted by 1 g of fine quartz chips(60/80 mesh)to avoid any hot spots.A thermal couple protected by a one-end closed quartz-tube was directly inserted into the catalyst bed to measure the sample temperature.For other xcAl-Au/TiO2catalysts,the amount of catalyst was adjusted to keep the same Au content with the uncoated Au/TiO2catalyst. After loading a catalyst to the quartz reactor,the catalyst was first calcined in 10%O2in He at a flow rate of 40 mL/min at a specified temperature for 1 h.Next, the reaction gas consisting of 2%CO and 8%O2balanced in He was fed to the reactor at a total flow rate of 50 mL/min.The reaction products were analyzed by an on-line gas chromatograph(Fuli,GC-9790II)with a thermal conductivity detector.The CO conversion was calculated based on the ratio of the consumed CO to the fed CO.
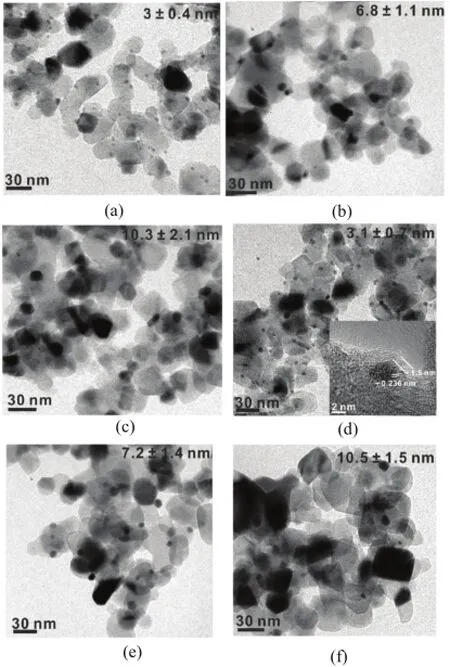
FIG.1 TEM images of(a)uncoated Au/TiO2catalyst asprepared,calcined at(b)500°C and(c)600°C,respectively. TEM images of(d)50cTi-Au/TiO2catalyst as-prepared, calcined at(e)500°C and(f)600°C,respectively.The TiO2overcoat is shown in the inset of(d).
III.RESULTS AND DISCUSSION
A.Thermal stability of xcTi-Au/TiO2and xcAl-Au/TiO2catalysts
An Au/TiO2catalyst with a particle size of 3±0.4 nm was synthesized using the DP method[38],as shown in Fig.1(a).The Au loading of the catalyst was determined to be 2.5%using ICP-AES.Remarkable sintering by increasing the Au particle size to 6.8±0.4 and 10.3±2.1 nm was observed after calcining the Au/TiO2catalyst in 10%O2in Ar at 500 and 600°C for 1 h,respectively(Fig.1(b)and(c)),consistent with previous observation[13].Applying TiO2overcoating onto the Au/TiO2catalyst by ALD at 150°C would not change the Au particle size,indicated by Fig.1(d)[34]. The insert of Fig.1(d)shows that the thickness of TiO2overcoat on 50cTi-Au/TiO2was about 1.5 nm,which indicates the growth rate of TiO2ALD was about 0.3˚A/cycle,consistent with reports in Ref.[34].After calcining 10cTi-Au/TiO2(not shown here)and 50cTi-Au/TiO2in 10%O2in Ar at 500 and 600°C for 1 h, significant sintering occurred,showing nearly identical Au particle sizes with the uncoated Au/TiO2catalyst after the same treatment(Fig.1(e)and(f)).Obviously, the TiO2overcoat did not considerably improve the stability of Au nanoparticles,which is different from the observation by Biener et al.,where TiO2ALD overcoat can stabilize nanoporous bulk Au up to 600°C.This might not be surprising,since Au nanoparticle is more unstable than bulk Au.
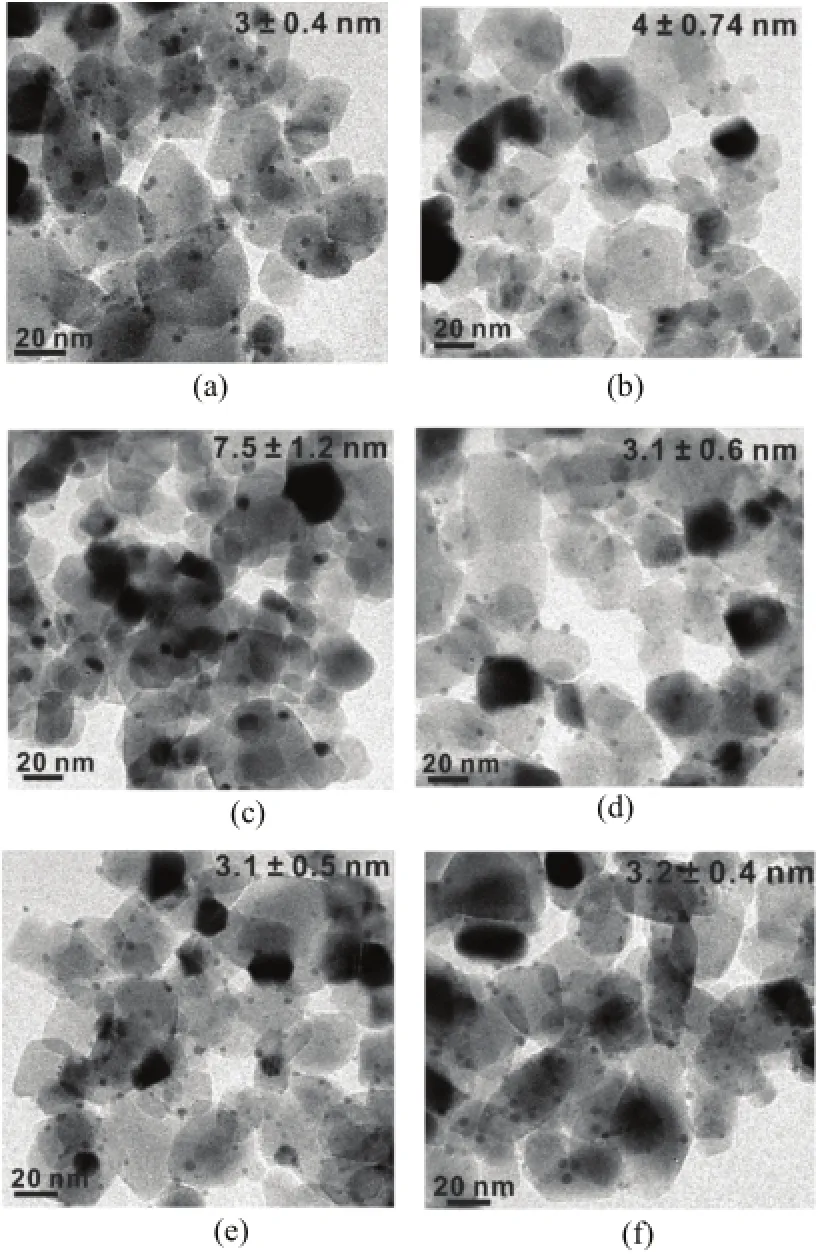
FIG.2TEM images of(a)2cAl-Au/TiO2catalyst asprepared,calcined at(b)500°C and(c)600°C,respectively. TEM images of(d)4cAl-Au/TiO2catalyst as-prepared,calcined at(e)500°C and(f)600°C,respectively.
On the contrary,the stability of Au nanoparticles was largely improved by even two ALD cycles of Al2O3overcoat(2cAl-Au/TiO2),where calcination at 500°C only slightly increased the Au particle size from 3±0.4 nm to 4±0.74 nm(Fig.2(a)and(b)).And very surprisingly,we found that four ALD cycles of Al2O3overcoat completely inhibited the sintering of Au particles up to 600°C,where no changes in Au particle size were observed(Fig.2(d−f)).According to the growth rate reported in Refs.[27,39],two and four cycles of Al2O3ALD should produce approximately 0.3 and 0.5 nm thickness of amorphous alumina films respectively,even though we did not observe the Al2O3overcoating layer on our samples under current resolutions.

FIG.3 DRIFT spectra of CO chemisorption on(a)10cTi-Au/TiO2and(b)2cAl-Au/Al2O3catalysts after calcination at different temperatures of 250,350,and 450°C.
B.DRIFTS of CO chemisorption
DRIFTS of CO chemisorption measurements were further carried out to investigate the accessibility of embedded Au nanoparticles under the TiO2and Al2O3overcoat.As shown in Fig.3,the uncoated Au/TiO2sample showed a strong CO peak at 2103 cm−1,which is assigned to linear CO on low-coordination neutral Au sites[40−43].On both 10cTi-Au/TiO2and 2cAl-Au/TiO2catalysts after calcination at 250°C,the intensity of CO peak both decreased dramatically,directly implying the presence of oxide overcoats on Au nanoparticles(Fig.3).In both cases,the shifts of CO peak by overcoat were minor,thus the electronic properties of Au nanoparticles are not considerably altered by these overcoats.Meanwhile,we also noticed that increasing calcination temperatures could increase the in-tensity of CO chemisorption peaks,which suggests that high temperature treatment caused the change in the structures of TiO2and Al2O3overcoats and allow the embedded Au nanoparticles more accessible.Such observation is consistent with the results of alumina overcoated Pd and Cu catalysts[29,44].
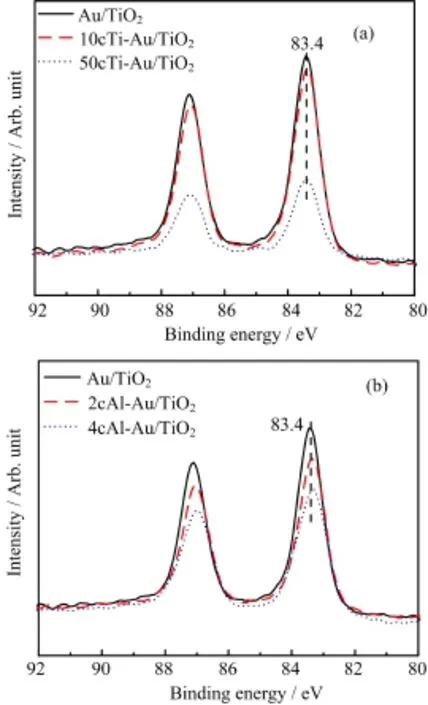
FIG.4 XPS spectra of(a)as-prepared xcTi-Au/TiO2and (b)xcAl-Au/TiO2in the Au 4f region.
C.XPS studies
XPS was also carried out to study the electronic properties of Au nanoparticles after applying different cycles of ALD overcoat.As shown in Fig.4,the Au 4f binding energy remained nearly constant at 83.4 eV after either TiO2or Al2O3ALD overcoat,implying that the ALD overcoat does not considerably change the electronic properties of Au nanoparticles,similar to the case of TiO2overcoated Au/Al2O3catalysts[34]and others reported in literature[45,46].This results also explain the minor shifts of CO chemisorption peak on both 10cTi-Au/TiO2and 2cAl-Au/TiO2in Fig.3.Moreover, the gradual decrease of the intensity of Au 4f binding energy with the increasing number of ALD cycles again confirms the successful deposition of TiO2and Al2O3overcoat on Au nanoparticles.
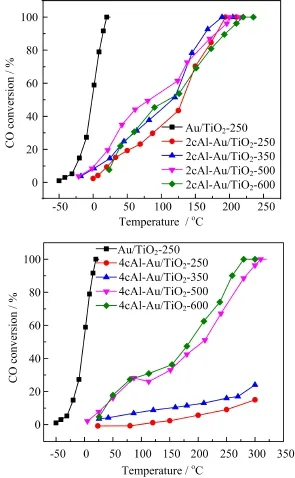
FIG.5 Catalytic activities of(a)2cAl-Au/TiO2and(b) 4cAl-Au/TiO2catalysts in CO oxidation reaction after calcination at different temperatures of 250,350,500 and 600°C. The catalytic activity of uncoated Au/TiO2was shown in both(a)and(b)for comparison.
D.Catalytic activity
Our results have clearly demonstrated that subnanometer Al2O3ALD overcoat can remarkably increase the thermal stability of Au/TiO2catalyst up to 600°C.Here,the catalytic activity of 2cAl-Au/TiO2and 4cAl-Au/TiO2catalyst was further examined using CO oxidation as a probe reaction as shown in Fig.5. Compared with the uncoated Au/TiO2catalyst,the activity largely decreased on 2cAl-Au/TiO2and became almost inactive on 4cAl-Au/TiO2,which is likely due to the blockage of perimeter sites at the Au-TiO2interface,since the perimeter sites are suggested to be the active sites[47,48].Nearly no activity of 4cAl-Au/TiO2in CO oxidation also implies that~0.5 nm thickness of Al2O3ALD overcoat on Au nanoparticles is very dense, making the embedded Au nanoparticles almost inaccessible for catalytic function.This result is consistent with the observation of 1 nm thick Al2O3overcoat on nanoporous gold by Biener et al.[33].Nonetheless, calcining the catalysts at higher temperatures of 350, 500,and 600°C,respectively could make the Al2O3ALD overcoat become porous and activate the catalystto a large extent.It is worthy to note that the 4cAl-Au/TiO2catalyst calcined at 600°C showed a very similar catalytic activity with an Au/Al2O3catalyst(Au particle size:2.9±0.45 nm)in our previous work[34].
IV.CONCLUSION
In order to improve the stability of Au nanoparticles,TiO2and Al2O3ALD were performed in this work to precisely deposit an oxide overcoating layer to an Au/TiO2catalyst.We found that the enhancement of Au nanoparticle stability by TiO2ALD overcoat (<1.5 nm thickness)is very limited below 600°C.On the contrary,sub-nanometer-thick Al2O3overcoat can sufficiently inhibit the aggregation of Au particles up to 600°C during calcination in oxygen.DRIFT of CO chemisorption and XPS measurements both confirmed the ALD overcoat on Au particles surface according to the attenuated intensity of CO peak and Au 4f peak, respectively,and suggested that the presence of TiO2and Al2O3ALD overcoat on Au nanoparticles does not considerably change the electronic properties of Au nanoparticles.In CO oxidation,the nearly complete loss of catalytic activity of 4cAl-Au/TiO2implies that the~0.5 nm thickness of Al2O3ALD overcoat on Au nanoparticles is very dense,making the embedded Au nanoparticles almost inaccessible for catalytic function. Nonetheless,high temperature calcination could make the Al2O3ALD overcoat become porous and activate the catalyst to a large extent.In summary,our work has demonstrated an effective method to stabilize supported Au catalyst using Al2O3ALD overcoat,which might open new opportunities to apply the stabilized Au catalysts in other reactions under severe conditions.
V.ACKNOWLEDGMENTS
This work was supported by the One Thousand Young Talents Program under the Recruitment Program of Global Experts,the National Natural Science Foundation of China(No.51402283 and No.21473169), the Fundamental Research Funds for the Central Universities(No.WK2060030014,No.WK2060190026 and No.WK2060030017),and the Startup Funds from University of Science and Technology of China.
[1]M.Haruta,T.Kobayashi,H.Sano,and N.Yamada, Chem.Lett.16,405(1987).
[2]I.X.Green,W.J.Tang,M.Neurock,and J.T.Yates, Science 333,736(2011).
[3]X.Liu,L.He,Y.M.Liu,and Y.Cao,Acc.Chem.Res. 47,793(2014).
[4]D.Andreeva,V.Idakiev,T.Tabakova,A.Andreev,and R.Giovanoli,Appl.Catal.A 134,275(1996).
[5]P.Landon,P.J.Collier,A.J.Papworth,C.J.Kiely, and G.J.Hutchings,Chem.Commun.2058(2002).
[6]M.M.Schubert,V.Plzak,J.Garche,and R.J.Behm, Catal.Lett.76,143(2001).
[7]A.Sinha,S.Seelan,S.Tsubota,and M.Haruta,Top. Catal.29,95(2004).
[8]M.Haruta,Faraday Discuss.152,11(2011).
[9]G.J.Hutchings,M.Brust,and H.Schmidbaur,Chem. Soc.Rev.37,1759(2008).
[10]G.Pattrick,E.van der Lingen,C.W.Corti,R.J.Holliday,and D.T.Thompson,Top.Catal.30,273(2004).
[11]W.Yan,S.M.Mahurin,S.H.Overbury,and S.Dai, Top.Catal.39,199(2006).
[12]T.W.Hansen,A.T.Delariva,S.R.Challa,and A.K. Datye,Acc.Chem.Res.46,1720(2013).
[13]W.F.Yan,S.M.Mahurin,Z.W.Pan,S.H.Overbury, and S.Dai,J.Am.Chem.Soc.127,10480(2005).
[14]M.Valden,X.Lai,and D.W.Goodman,Science 281, 1647(1998).
[15]J.L.Lu,H.J.Gao,S.Shaikhutdinov,and H.J.Freund, Catal.Lett.114,8(2007).
[16]A.Luengnaruemitchai,S.Osuwan,and E.Gulari, Catal.Commun.4,215(2003).
[17]B.Min,W.Wallace,and D.Goodman,J.Phys.Chem. B 108,14609(2004).
[18]C.M.Wang,K.N.Fan,and Z.P.Liu,J.Phys.Chem. C 111,13539(2007).
[19]V.Rodriguez-Gonzalez,R.Zanella,L.A.Calzada,and R.Gomez,J.Phys.Chem.C 113,8911(2009).
[20]K.Zhao,B.Qiao,J.Wang,Y.Zhang,and T.Zhang, Chem.Commun.47,1779(2011).
[21]S.H.Joo,J.Y.Park,C.K.Tsung,Y.Yamada,P.D. Yang,and G.A.Somorjai,Nat.Mater.8,126(2009).
[22]M.Cargnello,J.J.D.Jaen,J.C.H.Garrido,K. Bakhmutsky,T.Montini,J.J.C.Gamez,R.J.Gorte, and P.Fornasiero,Science 337,713(2012).
[23]A.J.Forman,J.N.Park,W.Tang,Y.S.Hu,G.D. Stucky,and E.W.McFarland,ChemCatChem 2,1318 (2010).
[24]Q.Zhang,I.Lee,J.B.Joo,F.Zaera,and Y.D.Yin, Acc.Chem.Res.46,1816(2013).
[25]M.Seipenbusch and A.Binder,J.Phys.Chem.C 113, 20606(2009).
[26]H.G.Zhu,Z.Ma,S.H.Overbury,and S.Dai,Catal. Lett.116,128(2007).
[27]J.L.Lu,J.W.Elam,and P.C.Stair,Acc.Chem.Res. 46,1806(2013).
[28]H.Yan,H.Cheng,H.Yi,Y.Lin,T.Yao,C.L.Wang, J.J.Li,S.Q.Wei,and J.L.Lu,J.Am.Chem.Soc. 137,10484(2015).
[29]J.L.Lu,B.S.Fu,M.C.Kung,G.M.Xiao,J.W. Elam,H.H.Kung,and P.C.Stair,Science 335,1205 (2012).
[30]H.Feng,J.Lu,P.C.Stair,and J.W.Elam,Catal. Lett.141,512(2011).
[31]X.Liang,J.Li,M.Yu,C.N.McMurray,J.L.Falconer, and A.W.Weimer,Acs Catal.1,1162(2011).
[32]Z.Ma,S.Brown,J.Y.Howe,S.H.Overbury,and S. Dai,J.Phys.Chem.C 112,9448(2008).
[33]M.M.Biener,J.Biener,A.Wichmann,A.Wittstock, T.F.Baumann,M.Baumer,and A.V.Hamza,Nano Lett.11,3085(2011).
[34]C.L.Wang,H.W.Wang,Q.Yao,H.Yan,J.J.Li,andJ.L.Lu,J.Phys.Chem.C 120,478(2016).
[35]R.Zanella,S.Giorgio,C.R.Henry,and C.Louis,J. Phys.Chem.B 106,7634(2002).
[36]L.Delannoy,R.L.Chantry,S.Casale,Z.Y.Li,Y. Borensztein,and C.Louis,Phys.Chem.Chem.Phys. 15,3473(2013).
[37]J.L.Lu,B.Liu,J.P.Greeley,Z.X.Feng,J.A.Libera, Y.Lei,M.J.Bedzyk,P.C.Stair,and J.W.Elam, Chem.Mater.24,2047(2012).
[38]M.Haruta,J.New.Mat.Electr.Sys.7,163(2004).
[39]H.Yi,H.Y.Du,Y.L.Hu,H.Yan,H.L.Jiang,and J. L.Lu,Acs Catal.5,2735(2015).
[40]F.Menegazzo,M.Manzoli,A.Chiorino,F.Boccuzzi,T. Tabakova,M.Signoretto,F.Pinna,and N.Pernicone, J.Catal.237,431(2006).
[41]J.D.Grunwaldt,M.Maciejewski,O.S.Becker,P.Fabrizioli,and A.Baiker,J.Catal.186,458(1999).
[42]K.Qian,L.F.Luo,H.Z.Bao,Q.Hua,Z.Q.Jiang, and W.X.Huang,Catal.Sci.Technol.3,679(2013).
[43]M.Boronat,P.Concepcion,and A.Corma,J.Phys. Chem.C 113,16772(2009).
[44]B.J.O’Neill,D.H.Jackson,A.J.Crisci,C.A.Farberow,F.Shi,A.C.Alba-Rubio,J.Lu,P.J.Dietrich, X.Gu,and C.L.Marshall,Angew.Chem.Int.Ed.52, 13808(2013).
[45]M.M.Gao,C.K.N.Peh,Y.L.Pan,Q.H.Xu,and G. W.Ho,Nanoscale 6,12655(2014).
[46]M.M.Ye,H.H.Zhou,T.Q.Zhang,Y.P.Zhang,and Y.Shao,Chem.Eng.J.226,209(2013).
[47]M.Haruta,Gold Bull.37,27(2004).
[48]M.Haruta,Chem.Rec.3,75(2003).
(Dated:Received on April 2,2016;Accepted on May 18,2016)
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Preparation of Bio-hydrogen and Bio-fuels from Lignocellulosic Biomass Pyrolysis-Oil
- Combination Computing of Support Vector Machine,Support Vector Regression and Molecular Docking for Potential Cytochrome P450 1A2 Inhibitors
- Working Condition Real-Time Monitoring Model of Lithium Ion Batteries Based on Distributed Parameter System and Single Particle Model
- Hydrodeoxygenation of Anisole over Ni/α-Al2O3Catalyst
- Highly Efficient and Selective Removal of Pb(II)ions by Sulfur-Containing Calcium Phosphate Nanoparticles
- Efficient Removal Phenol Red over Ternary Heterostructured Ag-Bi2MoO6/BiPO4Composite Photocatalyst
